Introduction
Mizuhopecten yessoensis (Mollusca, Bivalvia, Pteromorphia, Pterioida, Pectinidae) is a large, cold water, filter-feeding shellfish with double shells and fan-shaped shells, which mostly lives in high salinity and freshwater-free inner bays. M. yessoensis is native to Japan and South Korea, and has rich nutritional value. The taste of this scallop is delicious and unique, and thus, it has high economic benefits, making it strongly favored by breeding enterprises and consumers. M. yessoensis was introduced into China in the 1980s, and it is now extensively farmed and one of the most important shellfish species in northern China.1
In China, the two main methods for breeding M. yessoensis are raft farming and bottom-sowing farming, where bottom-sowing farming is the main breeding mode.2 Due to the expanded scale of shrimp and scallop cultivation, problems have occurred, such as a low recapture rate and high mortality rate during the bottom sowing cultivation process, severely affecting the healthy development of scallops.3 In particular, predation by enemy organisms is one of the main causes of the high mortality rate for scallops. N. cumingii is mainly distributed in the Yellow and Bohai Seas in northern China, and its living conditions are similar to M .yessoensis. N. cumingii mostly feeds on bivalves and other shellfish and is one of the main predators of scallops. During the bottom sowing and breeding process for M. yessoensis, significant economic losses were caused by N. cumingii. Thus, predation by enemy organisms significantly impacts the cultivation and survival of scallops.
The behavior of animals determines their survival, growth, and successful breeding.4 Thus, understanding the behavior of scallops can help to ensure targeted breeding, healthy development, and economic benefits. When encountering stimuli due to predators, scallops will behave by closing their shells, swimming, and jumping to avoid predators.5 Blood (coelom fluid) is an important biological component for assessing the responses of aquatic animals to external stimuli. The activities of various immune-related enzymes in the blood can be used as important physiological indicators to measure aquatic animals’ health status and immune capacity, which directly reflect their adaptability to the external environment.6
So far, few reports have reported M. yessoensis responding to predator predation. In this paper, we studied the behavior characteristics and ability of M. yessoensis to avoid enemy when N. cumingii stressed different specifications of M. yessoensis in the laboratory, compared the changes in enzyme activity of M. yessoensis tissues after no enemy organism stimulation and continuous enemy organism stimulation, and conducted deep transcriptome sequencing for tissues with significant differences, and randomly selected genes with significant differences were verified by qRT-PCR. The results of this study contribute to the in-depth understanding of M. yessoensis’ response mechanism to predation pressure, especially at the genetic and molecular levels, filling the gap in the biological response mechanism of M. yessoensis to avoid the enemy. At the same time, the results could provide data support and theoretical references for the prevention and control strategy of M. yessoensis enemies and the improvement of M. yessoensis bottom-sowing farming technology.
Materials and Methods
Experimental materials
M. yessoensis were caught in Longwangtang Bay (Lvshunkou District, Dalian, China) in November 2022 and transported back to the Key Laboratory of Mariculture and Stock Enhancement in North China’s Sea, Ministry of Agriculture in a wet state using a holding tank. Scallops were rinsed clean and temporarily cultured in tanks. They were fed with Spirulina powder at regular intervals every day. After 7 days of culture, scallops that could close naturally and were healthy and undamaged were selected for use in the experiment.
N. cumingii were also caught in Longwangtang Bay in November 2022. Healthy N. cumingii were cultured without feeding for 7 days. Individuals without obvious trauma and able to feed normally were selected for use in the experiment.
The shell length, height, and width of the scallops were measured using Vernier calipers (Marr Precision Measuring Instruments Co., Ltd., Suzhou, China) with an accuracy of 0.01 mm. The wet weight of each scallop was measured using electronic scales (Changshu Shuangjie Testing Instrument Factory, Jiangsu, China) with an accuracy of 0.01 g. Scallops were classified into three size groups: shell length, height, and width. Table 1 shows the basic biological indexes for the specimens.
Generally, the M. yessoensis release specifications are generally 30-60mm in shell length in China, and which can reach the market specifications after 2-3 years, so we chose 60,90 and 120mm three specifications in this experiment, which can more fully reflect the ability of M. yessoensis bottom to avoid enemy organisms at different stages after sowing.
Behavioral observations of scallops
We filmed each experiment described below for 72h using a webcam (Model: KS-X6-QG4, Police Vision Guard, Jiangsu, China). The camera was installed 3m above the tank, allowing filming under light-free conditions. For each group, a 1h window was randomly selected to count the number of swimming events (shell opening and closing > 3 times consecutively) and jumping events (shell opening and closing ≤ 3 times consecutively). During the experimental period, the experimental conditions were the same for all groups, and the water was minimally aerated to minimize other disturbances.
N. cumingii-scallop Experiment I consisted of one control group and three experimental groups. Control group: put 15 scallops (5 large scallops, 5 medium scallops and 5 small scallops) into the tank, without N. cumingii (I-L0). Experimental group: after 15 scallops were put into the experiment, small size N. cumingii (I-sL), medium size N. cumingii (I-mL) and large size N. cumingii (I-lL) were put into the experiment respectively. Three replicates per experiment were set to reduce errors.
