Introduction
The electric yellow cichlid (Labidochromis caeruleus), commonly known as the “African Prince”, belongs to the Perciformes order, Cichlidae family and Labidochromis genus. Their bodies typically exhibit a vibrant yellow hue and measure about 10-12 centimeters in length, with a black stripe on their dorsal fins extending directly to the tail. In recent years, due to their high ornamental value and strong environmental adaptability, they have been introduced to various countries around the world, becoming the most popular ornamental fish among aquarium enthusiasts and holding substantial economic value in the market.
The growth of fish is influenced by various factors such as feed and the aquaculture environment. Throughout the artificial feeding process, the type and quantity of feed, as well as the feces of fish contribute to the breeding environment. Electric yellow cichlids are common ornamental fish species, and their breeding feeds include both natural animal feeds and artificial feeds. Under different types of feed aquaculture conditions, the microbial communities in circulating water bodies exhibit differences, influencing the material and energy cycling of the water bodies. These differences can also impact the health of cultured fish. In recent years, more and more researchers have studied the structure and function of the bacterial communities in circulating aquaculture systems, and high-throughput sequencing has become a routine method for studying the structure and function of microorganisms (Johnson et al., 2005; Tringe et al., 2008; Mitchella et al., 2018; Bai et al., 2018; Tang et al.,1). By conducting in-depth research on the structure and function of microorganisms in water bodies, we cannot only understand the impact of microorganisms on aquaculture organisms, but also utilize their unique properties and functions to improve water quality conditions. A series of biological treatment methods and technologies that utilize certain characteristics of aquatic microorganisms to transfer, transform, and degrade pollutants in water have been widely recognized and utilized.2
By far, there is limited research on electric yellow cichlids at home and abroad. Fu et al.3,4 explored the effect of the fat levels in feed on the growth, digestion and immune enzymes of the electric yellow cichlids. This study selected six common feeds used in the cultivation of electric yellow cichlids and analyzed the quantity, diversity, metabolic functions, and corresponding relationships with different environmental conditions of microbial communities in the aquaculture environment using high-throughput sequencing technology based on 16S rRNA. It can also help control the occurrence of fish diseases, enhance the immunity of cultured fish, and improve the survival rate of fish, thus providing a theoretical foundation for microecological regulation of electric yellow cichlids in aquaculture water bodies.
Materials and Methods
Experimental Fish
The electric yellow cichlids (Labidochromis caeruleus) used in the experiment were obtained by artificial propagation of the same batch at the Qingdao Marine Science and Technology Museum. The circulating aquaculture experiment was carried out in the museum’s freshwater laboratory.
Methodology
Feeding Conditions and Operations
Three-month-old experimental fishes were selected with a body length of (5.20±0.08) cm and a body mass of (2.10±0.10) g. These fish were randomly divided into five groups, each containing 20 fish. Each fish culture system consisted of water tanks and filter tanks of the same specifications, with a water volume of about 360L. The breeding temperature was 26-28 ℃, and the pH was 6.8 -7.2. The filter materials were Yee bacterial houses and quartz sand, and the biofilter was membrane-hung using a natural feeding method. All procedures were conducted in accordance with the “Guiding Principles in the Care and Use of Animals” (China).
Each experimental group was fed with different types of feeds: nematode (Group 1), shrimp meat (Group 2), red worm (Group 3), brine shrimp (Group 4), water flea (Group 5) and compound feed (Group A) respectively. Adequate amounts were provided during each feeding, ensuring uniform total feeding across all six experimental groups. After feeding for one-hour, leftover feed and feces were siphoned, and water was changed every three days, with the replacement volume being 1/4 of the total water volume.
Daily Management and Statistics
Feed these fish twice daily at 9:00 and 16:00, with a daily feeding amount equivalent to 2% of the body weight. The fish’s activity was observed daily throughout the experiment, from June to September 2022.
Determination of Growth Index
Samples were taken four times at 120 days, 130 days, 150 days, and 180 days. The fish’s body length was measured using a vernier caliper (in cm, accurate to 0.01 cm), and the body mass was recorded using an analytical balance (in g, accurate to 0.01 g). At the end of the experiment, specific growth rate (RSG), weight growth rate (RWG), and food conversion efficiency (EFC) were calculated as follows:
RSG (%/d) = 100%×(lnWt–lnW0)/T
RWG (%) = 100%×(Wt–W0)/W0
Wt is the mass of the final juvenile fish (g), W0 is the initial mass of the juvenile fish in the experiment (g), and T is the experimental time (d).
