INTRODUCTION
Aquaculture is an essential human food sourc.1 With the rapid development of intensive aquaculture, unscientific feeding methods have resulted in frequent aquatic animal diseases and economic losse.2 Common diseases affecting aquatic animals include bacterial vira,3 and parasitic disease,4 and Vibrio harveyi is a common causative agent of bacterial diseases. Antibiotics and chemicals are frequently employed to treat illnesses, but these approaches lead to issues with drug resistance, environmental contamination, and food safet.5 Thus, alternatives to antibiotics in aquaculture have received widespread attention, and immune boosters have been used to prevent disease and improve the host’s resistance to stress.
Golden pompano (Trachinotus ovatus) is a fish belonging to the family Kamaridae and the order Perciformes and is widely distributed in tropical and subtropical seas. Due to its fast growth rate, delicious taste, and increasing market demand, it is among the most important species for marine-aquaculture in southern China.6 The most common culture model for golden pompano was high-density, large scale net cage culture in offshore waters. Therefore, golden pompano farming was vulnerable to extreme weather conditions, such as typhoon and disease breakouts afterwards. Unfortunately, the literature indicates climate change is likely to cause more frequent and intense extreme weather events and more severe stresses to offshore fish farming.7 Therefore, it is important to enhance the golden pompano’s stress tolerance and overall immunity so that it an survive extreme weather and pathogen infections.
MOS is a glucose protein complex derived from the cell wall of brewer’s yeast and is one of the most common prebiotics for aquatic animals.8 Several studies have shown that MOS positively affects aquatic organisms’ growth performance, immunity and resistance to stress and diseases.9 Dietary MOS has been reported to enhance anxiety-like behaviors and stress resistance in zebrafish (Danio rerio)10 and enhance tolerance to air exposure in hybrid grouper (Epinephelus fuscoguttatus ♀× Epinephelus lanceolatus♂),11 improve the resistance of bluntnose seabream (Megalobrama amblycephala)12 against Vibrio vulnificus and Vibrio alginolyticu and enhance the immune function against Aeromonas hydrophila in and grass carp (Ctenopharyngodon idella).13 So far, only one study reported MOS’ effect on pompano, enhancing its tolerance to low salinity stress and Streptococcus iniae.14 Being a saltwater fish species cultured in net cages in the subtropical seas, Pompano is more prone to Halophilic pathogens such as Vibrio bacteria and natural stresses such as typhoons. Therefore, it is important to evaluate how MOS might protect T. ovatus against typhoon and Vibrio infection.
In the present study, different supplementary levels of MOS were added to the feed of T. ovatus. The effects of MOS on the immune response and stress resistance of T. ovatus were investigated by measuring antioxidant capacity, immune capacity, and survival rates after typhoon stress and after V. harveyi challenge tests. The findings of this study will provide a theoretical basis for applying MOS in T. ovatus aquaculture.
MATERIALS AND METHODS
DIET PREPARATION
Commercial feed (52.0% crude protein, 9.0% crude fat, 3.0% crude fiber, 10.0% crude ash, 2.0% calcium, 1.8% total phosphorus, 10.0% moisture, and 3.0% lysine) was used as the basal feed (Guangdong Yuequn Marine Biological Research and Development Co., Ltd., China). Groups with different diets were set up: (1) the control group (C group) was fed a diet containing only basal diet; (2) the M1 group was given a meal that included 3g MOS/kg basal diet; (3) the M2 group was given a meal that included 6g MOS/kg basal diet; and (4) the M3 group was given a meal that included 10g MOS/kg basal diet.15 To prepare the experimental group rations, 0.3%, 0.6%, and 1% (g/kg) solutions of MOS were used. An equal amount of pure water was sprinkled onto the basic feed for the control group. The same purified water was used to dissolve MOS, which was then uniformly sprayed on the feed. Each mixed meal was allowed to air dry at room temperature. MOS supplement formulations were prepared as needed every 7 days during the feeding trial and stored in plastic bags at 4°C.