In N. cumingii-scallop experiment II: Five scallops of the same size put into their respective tanks, to which different size N. cumingii was added for predation stimulation small (II-sLsB, II-sLmB, and II-sLlB), medium (II-mLsB, II-mLmB, and II-mLlB), and large (II-lLsB, II-lLmB, and II-lLlB). Control groups without a crab present were set up for each size class (II-LsB0, II-LmB0, and II-LlB0). Each group had three replicates.
Force of clap measurement
The scallop shell closure force was measured following a previously described force gauge method.4 The experimental setup included a digital force gauge (Nscing SH-III-100N, digital dorce gauce, Suce, Nanjing, Jiangsu, China), water tank, and stand (Figure 1). The lower valve of a scallop was fixed on the experimental table in the water tank, and the upper valve was able to open and close freely. The force meter was fixed with a bracket. One end consisted of a hooked rod fixed between the upper and lower valve, which could accurately capture each time the scallop closed its valve, and the other end was a data port connected to a personal computer using a USB port. Soft-SH software was used to collect and store the data.
The scallops were fixed to the test bench, and the force measuring device was installed to assess the movement state of different sizes of scallops in their natural state and during continuous stimulation by the presence of an N. cumingii. The contraction of the adductor muscles is mainly divided into two stages.4 Phasic contractions (i.e., the rapid opening and closing of the shell) are generated by the contraction of the transverse striated muscle in the adductor muscles; in the force output diagram, every peak value represents one clap of the scallop. The force generated by the process is the force of the clap (Fclap), and the tense contraction (i.e., sustained for > 0.5 s) is the contraction of the shell. The second stage is tonic contractions (i.e., sustained for > 0.5 s), which result in slow opening and closing of the shell. They are generated by the contraction of the smooth muscles in the adductor muscles, and they show an obvious and sustained value in the output graph of the force measurement (Figure 1). The force generated by this process is the tonic contraction force (Ftonic). Typically, a scallop undergoes a cycle of motion that consists of rapidly clapping n times within a short period, followed by one more sustained slow contraction. The force generated by this process is called the phasic contractile force (Fphasic), which is the sum of Fclap.
According to Zhang et al.4 and the results of a pre-test, M. yessoensis stops moving after 3 min of continuous stimulation by a predator, and a period of recovery time is needed before it can attempt to evade again. Therefore, the measurement time for each scallop in this experiment was set at 180.2±0.2 s. First, the movement of each scallop in each size class was measured in the natural state (i.e., no N. cumingii). After 48h, the shell closure of each scallop in each size class under continuous stimulation by the presence of N. cumingii was measured. The data indexes were the maximum force of clap (Fmax), number of phasic contractions (Tphasic), number of tonic contractions (Ttonic), and frequency of shell closure (the number of times the scallop closes its shell per minute).
Because the initial response and strength of the scallop is important for its survival when it encounters a predator, the number of shell closures in the first 30 s as a percentage of the total number of shell closures (P30s) was also calculated. In order to minimize inter-individual errors, 15 parallels were set up for each set of experiments.
Enzyme activity assay
The adductor muscles, gills, and mantle tissues were removed from three scallops in the unstimulated control group and three scallops from each size group after exposure to N. cumingii, frozen in liquid nitrogen, and preserved at −80℃ for subsequent enzyme activity assays. Activities of superoxide dismutase (SOD), catalase (CAT), octopine dehydrogenase (ODH), and arginine kinase (AK) activities in each tissue from each scallop were measured using the FANKEW ELISA kit.
Statistical analysis
SPSS25.0 software was used to conduct one-way ANOVA on the motor behavior indicators of the same state (normal state or N. cumingii stimulated state) with different specifications or the same specifications with different states, and LSD was used to make a two-by-two comparison of the indicators with significant differences; two-way ANOVA was conducted to analyze the interactions between the specifications and the states, with P<0.05 regarded as the difference being significant, and P<0.01 regarded as the difference being extremely significant.
Transcriptome analysis
Total RNA was used as input material for the RNA sample preparations. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was performed using divalent cations under elevated temperature in First Strand Synthesis Reaction Buffer(5X). First strand cDNA was synthesized using random hexamer primer and M-MuLV Reverse Transcriptase (RNase H-). Second strand cDNA synthesis was subsequently performed using DNA Polymerase I and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase activities. After adenylation of 3’ ends of DNA fragments, Adaptor with hairpin loop structure were ligated to prepare for hybridization. To select cDNA fragments of preferentially 370~420 bp in length, the library fragments were purified with AMPure XP system (Beckman Coulter, Beverly, USA). Then PCR was performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers and Index (X) Primer. At last, PCR products were purified (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2100 system.All RNA clean data were submitted to the Short Read Archive (SRA) Sequence Database at the National Center for Biotechnology Information (NCBI) (Accession No. PRJNA1048362).
According to the manufacturer’s instructions, the index-coded samples were clustered on a cBot Cluster Generation System using TruSeq PE Cluster Kit v3-cBot-HS (Illumia). After cluster generation, the library preparations were sequenced on an Illumina Novaseq platform, and 150 bp paired-end reads were generated.
