Introduction
The giant Chinese salamander (Andrias davidianus) is a relict animal endemic to China that retained morphological features from 165 million years ago and is the largest living amphibian worldwide.1 It has historically enjoyed widespread distribution across the Yangtze, Pearl, and Yellow River basins.2,3 However, anthropogenic activities, such as habitat degradation and overexploitation, have rapidly caused declines in wild populations of this species.3,4 As a result, the International Union for Conservation of Nature (IUCN) has designated this species “critically endangered”,5 making it a top priority for global amphibian conservation efforts.6–8 Despite its precarious conservation status in natural ecosystems, A. davidianus has been subject to more than 50 years of aquaculture and product development in numerous provinces across China owing to its considerable biological, medicinal, and culinary value.9–11 The increased intensity of artificial breeding practices and frequent trade activities have ushered in bacterial infections as an inescapable malady, thereby constituting a significant barrier to the sustainable development of the A. davidianus aquaculture industry, with substantial economic ramifications requiring focused mitigation.12,13
Acinetobacter species are ubiquitously distributed in various environmental matrices, such as water and soil, and are capable of thriving on biotic and abiotic surfaces.14,15 Notably, it is a primary carrier for hospital-acquired infections, including respiratory viral infections, septicemia, urinary tract infections, and wound infections.16,17 Its high mortality rate and multidrug resistance represent considerable threats to public health.18 Furthermore, as zoonotic pathogens, Acinetobacter species exhibit extensive transmission across many animal taxa, including mammals, avians, amphibians, and piscine species.19 This spread endangers global wildlife and domesticated animal populations, culminating in mass morbidity and mortality.20
Zhangjiajie City is renowned as the “Home of the Chinese Giant Salamander,” serving as a critical hub for the distribution and production of these wild creatures in China.21 Numerous farmers engage in the artificial rearing and trading of this species.22 However, as salamander farming has continued to grow in size and intensity, a number of diseases have subsequently surfaced, especially recently discovered illnesses that have severely harmed the industry’s bottom line.21,23 The revenue and viability of A. davidianus farming have been directly threatened by the epidemics that have resulted from this.7 Identification of the diseases and their causative agents will help to develop strategies to control and prevent the incidence of diseases in farmed A. davidianus, thus reducing loss to farmers.
Due to the higher number of sick patients and deaths among A. davidianus at the research site, this project has performed pathogen diagnostics, antibiotic sensitivity tests, reinfection experiment on diseased samples. The present study aimed to understand the characteristics and pathogenicity of this pathogen, it can provide scientific basic data for the healthy breeding of giant salamanders in the future.
Materials and Methods
2.1. Experimental Materials
In February 2023, specimens(n=10) exhibiting typical symptoms and characterized by overt health were collected from a designated A. davidianus aquaculture facility in Zhangjiajie. Subsequently, the specimens were meticulously partitioned into thermal containers and shipped to the Yangtze River Fisheries Research Institute under the Chinese Academy of Fishery Sciences for isolation, identification, and further experimental evaluation of etiological agents.
2.2. Pathogen isolation
Ailing A. davidianus was subjected to topical disinfection using 75% ethanol, followed by aseptic dissection within a biosafety cabinet to expose the abdominal cavity. Freshly dissected kidney, spleen, and intestine organ surfaces were inoculated with sterile inoculating loops before the sample was transferred to Brain-Heart Infusion (BHI; HopeBio, Qingdao, China) agar plates.24 For a full day, the plates were incubated at 28°C. To obtain pure colonies, dominant individual bacterial colonies were chosen after incubation and streaked onto fresh BHI agar plates for 24 hours under the same culture conditions. After being inoculated into 5 mL of BHI liquid media, a few selected individual colonies were grown for approximately 24 hours at 28°C with shaking at 200 rpm. After the bacterial culture was complete, aliquots were placed in 1.5 mL Eppendorf tubes and kept in an ultralow temperature freezer at −80°C. An equivalent volume of glycerol was then added. DN-3 is the designation given to the isolated strain of bacteria.
2.3. Morphological observation
Purified bacterial colonies were streaked onto fresh BHI agar medium and incubated at 28°C for approximately 24 h. After incubation, the colony morphology was assessed. Bacterial suspensions were prepared using phosphate-buffered saline (PBS; Procell, Wuhan, China) as the solvent, followed by Gram staining procedures.24 To examine the ultrastructure of each colony under a scanning electron microscope, we fixed, dehydrated, dried, and coated the cells with gold sputtering agent (Hitachi, Tokyo, Japan).