EXPERIMENTAL FISH AND SAMPLE COLLECTION
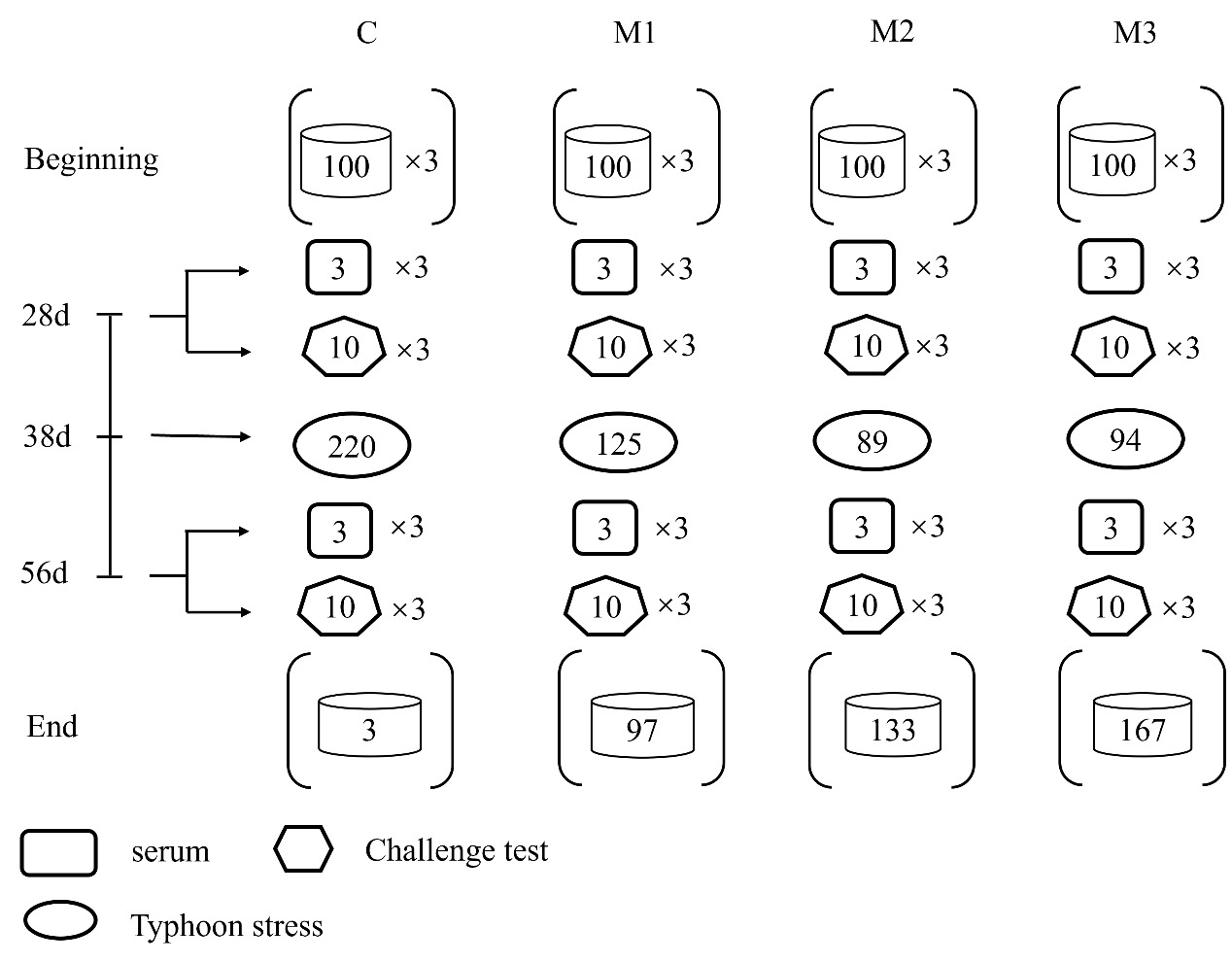
The feeding trial of T. ovatus was carried out on fish rafts in the Lingao Bay, Hainan Province, using small seawater net cages. Before the experiment, the primary feed was administered for one week to allow the fish to adapt to the feed and environment. After 24 hours of fasting, 1200 T. ovatus (mean weight 14.41±0.31 g) were randomly assigned to 12 cubic net cages (100 fish/net cage), and three replicates were set up in each group. The fish were fed approximately 3% of their body weight twice a day, at 8:00 and 17:00. The water’s temperature varied between 28°C and 30.7°C, with a salinity of 35%, pH of 7.9 ± 0.2, and less than 0.2 mg/L of ammonia nitrogen.
