Introduction
Crucian carp (Carassius auratus) holds significant importance in freshwater aquaculture in China, highly preferred by farmers for its brief breeding cycle and delectable meat.1 In 2021, China’s crucian carp output reached 2.78 million tons. However, the rapid expansion of crucian carp aquaculture has led to escalating environmental degradation, posing a threat to the industry’s sustained development. Diseases stand out as a pivotal factor impeding the growth of the crucian carp aquaculture sector.2 Additionally, the excessive use of antibiotics raises concerns about pathogen drug resistance, the proliferation of multidrug-resistant bacteria, disruptions to gut microbiota, environmental pollution, and related issues.3 Therefore, developing environmentally friendly, effective medications free from residues is crucial for disease prevention in crucian carp.
Chinese herbal medicine has shown promise in enhancing fish health, elevating survival and growth rates, and enhancing immune responses.4 An increasing body of evidence suggests that combining microorganism fermentation with Chinese herbal medicine can enhance the therapeutic effects of drugs.5 The microbial fermentation process of Chinese herbal medicines can produce numerous secondary metabolites, which has high efficiency, low toxicity, minimal residues, and mild reaction conditions.6 This process degrades macromolecules into smaller, less harmful compounds, minimizing side effects.7
Astragalus, known for its immune-boosting properties and ability to protect kidneys and regulate antiviral reactions, is commonly used to treat aquatic animal diseases. Lactobacillus plantarum (L. plantarum) and Bacillus coagulans (B. coagulans) prevalent in the fermentation products of animals and plants,8 have been extensively used in shrimp and fish farming.9 Previous research has indicated that mixed fermentation can enhance Astragalus polysaccharide (APS) content and degrade high molecular weight proteins.10 However, the effects of supplementing fish diets with fermentation products of Astragalus, L. plantarum, and B. coagulans remain unexplored. This study aimed to investigate the potential effects of incorporating fermentation products of L. plantarum, Astragalus, and B. coagulans on crucian carp. We provided hitherto undocumented evidence of the impact of different fermentation products on growth performance, serum indexes, liver and intestine morphology, and the expression of immune-related genes in crucian carp. Additionally, the study explored the effects of different diets on the survival rate of Aeromonas hydrophila (A. hydrophila)-infected crucian carp. Notably, the cumulative survival rate in the mixed fermentation group by L. plantarum, Astragalus, and B. coagulans reached 60% after infection with A. hydrophila. These findings hold implications for applying mixed fermentation products by L. plantarum, Astragalus, and B. coagulans in aquaculture and provide directions for future research.
Materials and Methods
Experimental Diets
The experimental diet comprised standard feed with basic components, including fat, protein, water, and ash content, with a feed diameter of 2 mm. L. plantarum (YFI-7, CCTCC: M 2019656) and B. coagulans (YFI-NJ2, CCTCC: M 2021313) powders were prepared by freeze-drying to 1.0×109 colony-forming units (CFU)/g. After resuspending in phosphate-buffered saline (PBS), L. plantarum and B. coagulans concentrations were 1.0×108 CFU/mL,10 respectively. A mixture of 0.5 mL L. plantarum, 0.5 mL B. coagulans, and 1 g Astragalus was sealed in a 50 mL centrifuge tube, incubated at 37°C for 48 h and fermentation products were mixed with common feed at a ratio of 1:99.10 The feed was freeze-dried for 24 h, stored in sealed bags at 4°C. It is produced twice a week to maintain the cell count of L. plantarum and B. coagulans.
Experimental Fish
Experimental crucian carp (29 ± 1 g) were obtained from the Wuhan Academy of Agricultural Science (Wuhan, China) and temporarily cultured in breeding boxes for 14 days before feeding experiments. Aquaculture water conditions in the breeding tank were maintained at a pH of 7.8 ± 0.2, temperature of 25 ± 1°C, and dissolved oxygen level of 6.5 ± 0.5 mg/L, and the water used was sourced from a tap that had been aerated for more than 48 hours. Crucian carp were fed at a rate of 2% of their body weight and twice a day at 9:00 and 17:00, respectively.