The quality of the raw data was assessed using FastQC. Raw data (raw reads) were processed using Trimmomatic to remove adapters, reads containing ploy-N, and low-quality reads to obtain clean reads. Then the clean reads were mapped to the M. yessoensis genome (https://www.ncbi.nlm.nih.gov/genome/12193) using HISAT2. The FPKM (Fragments Per Kilobase of exon model per Million mapped fragments) value of each gene was calculated using cufflinks, and the read counts of each gene were obtained by the-count. DESeq was used to compare RNA-seq data between the mBJ and L-mBJ groups. Differentially expressed genes (DEGs) in mBJ versus L-mBJ were identified using the DESeq functions estimateSizeFactors and nbinom Test. The false discovery rate (FDR) control method was used to identify the p-value threshold in multiple tests to compute the significance of the differences. Threshold values were set as p < 0.05 and fold change >2. Gene ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed. The stress-related genes were selected, and the sequences of selected DEGs and annotated genes were verified and compared by BLAST to ensure their similarity. Beijing Novozymes Technology Co conducted the bioinformatic analysis.
To examine the reliability of the RNA-Seq results, 9 DEGs were randomly selected for qRT-PCR validation. They included C1QL4, CYP2C8, ZCCHC8, TRXL, RAD17, OTOF, C25B8.10, PROM1A, and β-GLB. The housekeeping gene Gapdh was used as the reference gene. The primers were designed by Primer Premier 6, with lengths of 18-27 bp, GC (ratio of two bases, guanine, and cytosine, to the total base) content of 45%–55%, and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The RNA was reverse transcribed to cDNA using TIANGEN® FastKing gDNA Dispelling RT SuperMix (Tiangen Biotech, China). Suitable primers were designed using Primer 6 (Table 2) and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). qRT-PCR was performed with TIANGEN® Talent qPCR PreMix (SYBR Green) (Tiangen Biotech, China) on a Roche LightCycler®96 (Roche Diagnostics, Switzerland) real-time PCR system according to the manufacturer’s protocol. Samples from the same batch experiment of the RNA-seq were used for the confirmation experiment.
Results
Scallop behavior
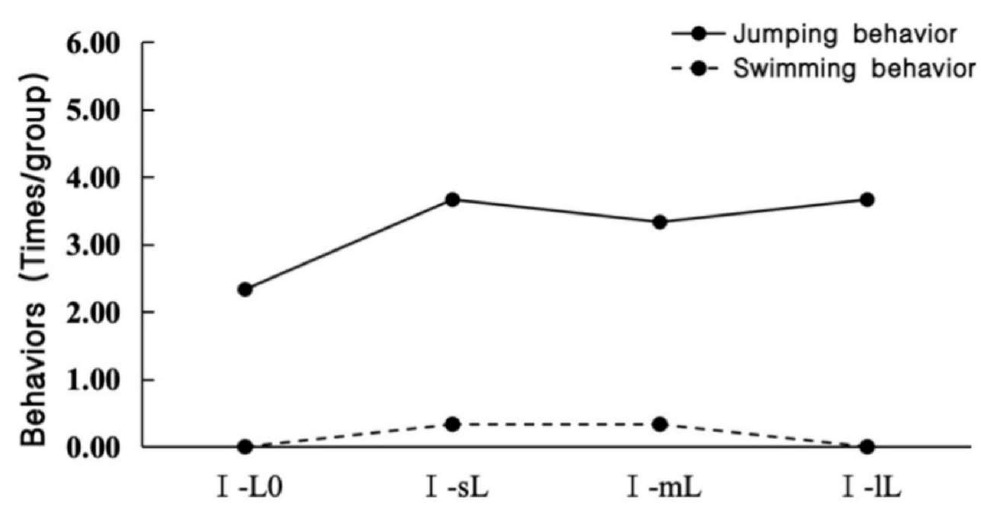
Table 3 shows the predation of scallops by N. cumingii in Experiment I. The small N. cumingii group (I-sL) did not predate scallops, whereas the medium N. cumingii group (I-mL) and large N. cumingii group (I-lL) exhibited relatively more predation of scallops. The number of motor behaviors by scallops was slightly higher in the presence of predators than in the control group, but the differences were insignificant (Figure 2).
Scallops were also exposed to different N. cumingii group sizes in the farming environment (N. cumingii predation of scallops in experiment II). The small N. cumingii group predated no scallop, whereas the medium N. cumingii group predated three small scallops and one medium scallop. In addition, four small scallops were predated by the large-sized N. cumingii group (Table 4). Figure 3 shows the movement behaviors of the scallops of different sizes. In the presence of small and medium-sized N. cumingii groups, the frequency of movement behaviors by scallops was significantly greater than that of the control group. Still, the change was not significant with the large N. cumingii group.
Force of shell closure
After observing images of the shell closure force by M. yessoensis of different sizes under natural conditions and under stimulation by N. cumingii (Figure 4), the results showed that the shell closure force was greater under stimulation by N. cumingii than the control group for scallops of the same size, and the number or duration of shell closure was also higher. The response patterns of scallops with different sizes to stimulation were slightly different. Under stimulation, the Tphase values for the small and medium-sized scallops were significantly higher than the natural state (Figure 4; L-sB, L-mB), and the Ttonic values were longer for larger scallops (Figure 4; L-lB). The shell closure capacities of scallops with various sizes tended to decrease over time.