After 4 weeks and 8 weeks of the feeding experiment, the fish were sampled following 24 hours of fasting. Three sample fish were taken randomly from each net cage (nine fish per group). The sampled fish were first anesthetized with eugenol (100–200 mg/L; Shanghai Medical Instruments Co., Ltd., Shanghai, China).16 After weighing and disinfecting the fish body surface with alcohol cotton balls, blood was collected via tail vein puncture with a 1ml disposable sterile syringe. The blood was transferred to a centrifuge tube to obtain serum. The blood specimens were left to rest at room temperature for 2 hours, then at 4°C overnight, and finally centrifuged (4°C, 1500rpm, 15 minutes) to separate the serum. The samples were then stored at -80°C for immunological index analysis.11,15 The head kidney was preserved in Leibovitz’s L-15 cell medium (L-15), and respiratory burst was measured. The unsampled fish were weighed and returned to their original cages for subsequent experiments.15 The research design diagram (Fig. 1) provided illustrations for each of the aforementioned primary sampling processes. All animals were handled strictly in compliance with the rules established by the animal experimentation ethics committee at Hainan University.
GROWTH PERFORMANCE
At the beginning and end of the 56-day feeding trial, the total weight of all fish in each cage was measured, and the average weight (the total weight/ the total number of fish) in each cage was used as one replicate for the average weight of each group.11,15 Specific growth rate (SGR) and weight gain rate were computed.
Weight gain rate (WGR)(%) = 100% × (final average weight – initial average weight) / initial weight
Specific growth rate (SGR)(%) = 100% ×[ln(final average weight) – ln(initial average weight)] / feeding days
THE ACTIVITY OF ANTIOXIDASE AND IMMUNE-RELATED PARAMETERS
A biological replication of the appropriate group was combined with serum samples from three fish in the same experimental group (9 fish/group ÷ 3 fish/pool = 3 pools/group). Serum from fish fed for 4 weeks and 8 weeks was assayed by the instructions of enzyme activity assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China): total antioxidant capacity (T-AOC) (Cat. No. A015), superoxide dismutase (SOD) (Cat. No. A001–3), acid phosphatase activity (ACP)(Cat. No. A060-2), alkaline phosphatase assay kit (AKP) (Cat. No. A059-2), lysozyme (LZM) activity (Cat. No. A050), catalase (CAT) (Cat. No. A007-1-1), albumin (ALB) (Cat. No. A028-2-1), and total protein (TP) (Cat. No. A045-4). Hong et al. followed the approach of measuring macrophages’ respiratory burst (RB) using nitroblue tetrazolium (NBT).17 Using potassium hydroxide (KOH)+ dimethyl sulfoxide (DMSO) as a blank, absorbance was measured at a wavelength of 620 nm.
CHALLENGE TEST
V. harveyi 385 was acquired from Hainan University’s (Hainan, China) Collaborative Innovation Center of Marine Science and Technology. The bacteria were grown in Marine Broth 2216E medium and shaken for the entire night at 180 rpm at 30°C. The broth culture was centrifuged at 4℃ and 3000rpm/min for 20 minutes. After removing the supernatant, the bacterial pellet was cleaned and added to the phosphate buffer saline (PBS).18 LD50 (1.44×107 CFU/g fish weight) was obtained by preliminary experiments and referring to previously published literature.19 After 4 and 8 weeks of the feeding experiment, 30 tails in each group were randomly selected for intraperitoneal injection of 100 uL bacterial suspension 1.44×107 CFU/g fish body weight.20 The fish were observed for seven days after the bacterial challenge, and mortality was recorded daily. V. harveyi 385 was reisolated from dead and clinically infected fish and identified using standard biochemical tests (as described in Ber-gey’s Manual of Systematic Bacteriology) and 16S rDNA sequencing methods.18
The typhoon stress on the 32nd day of the feeding trial, a Typhoon hit the experimental site, which brought tremendous stress to the experimental fish in the net cages. The cumulative mortality of each group was recorded for 48 hours after the typhoon.
STATISTICAL ANALYSIS
The three replicates’ mean ± standard deviation (SD) values were used to express all data. To examine the data, Duncan’s multiple-range tests were employed. Significant statistical differences were indicated by p<0.05. SPSS 25.0 was used to analyze the data (SPSS Inc., Chicago, USA).
RESULTS
GROWTH OF T. ovatus
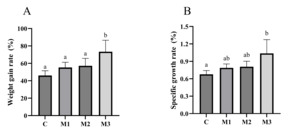
After 8 weeks of MOS feeding, the WGR and SGR were calculated. The results showed that the addition of different concentrations of MOS increased WGR and SGR. Groups M1, M2, and M3’s WGR and SGR were significantly higher than group C (p<0.05; Fig. 2).