Feeding Frequency
The fish were fed regularly with common feed during the acclimatization period. After 14 days of acclimation, nine hundred fish were randomly divided into five groups (each with three boxes) and fed with common feed (C), C + Astragalus (A), A + Lactobacillus plantarum (AL), A + Bacillus coagulans (AB), and AL + Bacillus coagulans (ALB) for 28 days. Sampling analysis was conducted on days 3, 7, 14, 21, and 28 to study the crucian carp’s response to fermentation products.
Growth Performance
During the trial, the weights of the crucian carp were measured at the beginning and end for each group. Growth performance parameters were calculated for crucian carp: weight gain ratio (WGR, %), specific growth rate (SGR, %/day), condition factor (CF, %), feed conversion ratio (FCR), and survival rate (SR, %).
WGR (%) = ((Final average body weight − initial average body weight)/initial body weight) × 100
SGR (%/day) = ((ln (final average body weight) − ln (initial average body weight))/days) × 100
CF (%) = (Final body weight/ (body length (cm))3) × 100
FCR = Total feed intake / (final average body weight − initial average body weight)
SR (%) = (Number of fishes that survived at the end of experimentation/Number of fishes at the initiation of experimentation) × 100.
Serum Biochemical Analysis
Three crucian carps were selected from each tank; their tail vein blood was collected with a 1 mL syringe and injected into the plastic Eppendorf tubes. The blood was left at 4°C for 6 h and then centrifuged at 3000 × g for 10 min. The 0.2 mL of upper serum was taken from each sample for serum biochemical index analysis (serum was analyzed immediately after collection), and excess serum was stored at -80°C. The Olympus 600 automatic biochemical analyzer (Olympus, Tokyo, Japan) was used to measure glucose (GLU), aspartate aminotransferase (AST), albumin (ALBN), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total protein (TP) in serum by using a commercial kit (Dibao, Guangzhou, China). The specific detection wavelengths for GLU, AST, ALBN, ALT, ALP, and TP are 500 nm, 340 nm, 630 nm, 340 nm, 405 nm, and 540 nm, respectively.
Histopathological Analysis of Liver and Intestine of Crucian carp
The liver and intestine tissues of crucian carp were collected from C, A, AL, AB, and ALB groups. The tissues were fixed with 4% paraformaldehyde, dehydrated with a gradient series of ethanol, embedded in paraffin, and sliced using microtomes (Thermo, Waltham, USA). Hematoxylin-eosin was used to stain the tissue (Solarbio, Beijing, China), and optical microscopy was used for observation (Olympus, Tokyo, Japan).10
Intestine Digestive Enzyme Activities
To determine digestive enzyme activity, three randomly selected crucian carps were sampled from each tank, and foregut tissue was taken and placed in Eppendorf tubes stored at -80°C. The activities of amylases, lipases, and proteases in the intestine were determined by a UV spectrophotometer (Thermo, Waltham, USA) and a microplate reader (Biotek, Winooski, USA) using an enzyme activity test kit (Jiancheng, Nanjing, China).
Expression of Immune-related Genes in Crucian carp
Three fish were randomly selected to be euthanized with tricaine methanesulfonate MS222 (20 mg/L, sIgMa). Total RNA was isolated from the liver, spleen, kidney, and midgut tissues of crucian carp with TRIzol reagent (Invitrogen, Carlsbad, USA). Samples were stored at -80°C, and the quality and quantity of RNA were assessed by a gel imaging system (Bio-Rad, Hercules, CA, USA) and spectrophotometers (Thermo Fisher, Massachusetts, USA). According to the manufacturer’s instructions, RNA was reverse transcribed into cDNA using a kit (TaKaRa, Dalian, China). Primers were verified to be specific; the melt curve displayed a single peak, and the threshold period was less than 30 min. The PCR thermal cycling settings included a 5-minute denaturation step at 95°C, followed by 30 cycles of denaturation at 95°C for 20 s, annealing at 56°C for 20 s, and a final extension at 72°C for 20 s. The same program was applied to all primers. The expression levels of the target gene were calculated by the 2-∆∆Ct method using the primers β-actin,11 IL-10,4 IL-1β,11 IFN-α,4 C3,12 IgM.13 β-actin was the housekeeping gene, and all genes used are listed in Table 1.