The Fmax, Tphase, Ttonic, ttonic, closure frequency, and P30s values for each group of scallops are shown in (Figure 5). As the size of the scallops increased, Fmax tended to increase, and the values were slightly higher for the control group, which was the same size as the experimental group. However, the difference was not significant (Figure 5-A). Compared with the control group, the Tphase and Ttonic levels tended to increase in small and medium scallops after stimulation, whereas the changes were insignificant in large scallops. The ttonic values increased significantly, with extremely significant increases in both small and large scallops, and the duration of stimulation for large scallops was almost twice that in the natural state (Figure 5-C). The closure frequency increased significantly for small and medium shellfish, and it was twice that in large scallops (Figure 5-D), where the change in large scallops was insignificant. Figure 5-E shows that after stimulation, the P30s values were relatively large for small scallops, but there were no significant differences between medium and large scallops.
Changes in enzyme activities in tissues
Figure 6 shows changes in the enzyme activities (SOD, CAT, ODH, AK) in the gills, adductor muscle, and mantle of scallops under continuous stimulation by N. cumingii. The enzyme activities varied in different scallop tissues compared with those under no stimulation, with significant differences in the SOD activity in gill tissues and the SOD, CAT, and AK activities in the mantle (p < 0.05). The activities of the four enzymes changed significantly or extremely significantly in the adductor muscle under stimulation (p < 0.01).
Figure 7 shows the enzyme activities in the adductor muscles of scallops of various sizes. The ODH activities in the adductor muscles of largescale scallops after stimulation were 44.46 ± 0.69 U/mL in the control group and 37.53 ± 1.36 U/mL in the experimental group, and the difference was significant compared with the control group (p < 0.01). After stimulation, extremely significant differences (p < 0.01) were found in the activities of the four enzymes in the adductor muscles of medium-sized scallops. The activities of SOD, CAT, ODH, and AK in the control group were 36.82 ± 1.24 IU/mL, 8.46 ± 0.08 U/mL, 38.57 ± 0.28 U/mL, and 176.87 ± 2.89 U/L, respectively. The activities of SOD, CAT, ODH, and AK in the experimental group were 39.99 ± 0.80 IU/mL, 7.69 ± 0.32 U/mL, 43.38 ± 1.14 U/mL, and 158.82 ± 4.50 U/L, respectively. No significant differences were found in the CAT activities in small scallops. Still, significant differences were found in the SOD enzyme activities (41.28 ± 1.30 and 38.45 ± 1.11 IU/mL in the control group and experimental group, respectively), and extremely significant differences in the ODH and AK enzyme activities, where the activities in the control group and experimental group were 37.27 ± 0.37 and 42.73 ± 1.08 U/mL, and 187.92 ± 1.78 and 175.62 ± 1.70 U/L, respectively.
Transcriptome results
The total RNA concentration, integrity, and purity were detected in the scallop samples. The RNA concentrations in mBJs and L-mBJs (L-mBJ1, L-mBJ2, and L-mBJ3) in the closed shell muscle tissues ranged from 61.000 ng/mL to 166.0000 ng/mL, and the total RNA contents ranged from 1.9520 mg to 5.8100 mg. An RIN value above 6.0 indicates that the sample quality met the quality requirements for library construction and sequencing. The total amounts satisfied the requirements for library construction in this study.
In total, 58.41–72.95 × 106 raw reads were obtained by transcriptome sequencing. After removing the connectors and low-quality reads, 55.62–71.19 × 106 clean reads were obtained, with a data volume of 56.63 G. The GC content ranged from 41.67% to 43.67%, and Q20 and Q30 exceeded 95.30% and 88.27% respectively (Table 5). Thus, the reliability of the sample data was high and they could be used for subsequent analyses.
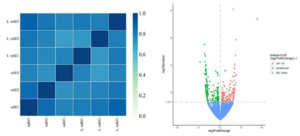
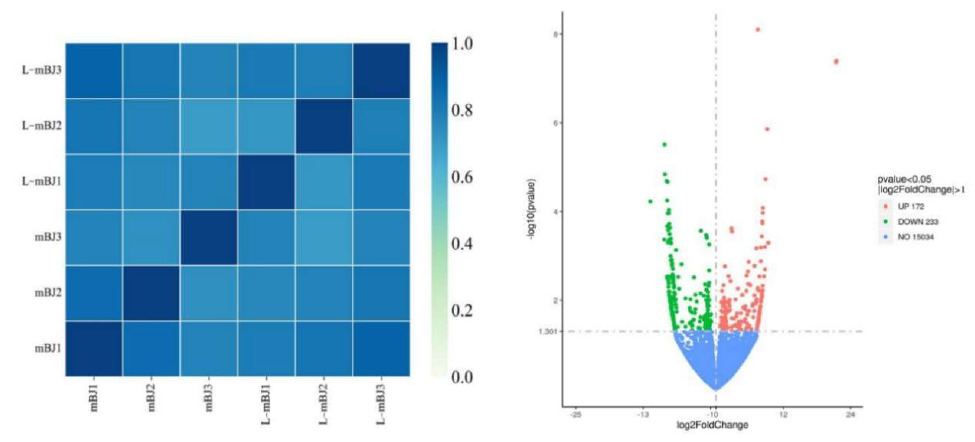
The correlation coefficients were calculated between and within groups of samples based on the FPKM values for all genes in each sample to draw a heat map illustrating the differences and similarities between samples. A correlation coefficient closer to 1 indicated higher similarity of the expression patterns between samples. According to the heat map, differences were found between the mBJ and L-mBJ groups (Figure 8, left). The RNAseq data obtained for the L-mBJ and mBJ groups were screened (DESeq2 p-value £ 0.05 log2FoldChange | ³ 0.0) and compared to obtain the distribution of DEGs in the adductor muscle of scallops after stimulation by N. cumingii (Figure 8, right). The results in the volcano plot in Figure 8 show that 172 DEGs (red) were upregulated and 233 DEGs (green) were downregulated.