ANTIOXIDANT CAPACITY OF T. ovatus AFTER MOS SUPPLEMENTATION
4 WEEKS
The SOD, T-AOC, and CAT activity levels in the serum were measured, and the results are shown in Figure 3. Compared to group C, group M1 showed significantly reduced T-AOC levels (p<0.05); group M2 showed significantly increased T-AOC levels (p<0.05); and group M3 showed significantly increased T-AOC levels (p<0.05) and significantly reduced SOD levels (p<0.05).
8 WEEKS
The SOD, T-AOC, and CAT activity levels in the serum were measured and shown in Figure 3. Compared to group C, group M1 showed significantly reduced SOD and T-AOC levels (p<0.05); group M2 showed significantly increased CAT levels (p<0.05) and significantly reduced T-AOC levels (p<0.05). Group M3 showed significantly increased CAT level (p<0.05) and significantly reduced SOD and T-AOC levels (p<0.05)
NON-SPECIFIC IMMUNITY OF T. ovatus AFTER MOS SUPPLEMENTATION
4 WEEKS
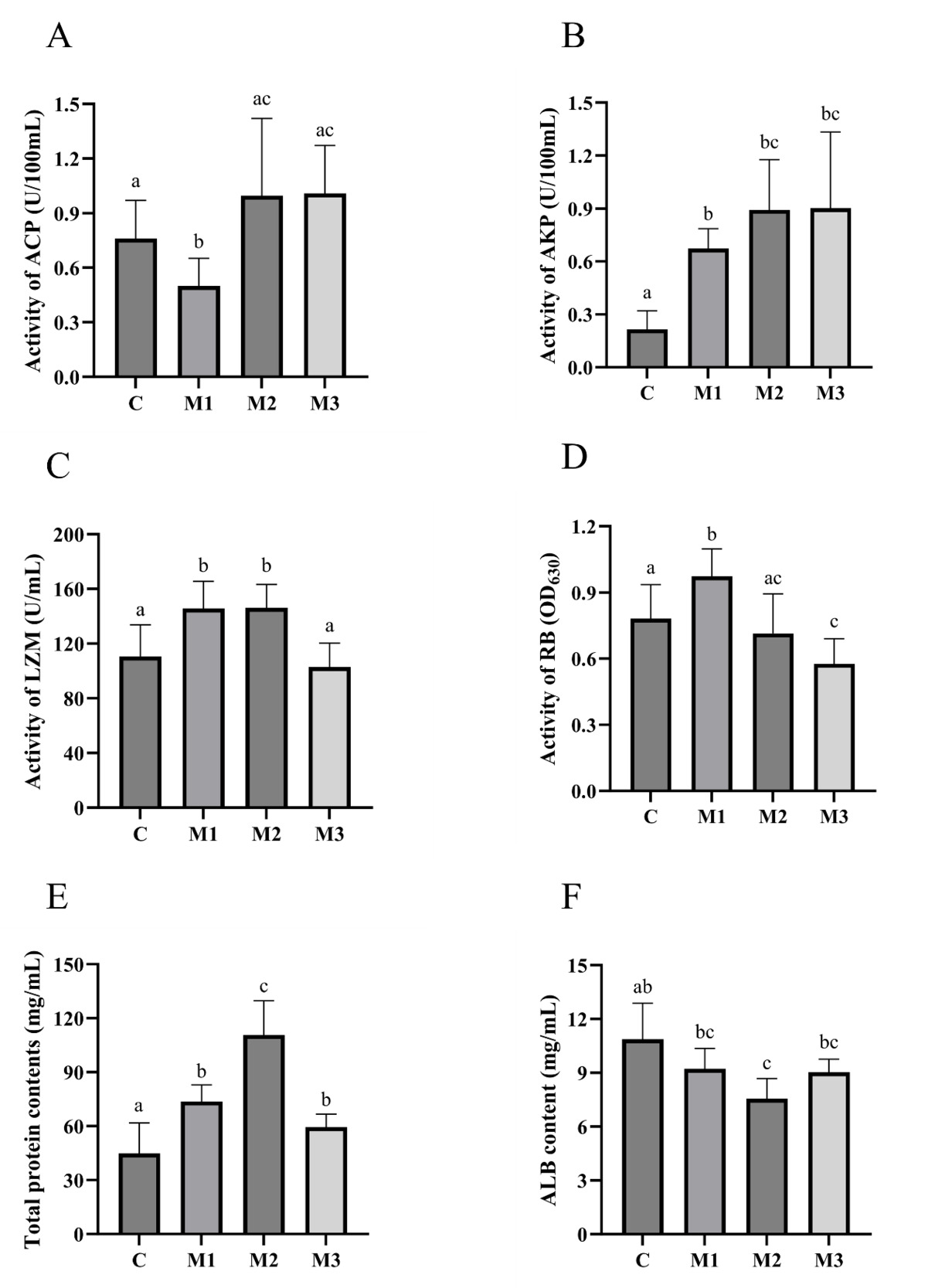
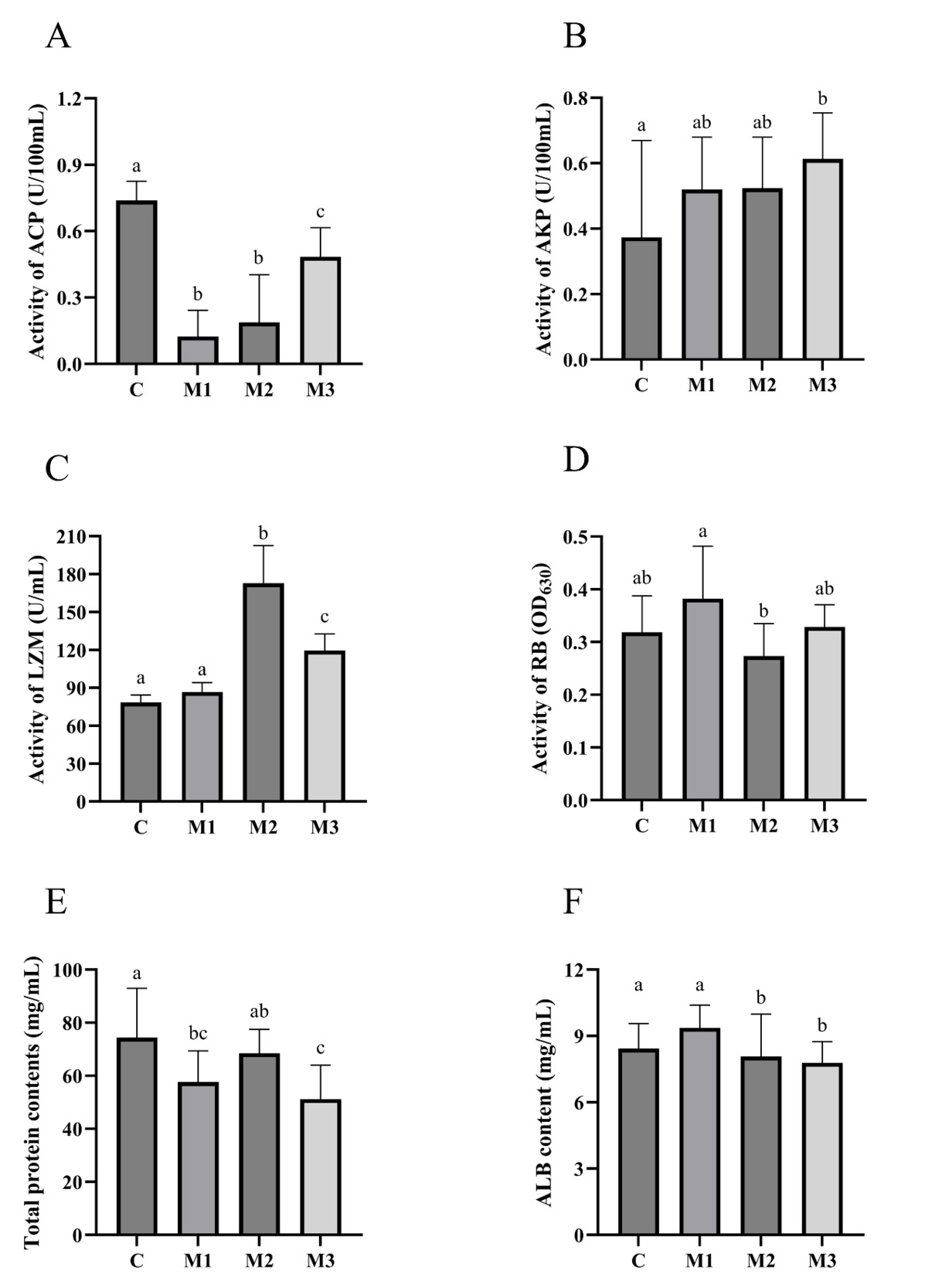
The serum ACP, AKP, LZM, RB, TP, and albumin (ALB) activities were assayed. As shown in Figure 5, compared to group C, group M1 showed 4 significantly up-regulated non-specific immunity (AKP, LZM, RB, and TP) (p<0.05) and one significantly down-regulated non-specific immunity (ACP) (p<0.05). Compared to group C, group M2 showed three significantly up-regulated non-specific immunity (AKP, LZM, and TP) (p<0.05) and one significantly down-regulated non-specific immunity (ALB) (p<0.05). Compared to group C, group M3 showed two significantly up-regulated non-specific immunity (AKP and TP) (p<0.05) and one significantly down-regulated non-specific immunity (RB) (p<0.05).