Challenge Test
After feeding for 28 days, the challenge test was conducted with A. hydrophila for each group. Thirty fish were randomly selected in each group, and 0.2 mL A. hydrophila (1.0×107 CFU/mL) was injected into the fin intraperitoneally. Counts of deceased crucian carp were conducted every 24 hours for ten days after the challenge. It was necessary to collect diseased fish, and then conduct pathogen detection and identification to ensure that the crucian carp was killed by A. hydrophila.
Statistical analysis
The results were expressed as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 8.0 Version X (La Jolla, USA). The one-way analysis of variance (ANOVA) was applied, followed by post hoc analyses using Bonferroni test. Statistical significance was considered significant when p < 0.05. To ensure robustness, at least three biological replications were performed for each analysis.10
Results
Growth Performance
The survival rate of crucian carp in all five groups remained at 100% throughout the 28-day feeding trial (Table 2). Notably, the final weight of fish in the AL, AB, and ALB groups showed a significant increase compared to group C. However, no significant differences were observed in either the Weight Gain Ratio (WGR) or Specific Growth Ratio (SGR) among the fish in the different groups. Furthermore, the additives did not significantly influence the CF and FCR across all groups.
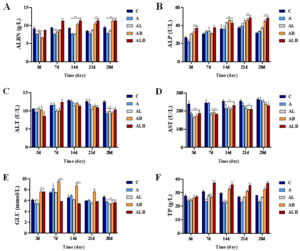
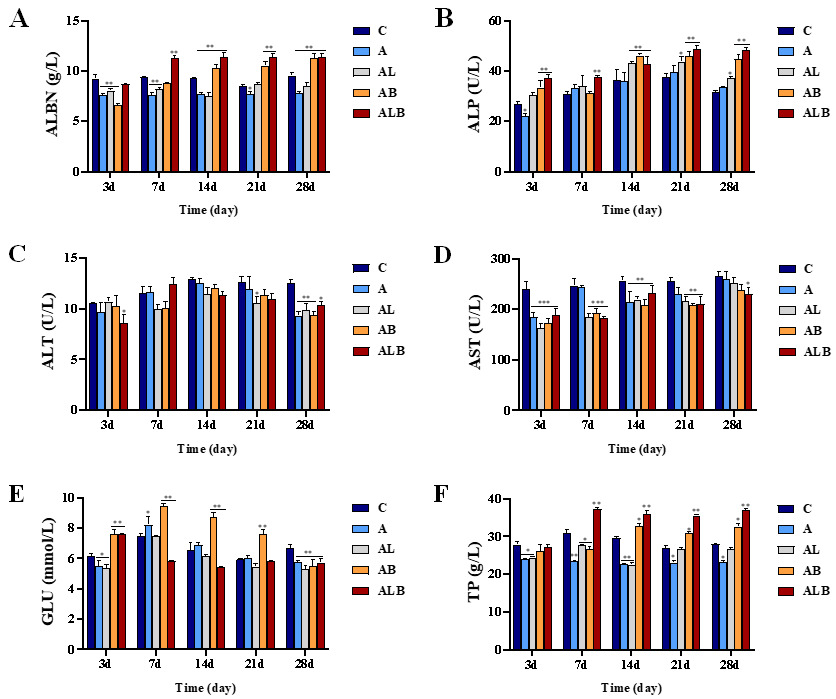
Serum Biochemical Parameters
Compared to group C, the ALB group exhibited a significant reduction in ALT, AST, and GLU indices, while ALBN, ALP, and TP showed significant increases (Figure 1). By day 28, ALT and GLU levels in groups A, AL, AB, and ALB were lower than in group C. AST levels in groups A, AL, AB, and ALB were lower than in group C on day 3, with only the ALB group showing a significant decrease by day 28, demonstrating 0.88-fold lower AST compared to the C group. The ALBN levels in groups AB and ALB remained consistent and higher than those in group C after 14 days. ALP levels in groups AB and ALB increased as the feeding trial progressed, reaching 1.41 and 1.51-fold higher than in group C on day 28. Furthermore, the TP content in the AB and ALB groups increased throughout the feeding trial, surpassing that of group C after 14 days of feeding.