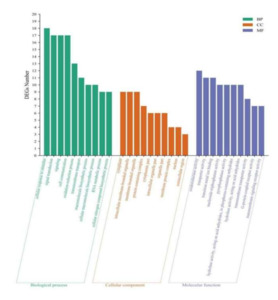
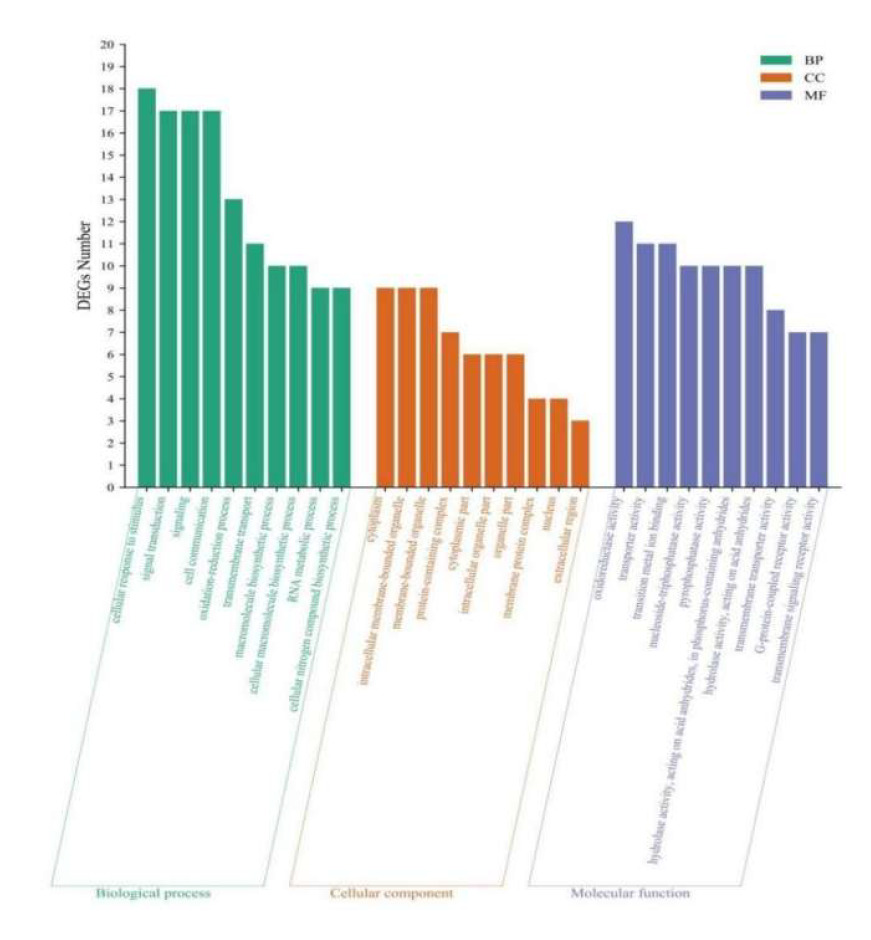
The GO database was used to classify DEGs, and 90, 50, and 149 DEGs were annotated as BP, CC, and MF processes. The top 10 GO entries with enriched DEGs in each process are shown in Figure 9. Among BP processes, DEGs were mainly enriched in stimulating cell response to stimuli, signal transduction, and cell communication. Among CC processes, DEGs were mainly enriched in the cytoplasm, intracellular membrane-bound organelles, membrane-bound organelles, and protein-containing complexes. Among MF processes, DEGs were mainly enriched in oxidoreductase and transporter activity, transition metal ion binding, and other processes.