2.4. 16S rRNA Sequence Analysis
Single bacterial colonies were selected and transferred to 1.5 mL Eppendorf tubes inside a biosafety cabinet. The Bacterial DNA Kit (Tiangen, Beijing, China) procedure was used to extract genomic DNA. Amplification of the 16S rRNA region of the isolated bacterial strain was conducted via polymerase chain reaction (PCR) utilizing universal 16S rRNA primers (27F: AGAGTTTGATCATGGCTCAG; 1492R: TACGGTTACCTTGTTACGACTT). A 25 μL reaction mixture comprising 12.5 μL of PCR mix, 1 μL of upstream and downstream primers at a concentration of 10 μmol/L, 1 μL of template DNA, and 9.5 μL of nuclease-free water was used for PCR. The settings for thermocycling were as follows: a five-minute initial denaturation at 94°C; thirty cycles of denaturation at 94°C for thirty seconds, one minute of annealing at 55°C, thirty seconds of extension at 72°C, and five minutes of final elongation at 72°C.
The amplified products were electrophoresed on a 1% agarose gel for verification. Sequence homology analysis was performed on the resulting sequences using the National Center for Biotechnology Information Basic Local Alignment Search Tool (BLAST, www.ncbi.nlm.nih.gov). MEGA 11.0 created a phylogenetic tree via the neighbor-joining (NJ) technique. Next, 1000 bootstrap replications were used to confirm the confidence level of the trees.
2.5. Antimicrobial susceptibility assay
After the experimental bacterial strains were cultured on an orbital shaker, the bacterial concentration was standardized with aseptic PBS. A 100 μL aliquot of the bacterial suspension was uniformly spread across the surface of a Mueller–Hinton (MH) agar plate within the confines of a biosafety cabinet. Following thorough absorption of the bacterial inoculum into the agar medium, prearranged antibiotic disks were meticulously placed on the surface of the agar plate. The plate was incubated at a consistent 28°C for 24 h in an inverted orientation. After incubation, the diameters of the inhibition zones were accurately measured in mm and interpreted for antimicrobial susceptibility, as delineated in the guidelines provided by the antibiotic sensitivity test kit.
2.6. Reinfection Experiments
After being separated and refined, the DN-3 bacterial strain was cultivated at 30°C with agitation at 200 rpm in liquid BHI medium. After the bacterial culture reached an optical density (OD) of 0.5, the supernatant was discarded, and the culture was centrifuged for two minutes at 4,000 rpm. The process was carried out three times, aseptically washing the bacterial pellet with PBS. Subsequently, the bacterial concentration was adjusted to 1 × 104, 1 × 105, 1 × 106, or 1 × 107 CFU/mL using aseptic PBS. The specimens of A. davidianus temporarily housed in a laboratory setting were arbitrarily segregated into four groups, each consisting of 30 individuals. Intramuscular injections of various concentrations of A. schindleri bacterial suspensions were administered to A. davidianus, and all procedural steps were meticulously documented.
Results
3.1. Clinical manifestations
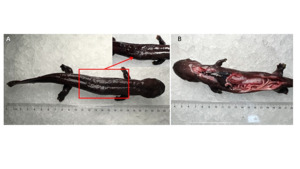
The affected A. davidianus exhibited an average body length of approximately 24 cm, pronounced lethargy and reduced surface mucus secretion (Figure 1). Postmortem anatomical inspection revealed conspicuous pathological alterations, including hepatic tissues exhibiting a darkened hue, mild darkening of the spleen, and nominal intestinal hemorrhage. discernible swelling of the internal organs was also observed.
3.2. Histopathological analysis
Tissue sections of renal, splenic, and intestinal organs were procured from afflicted and healthy A. davidianus for microscopic evaluation. The healthy subjects’ renal tissue architecture was intact, exhibiting normal intercellular spacing and the absence of discernible tissue edema (Figure 2A). Conversely, the afflicted A. davidianus renal tissues exhibited prominent vacuolization, accompanied by widened interstitial spaces between the glomerular and tubular components (Figure 2D). In the context of splenic tissues, the healthy specimens exhibited closely packed lymphocytes and a comparatively well-organized structural morphology (Figure 2B). In contrast, the splenic tissue of the afflicted specimens demonstrated a notable decrease in lymphocyte density, widespread cellular necrosis, and a filamentous, loosely arranged structure (Figure 2E). For intestinal tissues, the mucosal, submucosal, and serosal layers and epithelial cells of healthy A. davidianus plants were cultured under optimal conditions and characterized by closely arranged cells (Figure 2C). However, histological sections of intestinal tissues from afflicted A. davidianus showed signs of inflammatory infiltration in the mucosal and submucosal layers, laxity in the serosal layer, and extensive necrosis and disruption of the mucosal epithelial cells (Figure 2F).