8 WEEKS
The serum ACP, AKP, LZM, RB, TP, and albumin (ALB) levels were measured. As shown in Figure 6, compared to group C, group M1 showed two significantly down-regulated non-specific immunity (ACP and TP) (p<0.05). Compared to group C, group M2 showed two significantly up-regulated non-specific immunity (LZM and ALB) (p<0.05) and one significantly down-regulated non-specific immunity (ACP) (p<0.05). Compared to group C, group M3 showed two significantly up-regulated non-specific immunity (AKP and LZM) (p<0.05) and three significantly down-regulated non-specific immunity (ACP, TP, and ALB) (p<0.05).
CHALLENGE TEST
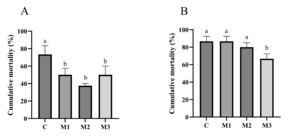
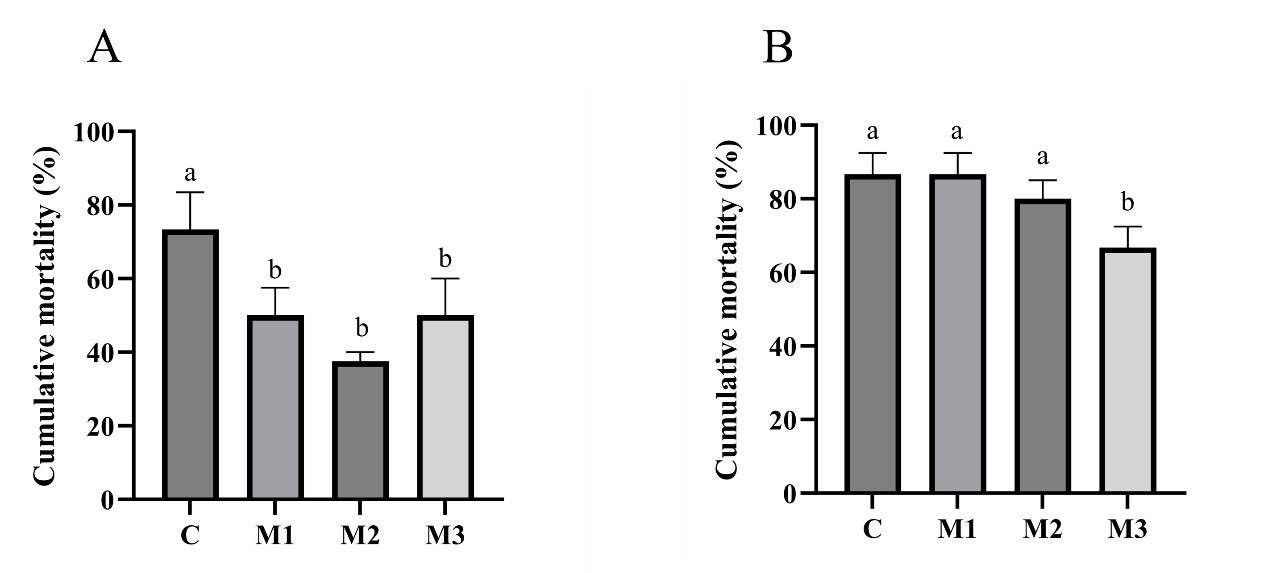
The challenge test results on day 28 revealed that, in comparison to group C, the cumulative mortality rates of all MOS-supplemented groups (M1, M2, and M3) were significantly lower (p<0.05). Only group M3’s cumulative mortality rates at day 56 were significantly lower than group C (p<0.05).
TYPHOON STRESS
Compared to group C, all three MOS-supplemented groups showed significantly lower cumulative mortality rates (p<0.05).