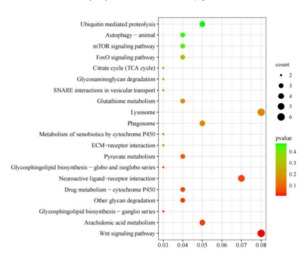
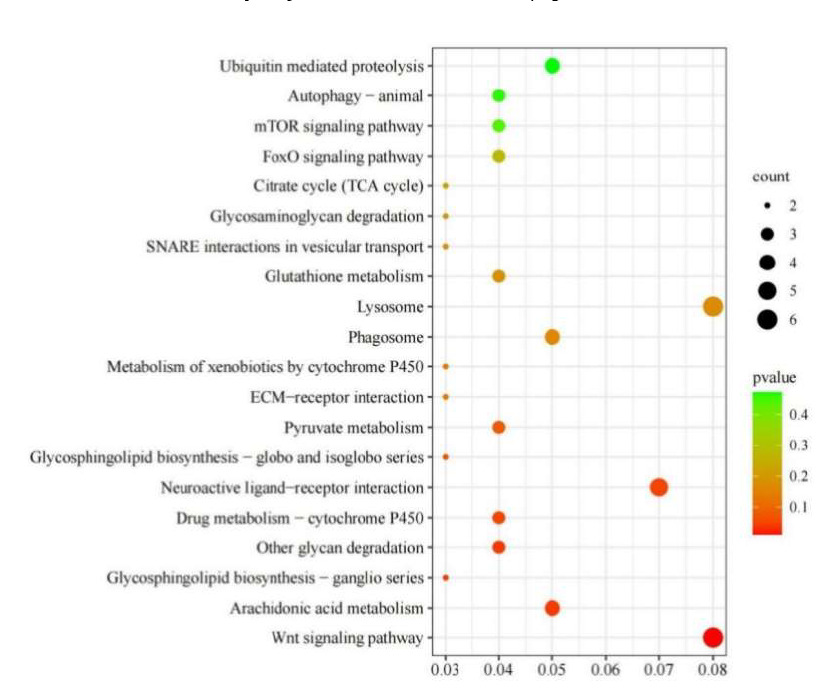
KEGG analysis was applied to understand further the biological functions of DEGs stimulated by N. cumingii in scallops. The results showed that 76 DEGs were enriched in 71 KEGG pathways, and the 20 most abundant pathways are shown in (Figure 10). In particular, the Wnt signaling pathway, Arachidonic acid metabolism, and Glycosphingolipid biosynthesis ganglion series. Other glycan degradation, Neuroactive ligand-receptor interaction, Drug metabolism cytochrome P450, and six other pathways were significantly enriched (p < 0.05).
Nine DEGs were subjected to qRT-PCR validation analysis, where the selected genes comprised C1QL4, OTOF, ZCCHC8, RAD17, TRXL, CYP2C8, C25B8.10, β-GLB, and PROM1A. The expression patterns were consistent with the RNA-Seq results, and the high correlation (R2 = 0.71) confirmed the reliability of the transcriptome analysis results (Figure 11).
Discussion
Effects of N. cumingii predation on the closed-shell force of scallops
The shell opening and closing behavior and mantle state of a bivalve can visually reflect the physiological condition of the shellfish, which is an effective behavioral indicator of the organism’s response to environmental changes. For example, a scallop can produce slow or fast movements to move away from predators through the contraction or diastole of the adductor muscles.7 In this study, the Fmax of large, medium, and small scallops was measured using a dynamometer, and the Tphasic, Ttonic, Fmax, and frequency of shell closure data were collected and analyzed. We found that the Fmax of scallops both with and without the presence of the predator increased with increasing scallop size and that the Fmax of scallops in the same size group produced a greater shell closure force in the presence of N. cumingii, Zhang et al reported similar results.4 We found that scallops would jump or swim away from potential predators. However, we tested the effects of different sizes of N. cumingii on M.yessoensis. We found that the number of jumps made by scallops increased significantly with increasing predator size, although increased swimming behavior was not obvious. Based on all of these findings, we speculate that scallops first choose the bitemporal contraction response when stimulated by predators (i.e., shells open and close quickly to produce jumping behaviors to escape from being eaten).
Scallops have two strategies to cope with predators: either they close their shells tightly or they jump quickly to escape. A thick, hard shell protects predators, but conversely, a heavy shell reduces the scallop’s ability to swim. Therefore, there is a trade-off between mechanical protection and swimming ability when scallops face threats from predators, and their escape behavior in the face of predatory stimuli may change over time during individual development. We found that the Tphasic and Ttonic values of N. cumingii-stimulated scallops were higher than those of the control group for both small- and medium-sized scallops. Additionally, the frequency of shell closure increased significantly upon stimulation, and small-sized scallops closed their shells frequently in the first 30s after stimulation. In contrast, no significant changes in large-sized scallops’ Tphasic and Ttonic values were detected after stimulation. The duration of shell closure in this experimental group was more than twice as long as that of the control group, and the increase in shell closure frequency was insignificant. These results suggest that small- and medium-sized scallops opted for locomotor behavior to escape from predators, whereas large-sized scallops preferentially used shell closure to escape from predators.
As scallops grow, the shell thickens and the ability to cope with predator attacks improves, but swimming ability may decline (Xia 2021). Changes in swimming behavior and ability with age and size vary among species. The locomotor ability (number of shell closures and magnitude of shell closure force) of all sizes of scallops tends to decrease with age. Tremblay et al. compared the swimming behavior of Amusium ballot, Placopecten magellanicus, Pecten formats, Mimachlamys asperrima, and Crassadoma gigantea and found that some scallops could avoid predation by staying still due to the advantage of their shell shape. In contrast, the more active scallops avoided predators by swimming.8 In a study of Aequipecten opercularis, shell closure during the first and second escape responses was more frequent in smaller individuals than in larger ones.9 This is consistent with our study of M.yessoensis. Therefore, we hypothesized that smaller-sized M. yessoensis were more active and avoided predation by N. cumingii through rapid shell closure. In contrast, larger scallops avoided predators by decreasing the frequency and increasing the duration of shell closure.