3.3. Colony morphology and bacterial characteristics
After 24 h of incubation on BHI agar plates, the DN-3 strain yielded colonies with a pale-yellow coloration, a diameter ranging from approximately 0.8–1.4 mm, and a shiny surface. Gram-staining assays confirmed that the organism was gram-positive. Scanning electron microscopy revealed that the DN-3 bacterial cells possessed a smooth surface devoid of any protuberances or indentations, conforming to a bacillus-shaped morphology and growing as individual cells (Figure 3).
3.4. 16S rRNA identification
PCR was used to amplify the 16S rRNA fragment of the isolated DN-3 strain. The resulting sequences were subjected to BLAST comparison against the NCBI database, which revealed 99% sequence similarity between the strains isolated from the liver of the afflicted salamander and A. schindleri. Phylogenetic trees were constructed using MEGA 11.0 software, which included nine other Acinetobacter species with high homology to the DN-3 strain, as indicated in Figure 4. The results demonstrated that the DN-3 strain (PP478637) exhibited 96% sequence similarity to A. schindleri with the GenBank accession number NR 025412.1, indicating close phylogenetic affinity (Figure 4).
3.5. Biochemical identification of bacteria
The DN-3 strain was subjected to identification utilizing the Biolog Automated Microbial Identification System. The resultant data confirmed that DN-3 belongs to the A. schindleri family, and its physiological and biochemical markers are presented in Table 1.
3.6. Antimicrobial susceptibility testing
Fourteen antimicrobial agents were selected for susceptibility testing against the DN-3 strain, as directed by the instructions provided by the antimicrobial susceptibility testing kit (Table 2). The assay results indicated that the DN-3 strain exhibited high sensitivity to six drugs: neomycin, midecamycin, doxycycline, piperacillin, nitrofurantoin, and carbenicillin. Additionally, the fungus exhibited moderate sensitivity to vancomycin and resistance to seven drugs, namely, erythromycin, gentamicin, flurbiprofen, streptomycin, norfloxacin, ciprofloxacin, and sulfonamides.
R, resistant; S, sensitive; I, intermediate sensitivity.
3.7. Reinfection Experiment
Healthy A. davidianus were inoculated with varying concentrations of the DN-3 bacterial suspension. No fatalities were observed in the control group (Figure 5). The fish injected with DN-3 died on the third day post-injection. A 100% mortality rate occurred under the 1 × 107 CFU/mL treatment with DN-3. The median lethal dose (LD50)16 of A. schindleri DN-3 for A. davidianus was calculated to be 6.25 × 104 CFU/mL. Bacterial isolates obtained from the infected group were subsequently identified, confirming the pathogenicity of the bacteria.
Discussion
Acinetobacter species have been detected more frequently in animals due to the significant increase in pathogenic bacteria infecting them. Nevertheless, less focus has been placed on the infection status and prevalence of resistance in Acinetobacter species from animals.16 In this study, diseased A. davidianus from a farm in Zhangjiajie were examined, and the isolated strain DN-3 was identified as A. schindleri through morphology staining, 16S rRNA sequencing, biochemical identification, and regression infection experiments. This is the first report of A. schindleri infection in A. davidianus, and this strain has caused many deaths. The LD50 of A. avidianus DN-03 was confirmed to be similar to that of A. baumannii,25 A. johnsonii,26 A. lwoffii,27 etc., causing pathologic lesions in several organs and tissues and exhibiting strong pathogenicity. Acinetobacter species can be transmitted between humans and animals.17,28 A. schindleri, which poses a zoonotic threat of pathogenicity, may be transmitted among breeders, transporters, and giant salamanders. This risk is particularly pronounced in Zhangjiajie city, a well-developed hub for A. davidianus aquaculture and trade. Consequently, it is crucial to reinforce the surveillance, prevention, and management of A. schindleri and related pathogens to curtail the mortality risk posed to the giant salamander population.
A. schindleri has recently been isolated and found in ungulates,29 fish,30 and poultry,31 revealing that the bacterium is widespread in the animal kingdom and that financial losses in the aquaculture sector frequently accompany its discovery. This bacterium causes red eye infection in the affected Pangasius sutchi, leading to symptoms such as gill damage, dermatitis, swollen and reddened eyes, or internal bleeding.30 This showed a phenomenon of group outbreak and demonstrated strong pathogenicity. Like other Acinetobacter infections in this study,25,26 A. schindleri induced salamander skin inflammation, internal organ enlargement, intestinal hemorrhage, secondary infections, and even death. These factors have made it challenging to prevent and control diseases in the breeding of A. davidianus because they are all drug-resistant.