DISCUSSION
Numerous aquatic animals have shown the growth-promoting properties of MOS: Japanese eel (Anguilla japonica),21 and grass carp (Ctenopharyngodon idellus)13 for example. On the contrary, no growth-promoting effects of MOS, regardless of dose levels, were discovered in juvenile blunt snout bream (Megalobrama amblycephala).12 These differences may be due to differences in factors, such as MOS chemical structure, feeding time and dosage, fish species, size, age, and culture environment.
The antioxidant response of a biological organism is an essential defense against environmental stress. According to Huang et al. (2015), SOD’s primary job is to convert superoxide radicals into molecular oxygen and hydrogen peroxide. In contrast, CAT catalyzes the conversion of hydrogen peroxide into water and oxygen.22 According to,23 T-AOC is a direct indicator of fish’s total antioxidant capacity. The current findings demonstrated that T. ovatus’s antioxidant capacity was raised by adding MOS to fish feed. This aligns with findings from prior research on aquatic creatures like the Nile tilapia (Oreochromis niloticus),24 and grass carp.13 In the present study, 0.6% and 1% dietary addition of MOS significantly increased the activity of serum T-AOC (after 4 weeks) and CAT (after 8 weeks) in T. ovatus. SOD activity exhibited no differences or significant declines in the three MOS-supplemented groups. This is inconsistent with MOS studies reported with different species: adding 0.4% MOS (4g/kg) to feed significantly increased the T-SOD activity of juvenile Penaeus vannamei.25 A possible reason for the discrepancy might be that MOS stimulates the production of various beneficial microorganisms and substances and an organism’s uptake of these substances selectively increases its T-AOC, CAT, or SOD levels.12
Non-specific immunity plays an essential role in the disease resistance mechanism of fish. LZM, AKP, ACP, respiratory burst (RB), ALB, and TP are all non-specific immunity indexes that play vital roles in the body’s defense. Lysozymes prevent bacterial growth and colonization by causing cell wall disintegration and, ultimately, bacterial death.26 When the amount of MOS in the diet increased, the LZM activities and RB of T. ovatus continually increased. In numerous studies, 14 MOS supplementation (at various doses and for different supplemental times) has demonstrated its LZM-promoting effects.2,6 Ren et al. (2020) observed a significant enhancement in the serum LZM level of hybrid grouper (Epinephelus lanceolatus ♂ × E. fuscoguttatus ♀) after a 9-week supplementation of MOS at doses ranging from 0.3% to 0.6%.15 In a study conducted by Pereira et al. (2018), it was shown that the introduction of MOS for 45 days, at concentrations ranging from 0.1% to 1.5%, resulted in a significant increase in the level of LZM in the serum of juvenile Nile tilapia.27 Our investigation findings were consistent with results from previous research studies. Significant increases in LZM levels in T. ovatus were seen following both 28-day supplementation of MOS at dosages ranging from 0.3% to 0.6% and 56-day treatment of MOS at doses ranging from 0.6% to 1%. Typical hydrolases that detoxify pollutants and prevent toxin invasion are AKP and ACP.28 The current study’s findings show that at 4 weeks, all three MOS-supplemented groups considerably raised T. ovatus’s AKP activities; at 8 weeks, only the 1% MOS group significantly raised the blood AKP levels of T. ovatus. Interestingly, AKP level changes seemed to be associated with the survival rates of the challenge test: all three groups exhibited enhanced survival rates at 4 weeks; at 8 weeks, only 1% of the MOS group showed significantly enhanced survival rates. The AKP-enhancing effect of MOS is also reported in many other studies on aquatic animals.28 For instance, Ding et al. (2022) found that supplementing with 0.02% MOS significantly increased the levels of AKP activities in the serum of juvenile blunt snout bream.12 The ACP-decreasing effect of MOS shown by the present study was not by the ACP-enhancing effect reported in juvenile blunt snout bream and grass carp. Ding et al. reported that Juvenile blunt snout bream (Megalobrama amblycephala) serum ACP levels were markedly elevated by an 8-week MOS supplementation at dosages of 0.02-0.04%.12 According to Lu et al., adding MOS for 60 days at 0.02-0.1% levels greatly raised the serum ACP level of grass carp (Ctenopharyngodon Idella).29 The variation in MOS doses or fish species may cause the disparity in the effects of MOS in fish. As a defense against pathogen invasion, phagocytosis causes phagocytes to create RB; hence, RB activity is a valuable indicator for assessing fish cellular immunity.26 The present study showed that after 28-day