Histopathological Analysis
The aspect ratio of midgut intestinal folds did not exhibit significant differences between the various groups (Figure 2). Histological analysis revealed that the aspect ratio of intestinal folds in group A was lower than in group C, but higher in groups AL, AB, and ALB. Goblet cells maintained an orderly arrangement across all groups, appearing more significant and prominent in the ALB group. Importantly, intestinal morphology remained uncompromised in all groups, confirming the safety of feeding Astragalus and its fermented products to crucian carp. While a slight amount of cellular vacuolation was observed in the liver of groups C and A, the liver cells of groups AL, AB, and ALB displayed a neat and tightly arranged structure.
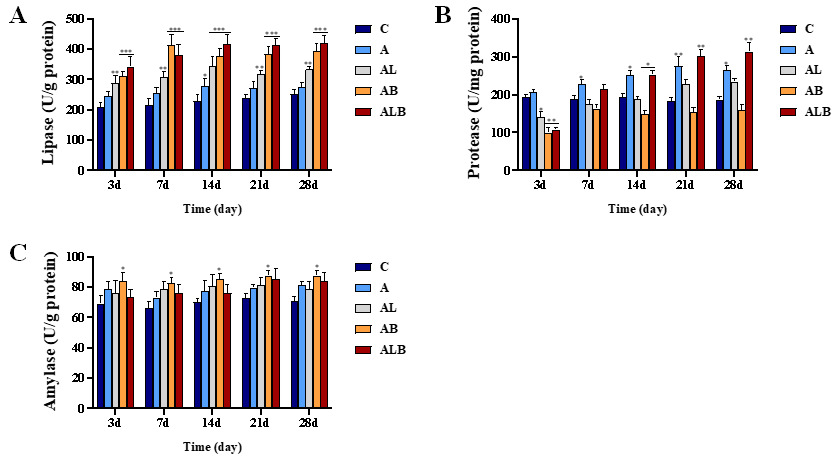
Intestine Digestive Enzyme Activities
A significant difference was observed between groups ALB and C concerning lipase and protease activities in the intestine (Figure 3). Within the initial three days, lipase activity in groups AL, AB, and ALB surpassed group C. Notably, on day 28, the lipase activity in the ALB group exhibited a remarkable 1.67-fold increase compared to group C. While the protease activity in group A peaked on day 21, it continued to rise steadily in the ALB group throughout the feeding trial, reaching a 1.68-fold higher level than in group C on day 28. Additionally, the intestinal amylase activity in the AB group surpassed that of group C on days 3, 7, 14, 21, and 28.
Changes in Immune-related Gene Expression
The expression levels of immune-related genes encoding interleukin (IL)-10, interferon (IFN)-α, IL-1β, C3, and IgM in the spleen, liver, kidney, and midgut of crucian carp were assessed on days 3, 7, 14, 21, and 28, respectively (Figure 4). Relative to group C, the expression levels of most immune genes increased following the consumption of fermented Astragalus products and probiotics. Notably, these upregulated expression levels were more pronounced in the liver and midgut compared to the spleen and kidney, with the heightened expression initiating earlier in the liver and midgut. In this study, significant upregulation was observed in the expression levels of IgM in the liver, spleen, and kidney, as well as C3 in the liver, spleen, and midgut. Furthermore, IL-10 and IFN-α expression levels in the liver, spleen, midgut, and kidney were significantly increased. Although the expression of IL-1β in the liver, spleen, midgut, and kidney did not exhibit a consistent pattern, a decrease was noted on day 28.
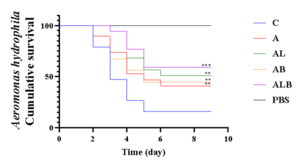
Pathogen Challenge
Following the 28-day feeding period with the fermentation products, crucian carp in the various groups were challenged with A. hydrophila. The findings revealed that after 10 days, the ALB group exhibited the highest cumulative survival rate, reaching 60%. In comparison, the cumulative survival rates for groups C, A, AL, and AB were 15%, 40%, 50%, and 45%, respectively (Figure 5).