One of the most severe public health issues in the 21st century is antimicrobial resistance, which threatens the efficient prevention and treatment of many diseases caused by pathogenic organisms.32 Acinetobacter, clinically induces a multiorgan inflammatory response in humans, triggering other diseases, and is often drug-resistant.33 Acinetobacter species of animal pathogens typically cause severe tissue damage to key organs, such as the kidney, liver, spleen, heart, and muscle.34 For instance, renal tubular epithelial cells of pathogenic A. davidianus exhibit vacuolar degeneration in the liver and macrophage infiltration (Figure 2D). This lesion adversely affects the health of A. davidianus, leading to weakened immunity, skin inflammation, cell necrosis, intestinal hemorrhage, and subsequent infection, ultimately resulting in death. A. schindleri has been shown to infect humans and animals, but this organism has been poorly characterized in antibiotic susceptibility studies of aquatic animals.24,35 Clinical trials have shown that A. schindleri is sensitive to antibiotics such as beta-lactams, aminoglycosides, chloramphenicol, and tetracyclines, among others.35 In the present study, the A. davidianus DN-3 strain was sensitive to neomycin, mesomycin, doxycycline, piperacillin, nitrofurantoin, and carbenicillin, similar to clinically sensitive drugs. Such consequences pose significant challenges to successfully breeding and maintaining healthy A. davidianus populations.
Zhangjiajie City, situated in Hunan Province, is renowned as the “Home of the Chinese Giant Salamander,” serving as a critical hub for distributing and producing these wild creatures in China.36–38 Numerous farmers engage in the artificial rearing and trading of this species. According to incomplete statistics, there are over a hundred aquaculture enterprises of A. davidianus in Zhangjiajie, with a cultivation volume exceeding one million22. This makes it a critical trading hub and production base for A. davidianus in China.21,39 However, as A. davidianus farming scales up and becomes more intensive, it faces a surge of various diseases, particularly newly emerging ones, inflicting substantial economic losses on the industry.40,41 The research into salamander diseases lags, with the primary approach being the application of fish disease treatments, which have proven largely ineffective.21,40 Consequently, it is crucial to delve deeper into the pathogenic testing and pathological analysis of newly discovered, highly pathogenic bacteria in salamanders, such as A. schindleri.42 This will enable the development of scientific medication and the formulation of prevention and control measures. By doing so, we can minimize the occurrence of diseases to the greatest extent and effectively control their spread.
Conclusions
Animal-origin A. schindleri strains are poorly understood, but they pose a serious risk to human and animal health.17,25 The bacterium A. schindleri DN-3 was isolated in this study from A. davidianus via physiological and biochemical identification, 16S rRNA gene sequencing, and scanning electron microscopy. The LD50 for A. davidianus was determined to be 6.25 × 104 CFU/mL. This pathogen exhibited a high level of pathogenicity, leading to internal bleeding in A. davidianus individuals. The findings of this investigation will provide valuable insights into identifying and managing infections caused by A. schindleri in A. davidianus. This will pave the way for developing scientific medications and formulating effective prevention and control measures.
Acknowledgments
We are very grateful to the Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences, for helping us with the experiments in this paper.
Authors’ Contribution
Conceptualization: Cheng Wang (Equal), Yong Zhou (Equal), Ying Wei (Equal). Formal Analysis: Cheng Wang (Equal), Yong Zhou (Equal). Investigation: Cheng Wang (Equal), Yixing Xie (Equal), Zhiyong Deng (Equal). Methodology: Cheng Wang (Equal), Yixing Xie (Equal), Yong Zhou (Equal). Resources: Cheng Wang (Equal), Yixing Xie (Equal). Software: Cheng Wang (Equal), Yixing Xie (Equal). Validation: Cheng Wang (Equal), Yong Zhou (Equal), Ying Wei (Equal). Writing – original draft: Cheng Wang (Lead). Writing – review & editing: Cheng Wang (Equal), Yong Zhou (Equal), Ying Wei (Equal). Supervision: Huanyan Yuan (Equal), Mingzhu Tian (Equal), Pan Mao (Equal). Funding acquisition: Ying Wei (Lead). Project administration: Ying Wei (Lead).
Competing of Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
The article adheres to the Convention on Biological Diversity and the Convention on Trade in Endangered Species of Wild Fauna and Flora Research.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.


_gram_staining_of_dn-3_cells_(scale_bar__1.jpeg)




_gram_staining_of_dn-3_cells_(scale_bar__1.jpeg)