supplementation of MOS at doses of 0.3%, RB levels were significantly increased in T. ovatus. This is in line with MOS studies in sea bass. It was reported that the 45-day addition of MOS at doses of 0.2-0.6% significantly enhanced the RB level of the sea bass (Dicentrarchus labrax) serum.30 The increased levels of TP and ALB can improve protein-beating digestibility and inhibit bacterial toxins.26 Our study result showed that 28-day supplementation of MOS at the doses of 0.3-1% induced significant increases in TP levels in T. ovatus. A similar TP-enhancing effect of short-term MOS supplementation was observed in Nile tilapia, which was supplemented with MOS for 30 days.26 Our study result showed that 56-day supplementation of MOS at doses of 0.6% significantly increased ALB levels in T. ovatus. 8-week supplementation of MOS at doses of 3% also significantly enhanced the ALB level of juvenile great sturgeon (Huso huso) serum.31 Based on the present study’s result and those from previous studies, we noticed that the impact of MOS on non-specific immunity parameters of aquatic animals was not always consistent. The divergent findings from different research could be attributed to variations in fish species, MOS dosages, feeding times, temperatures, and other factors.26,31
An aquatic animal’s resistance to disease can be considered a comprehensive indicator of the organism’s immunity. V. harveyi is one of the main disease-causing pathogens that affect T. ovatus. The present study showed that after 4-5 weeks of MOS supplementation, all tested MOS groups exhibited significantly increased survival rates after the challenge test and typhoon stress resistance; after 8 weeks of MOS supplementation, however, only one of the three MOS groups showed significantly enhanced survival rates after the challenge test. This “shorter is better” effect of MOS was also observed in hybrid grouper and Atlantic salmon. In hybrid grouper (Epinephelus lanceolatus ♂ × E. fuscoguttatus ♀), 4-week supplementation of MOS (0.2% and 0.3%) induced enhanced disease resistance and multi-stress resistance (ammonia-nitrogen stress and crowding stress);32 T. ovatus fed MOS had a higher tolerance to low salinity stress and Streptococcus iniae challenge14; yet at 9 weeks, only one of the 4 MOS groups exhibited increased tolerance to bacterial pathogens.12 Similarly, in Atlantic salmon, increased disease resistance was observed at MOS supplementation of 5 weeks, yet a non-significant protection effect against disease was observed at 10 weeks.33 On the other hand, however, different MOS effect pattern was also reported. In channel catfish (lctalarus punctatus), for example, the “longer is better” effect of MOS was observed. Although 4 weeks of MOS addition did not induce any protection against Edwardsiella ictaluri in Channel catfish, 6 weeks of MOS addition at the same dosage illustrated a significant protection effect against the same pathogen.34 Therefore, the above study results illustrated that the function pattern of MOS might be closely associated with fish species.
Our study indicates that MOS supplementation in T. ovatus diets could improve growth performance, antioxidant capacity (T-AOC, CAT), non-specific immunity (AKP, LZM, RB, TP, and ALB), and stress resistance (challenge test and typhoon stress) of T. ovatus. In addition, compared to long-term (56 days) supplementation, short-term (28 days) supplementation of MOS seemed to have an overall better-enhancing effect on antioxidant capacity, non-specific immunity, and disease resistance of T. ovatus fish. In summary, MOS can be an immunostimulant in the T. ovatus culture.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (32060031), the Natural Science Foundation of Hainan Province (320RC516), and the Hainan University Collaborative Innovation Center Fund (XTCX2022HYB03).
AUTHORS’ CONTRIBUTION
Data curation: Zihan Chen (Lead). Methodology: Zihan Chen (Equal), Yue Wu (Equal), Xin Chen (Equal). Writing – original draft: Zihan Chen (Equal), Yan Cai (Equal). Investigation: Yan Cai (Equal), Jianlong Li (Equal). Resources: Yongcai Zhou (Lead). Supervision: Yongcai Zhou (Lead). Conceptualization: Yongcai Zhou (Equal), Shifeng Wang (Equal). Funding acquisition: Zhenjie Cao (Supporting), Shifeng Wang (Lead). Formal Analysis: Jianlong Li (Lead).
ETHICAL APPROVAL – IACUC
In this study, the Instructional Animal Care and Use Committee of Hainan University permitted the animal experiments in advance. All possible efforts were made to reduce the suffering of the experimental animals.
DECLARATION OF COMPETING INTEREST
The authors state no competing interest in the present study. The common corresponding authors are Shifeng Wang and Yan Cai.
DATA AVAILABILITY
Data will be made available on request.