Discussion
The fermentation products could significantly increase the final weight of fish
When used as feed additives, probiotics can positively affect the host, including disease prevention, growth promotion, and enhancement of immune responses.14 Consequently, they have been extensively used in aquaculture. Specifically, B. coagulans and L. plantarum have been shown to increase the WGR and SGR of Litopenaeus vannamei.8 Additionally, dietary supplementation of APS has demonstrated a significant increase in the SGR, feed conversion ratio (FCR), WGR, and feed intake (FI) of Oreochromis niloticus.15 Our study results align with the observed increase in the final weight of fish in the AL, AB, and ALB groups, although no significant differences were noted in WGR, SGR, FCR, and CF. It is essential to acknowledge that significant differences in our results could stem from variations in experimental design, forms, administration methods, and sampling methodologies.16 The fermentation products could significantly increase the expression level of albumin (ALBN), alkaline phosphatase (ALP), and total protein (TP).
Serum biochemical indicators serve as reflective measures of the health conditions of fish.17 Besides its role in enhancing immunity and mediating resistance to pathogenic bacteria, ALP is an essential metabolic regulator enzyme.4 APS and L. plantarum have been shown to increase ALP activity in crucian carp and rainbow trout.4 In our study, the ALP level in the ALB group was the highest, exhibiting a 1.51-fold increase compared to group C on day 28. APS has demonstrated a significant reduction in ALT and AST levels in the serum of crucian carp.1 GLU serves as a sensitive indicator for evaluating the stress state of organisms.18 Herein, the GLU content in group AL was the lowest, showing a 0.80-fold decrease compared to group C on day 28. The gradual decrease in GLU may be attributed to metabolic changes.19 Dietary B. coagulans has been linked to increased TP and ALBN levels in the serum of Litopenaeus vannamei and Oreochromis niloticus.20 In our investigation, TP and ALBN in groups AB and ALB experienced significant increases, possibly attributable to the increased abundance of B. coagulans during the fermentation process with Astragalus.10 In addition, different additives may have varying effects on fish, dependent on factors such as consumption amounts, changes in consumption time, the sources of additives, and the specific species involved.21
The fermentation products could improve intestinal and liver morphology
The liver plays an important role in innate immunity in fish and mammals. In our study, liver cells in groups AL, AB, and ALB exhibited a more homogeneous and tightly arranged structure than group C. Living lactic acid bacteria produce short-chain fatty acids in the host digestive tract, serving as the primary energy source for intestinal epithelial cells.22 This production increases intestinal villi height, providing a larger surface area for nutrient absorption.22 Previous studies showed that L. plantarum and B. coagulans could enhance intestinal villus height in rainbow trout and Penaeus vannamei, thereby improving intestinal integrity.23 In our investigation, the aspect ratio of intestinal folds in groups AL, AB, and ALB experienced an increase, with the highest observed in ALB, reaching a 1.27-fold higher value than in group C. Additionally, goblet cells in the ALB group appeared clearer. This observation may be attributed to the mixed fermentation products of L. plantarum, B. coagulans, and Astragalus, which can potentially reduce damage to the intestinal structure caused by pathogenic bacteria. Furthermore, they may promote the secretion of mucin protein by goblet cells, hence contributing to the protection of intestinal health.24
Effects of Fermentation Products on Intestinal Digestive Enzyme Activities
In fish, the digestion and absorption of nutrients are profoundly influenced by digestive enzyme activity, with enzyme activity directly reflecting the level of digestive ability. Previous studies have indicated that L. plantarum could enhance protease and lipase activities in crucian carp.25 In the present study, the lipase activity in the ALB group was notably 1.67-fold higher than that of group C on day 28. It is now understood that lipases play a crucial role in digestion as they degrade fats, particularly triacylglycerol.15 APS has significantly increased the intestinal activities of protease, lipase, and amylase enzymes in crucian carp.1 Our results align with these findings, demonstrating that the activities of lipase and protease in the ALB group were significantly higher than those in the C group. Digestive enzymes are vital in helping fish digest nutrients, facilitating easier absorption.26