Effects of N. cumingii predation on the enzyme activities of scallops
Enzyme activity is the fundamental driver of all chemical changes in living organisms, so the regulation of enzyme activity is one of the most important ways to realize the regulation of biological metabolism. The immunity of scallops is affected by exposure to stressors, including predators. SOD and CAT are two important immunoenzymes in the shellfish immune system, and they play an important role in maintaining the balance of the antioxidant system in shellfish (Gao 2016; Liu et al.10). CAT and SOD scavenge and balance intracellular reactive oxygen radicals; thus they are important indicators of the immune defense ability of shellfish. SOD is also involved in defense against aging and biomolecular damage.11
In this study, we compared the SOD, CAT, ODH, and AK activities in the adductor muscles of control scallops and scallops exposed to N. cumingii. The SOD and CAT activities of different sized scallops were affected N. cumingii stimulation. Generally, stronger CAT and peroxidase activities are related to greater resistance and ability to eliminate free radicals. When the living environment of organisms changes, thereby putting them under stress, the activities of these two enzymes undergo changes to allow the organism to adapt to the new environment.12 In our study, the CAT activity decreased most obviously in medium-sized scallops. In contrast, the SOD enzyme activity of medium-sized scallops increased significantly. We speculate that CAT was preferentially involved in the scavenging of reactive oxygen radicals when scallops perceived the threat of predation and that the regulatory stress experienced by the scallops was too high. However, this theory requires further study.
Putative ODHs (OpDHs) play an important role in the anaerobic metabolism of marine invertebrates, especially shellfish. The production of octopus enzyme by ODH is analogous to the production of lactic acid by lactate dehydrogenase in vertebrate muscles. In their studies, the elevated ODH levels correlated with an increase in swimming ability, as adenosine triphosphate (ATP) production in this activity was supported by hydrolysis of phosphoarginine, which was subsequently followed by the continuation of oxidation through ODH-catalyzed NADH and L-arginine to produce octopus alkali, NAD+, and water to ensure the intracellular redox balance.13,14
Our analysis of the adductor muscle activity of scallops revealed a highly significant difference in the ODH activity between control and N. cumingii-stimulated scallops of all three sizes. In particular, we detected a highly significant increase in the ODH activity of medium- and small-sized scallops in the groups exposed to predation. Therefore, we hypothesized that the up-regulation of ODH synthesis may be related to acute stress or sudden swimming activity in M. yessoensis. Strahl et al also showed that the escape and swimming behaviors produced by bivalves in response to a predator are supported by the trans-phosphorylation of phospho-L-arginine and by anaerobic glycolysis to obtain ATP to support this intense muscle activity.15 During the escape response or subsequent recovery, bivalves restore phospho-L-arginine via anaerobic glycolysis, producing octopine. Octopine plays different roles in different tissues, and its level was higher in swimming scallops. The adductor muscle mass and ODH content Argopecten ventricosus, which are chronically exposed to predators, are high,16 which is consistent with the results of our study of M.yessoensis.
AK was first isolated from N. cumingii muscle in 1935, and it is widely found in lower and higher invertebrates. AK is one of the most important enzymes regulating energy metabolism in invertebrates, and it plays a role in the reversible transfer of phosphate groups between ATP via the enzyme-specific guanidinium receptor to keep ATP at relatively stable levels.17 It also can be directly or indirectly involved in related immune responses.18 AKs may be involved in maintaining normal life activities, in an organism’s defense against adverse external environments, and when an organism is subjected to environmental threats that result in locomotor behaviors.19 Our study found that AK activity in different tissues of scallops of all sizes was down-regulated after N. cumingii stimulation. AK expression in the adductor muscles of medium- and small-sized scallops was significantly down-regulated, indicating that scallops experience muscle stress and undergo accelerated energy metabolism when is exposed to predators. This result suggests that AK plays an important role in the muscle movements of stressed scallops. Voncken et al. reported similar findings.20 Predation stress causes scallops to produce explosive locomotor behavior, which requires much energy. Smits et al. (Smits et al. 2010) demonstrated that AK plays a role in vigorous muscle movements after analyzing the enzyme activity of scallop adductor muscles following escape or locomotor behavior. The decrease in AK expression under predation stress also reflects the increase in stress-related energy demand, and the elevated AK content accelerates the organism’s energy metabolism. This process provides energy for the organism to cope with predation stress, which is conducive to the scallop’s swimming ability and survival under predator attack.