The fermentation products could significantly increase the expression level of immune genes
Changes in immune parameters play a crucial role in contributing to fish resistance against pathogens.24 Interleukin-10 (IL-10), a cytokine known for inhibiting inflammation, has been reported to effectively suppress the release of cytokines that promote inflammation, thus playing a pivotal role in inflammation relief.27 Previous studies have shown that B. coagulans and Lactococcus lactis L-19 could upregulate IL-10 gene expression in the intestine of Cyprinus carpi and Channa argus,27 consistent with the findings in the present study. In the ALB group, the expression levels of C3, IgM, and IL-10 in the liver, spleen, kidney, and intestine were upregulated. C3 complement and IgM contribute to regulating fish immune responses through phagocytosis, exerting antibacterial effects. In the immune system, IL-1β plays an important role, and can influence the ability to resist invasion by bacteria and viruses.27 B. coagulans can increase the expression level of IL-1β in Oreochromis niloticus.20 APS can significantly upregulate IL-10, IL-1β and IFN-α in liver, spleen, kidney and midgut of crucian carp.4 The present study demonstrated that the expression levels of IFN-α and IL-1β were decreased, in contrast with the literature, which could be attributed to differences in the species and age of fish and differences in the dose and types of probiotic bacteria.17
The fermentation products could significantly improve the disease resistance of crucian carp
A. hydrophila is a major pathogen causing bacterial septicemia and inflammation in fish, posing a serious threat to crucian carp aquaculture (Wang et al., 2022). Over the years, different probiotics, including Lactobacillus rhamnosus (60.87%), Lactobacillus casei (56.09%), and L. plantarum (41.46%) have been used to ferment the traditional Chinese medicine Sanguisorba officinalis, and A. hydrophila-infected crucian carp exhibited a higher survival rate than control carp following dietary supplementation (Wang et al., 2022). The survival rate of CyHV-2 and Aeromonas veronii resistance of crucian carp increased by 80% and 65% following supplementation of Astragalus, L. plantarum and B. coagulans fermentation products.10 In the present study, the survival rate of fish in the experimental groups significantly improved after A. hydrophila infection, with the highest rate observed in group ALB (60%). This improvement may be attributed to multiple factors. APS potentially reducing bacterial and viral infections; probiotics producing lactic acid and acetic acid, inhibiting pathogenic microorganisms, and exerting bactericidal effects on intestinal pathogenic bacteria.28 Thus, the addition of fermentation products from probiotics and Chinese medicines exhibited a good biosafety profile and contributed to enhancing the immunity of crucian carp while preventing diseases.
In this study, the incorporation of Astragalus products fermented by L. plantarum and B. coagulans into the diet exhibited positive effects on the immune responses, growth performance, liver and intestinal morphology, intestinal digestive enzyme activities, serum parameters, and disease resistance of crucian carp. Consequently, fermented Astragalus products from L. plantarum and B. coagulans represent promising feed additives for crucian carp aquaculture.
Acknowledgments
This research was funded by the Earmarked Fund for CARS (grant number CARS-45), the Basic Scientific Research Funds of Central Public Welfare Research Institutes, the Chinese Academy of Fishery Sciences(2023TD46), the Achievement Transformation Project of Anhui Academy of Agricultural Sciences (grant number 2023YL023), and the National Freshwater Aquatic Germplasm Resource Center (grant number FGRC18537).
Authors’ Contribution - CRediT
Writing – original draft: Lisha Shi (Lead). Funding acquisition: Lisha Shi (Lead). Conceptualization: Mingyang Xue (Lead). Investigation: Mingyang Xue (Lead). Software: Yangyang Xing (Lead). Validation: Yangyang Xing (Lead). Formal Analysis: Nan Jiang (Lead). Supervision: Yuding Fan (Lead). Data curation: Jianwu Chen (Lead). Methodology: Wei Liu (Equal), Lingbing Zeng (Equal). Project administration: Yeying Wu (Lead). Visualization: Minglin Wu (Lead). Writing – review & editing: Yong Zhou (Lead). Resources: Yong Zhou (Lead).
Competing of Interest – COPE
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Conduct Approval – IACUC
Experimental procedures followed the guidelines of the Experimental Animal Center, Yangtze River Fisheries Research Institute, for ethical testing of animal experiments (ID Number: YFI2021-zhouyong-01-21).
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.