Effects of N. cumingii predation on scallop transcriptome
In this study, analysis of transcriptome sequencing data showed that scallops exhibited strong gene expression responses when stimulated by N. cumingii, where 405 significant DEGs were identified. These DEGs indicated that the predation stress on the scallops caused by N. cumingii stimulated a series of physiological activities, such as the significant upregulation of C1QL4. C1QL4 is a member of the C1q/TNF superfamily, and the presence of spherical C1q domains is a hallmark of the C1q/TNF superfamily.21,22 Members of the C1q family are involved in various important physiological functions, such as the innate immune response, insulin metabolism,23 and synaptic the classical activation pathway. C1q can bind to antibodies in antigen–antibody complexes and activate C1r and C1s. Therefore, C1q is an important bridge connecting acquired immunity and innate immunity. C1q like protein has been found in animals such as lampreys, amphioxus, sea squirts, and sea urchins. In addition to playing a role in classic activation pathways, C1q participates in many immune processes,24 such as clearing apoptotic cells to maintain immune tolerance,25 B cells,26 and T cells,27 C1q also plays a role in development,28 wound healing,29 and other processes. A study of rodents found that the expression of C1QL4 was regulated by its developmental status and hormonal factors, and C1QL4 could activate steroids to produce acute reactions.30 In the present study, C1QL4 was significantly upregulated in scallops after predation stimulation, suggesting that the immune system was affected under predatory stress from N. cumingii. Predation stress could have caused changes in energy metabolism and cellular functions in scallops, and C1QL4 could have been involved in the response of scallops to predatory stimuli.
Transcriptome sequencing for the scallop M. yessoensis under stimulation by predation also indicated that significant changes occurred in the expression levels of genes such as glutathione S-transferase (GST), 15-hydroxyprostaglandin dehydrogenase (PGDH), and FMRFaR in the arachidonic acid pathway. In particular, in the arachidonic acid pathway, the GST and PGDH-related genes were significantly upregulated and downregulated, respectively. GST has functions in clearing lipid free radicals and blocking lipid peroxidation.31 In addition, GST can promote the conversion of leukotrienes and prostaglandins. In previous studies, the oxidative stress caused by Corbicula javanicus and Mytilus galloprovincialis under different conditions led to increases in the GST enzyme activity.32–34 In the present study, downregulation of the GST gene may have affected the contents of prostaglandins, thereby influencing oxidative stress in scallops. In summary, we suggest that under continuous stimulation by N. cumingii, oxidative stress affected scallops, and the upregulation of GST played a role in clearing free radicals from the body. In addition, GST affects the conversion of prostaglandins, and the downregulation of PGDH indicates that oxidative stress reactions were initiated in scallops. Neuropeptides are involved in neuroregulation, neural transduction, and hormone function.35 Some neuropeptides have been shown to have strong inhibitory effects on muscle contraction in mollusks such as Meretrix lusoria and Aplysia kurodai.36 FMRFaR is a neuropeptide, and it was also significantly upregulated in these scallops, which may have been related to the strong contraction of the adductor muscle when scallops avoided enemy attacks. In addition, studies have shown that FMRFamide is involved in energy metabolism and the tension and amplitude of rectal contraction in Crassostrea gigas and Sepia officinalis,37 which are similar to our results. Prolactin-releasing peptide receptor (PrRP receptor) is a mediator of stress responses, and a study of rats showed that PrRP may regulate nociception through co-expression of its receptor.38 The differential expression of genes related to the neural active ligand-receptor interaction signaling pathway in scallops under the predatory threat of N. cumingii may help to sense external threats but also lead to a stress response when danger is perceived to regulate stress levels.
This study’s environmental conditions, such as water flow rate, temperature, and dissolved oxygen, are relatively stable as in a controlled laboratory environment, and the research results are ideal. However, under natural conditions, the changes in water flow, temperature, and dissolved oxygen may not be regular, which may be different from the results obtained in the laboratory. In our opinion, the results obtained from this study can still provide some technical reference for the bottom sowing and proliferation of scallops, especially when the size of the scallops exceeds 60mm. Their ability to avoid predators will be significantly enhanced, and the recovery rate of scallops will be improved, so as to improve the breeding benefits.
Conclusion
In summary, physiological responses occurred when scallops responded to predatory stimulation by N. cumingii, with slightly different response modes by scallops of different sizes after stimulation. The shell closure force was generally greater when the scallops were larger. Escape from predation by N. cumingii involved shifting from multiple, rapid opening and closing of the double shells to extending the closure time. In addition, physiological activities such as enzyme activity levels, material metabolism, and cell proliferation in various body tissues responded to predatory stress through self-regulation.
Acknowledgments
The authors wish to thank the staff of the Key Laboratory of Mariculture and Stock Enhancement in North China’s Sea, Ministry of Agriculture, China, for their help with the experiment. In addition, the author would like to thank the International Science Editing Company for helping to improve the language of this article. This study was supported by funds from the National Natural Science Foundation of China (42076101), the Marine Economy Development Special Project of the Liaoning Province Department of Natural Resources, the General Project of the Education Department of Liaoning Province (LJKZZZ0700), the Project of the Liaoning Provincial Natural Resources Department and Dalian key Research.
Authors’ Contribution
Conceptualization: Yang Liu (Lead). Writing – original draft: Yang Liu (Lead). Data curation: Danyang Li (Lead). Formal Analysis: Ying Tian (Lead). Investigation: Ying Tian (Lead). Methodology: Junxia Mao (Lead). Supervision: Xubo Wang (Lead). Writing – review & editing: Zhenlin Hao (Lead). Funding acquisition: Zhenlin Hao (Lead). Resources: Zhenlin Hao (Lead).
Competing of Interest
The authors declare that they have no conflicts of interest.
Ethical Conduct Approval – IACUC
All animal handling followed the guidelines for the care and use of animals for scientific purposes established by the Institutional Animal Care and Use Committee (IACUC) of Dalian Ocean University, China.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.