Collection of Bacterial Community Samples
1000mL of aquaculture water was collected at the same position in each circulating tank. After uniformly mixing three parallel water samples in each group, the samples were filtered through a SARTORIUS (Sedoris, Germany) triple microbial filter of 0.22 μm. The filter membranes containing bacteria from the water samples were stored in a -80℃ refrigerator.
Extraction and Detection of Genomic DNA
The OMEGA Soil DNA Kit extraction kit was used to extract total DNA from water samples of the circulating tanks. The extracted DNA’s concentration, purity, and integrity were assessed using the Qubit 3.0 fluorescence quantifier and 2% agarose gel electrophoresis.
PCR Amplification and High-throughput Sequencing
The amplification primer for the V3-V4 fragment of the bacterial 16S rRNA gene was TGTGTT. Complete sequence sequencing was performed on the Illumina MiSeq sequencing platform.
Data Analysis
Research was utilized for clustering non-repetitive sequences (excluding single sequences) into Operational Taxonomic Units (OTUs) based on a 97% similarity threshold. The RDP classifier was employed for species classification against the RDP database, using a threshold of 0.8; classification results below this threshold were categorized as ‘Unclassified.’ Mothur was employed for the diversity analysis of individual samples. R software was used to generate a bar chart depicting the relative abundance of dominant species, categorizing species with an abundance below a certain threshold (1%) across all samples as ‘Others.’ The R circle package created a collinear relationship graph, comparing the distribution proportions of dominant species among different samples. The PICRUSt was utilized for functional prediction analysis of bacterial communities by comparing the 16S rRNA gene sequence database.
Results
Effects of Different Feeds on the Growth of L. caeruleus
The growth parameter of juveniles fed with different feeds is illustrated in Table 1.
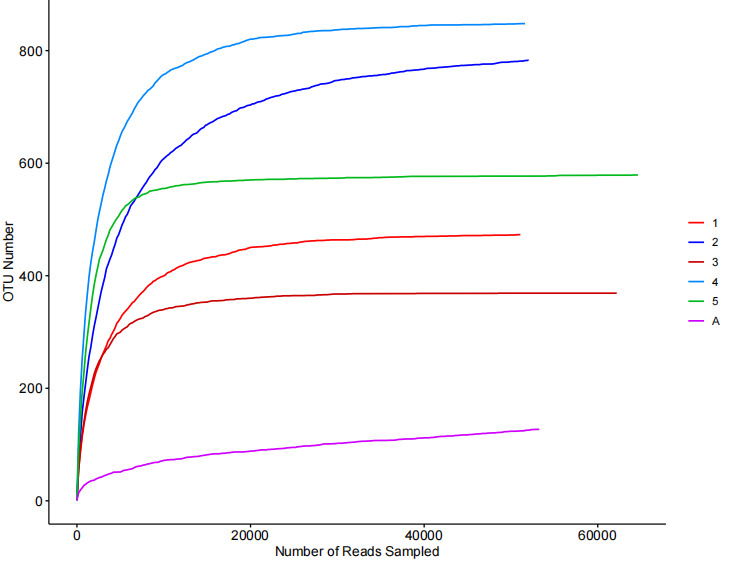
For samples obtained from different feed aquaculture circulating water pools, a total of 51,084 to 64,626 effective sequences were detected, clustering into 127 to 848 OTUs. The Good’s coverage index for each sample exceeds 99%, indicating that each sample has achieved sufficient sequencing coverage and the detected OTUs were also sufficient to represent the sampled population of the batch (Table 2). Meanwhile, the dilution curves of each sample exhibit a trend toward flattening, suggesting that the sequencing data is sufficient to represent the microbial information of each group (Figure 1).
High-throughput Sequencing Results
Analysis of Bacterial Community Diversity
Analysis of Alpha Bacterial Community Diversity
There were significant differences in the abundance and diversity of bacterial communities in the circulating water of electric yellow cichlids cultivated with different feeds (Table 2). According to the Chao index and ACE index, it is evident that the bacterial abundance was highest in the water system where water fleas were used as feed, while it was lowest in the water system where compound feed was used. According to the Shannon and Simpson indices, it can be concluded that the bacterial diversity is highest in the water system where water fleas were used as feed and lowest in the water system where compound feed was used.
Analysis of Beta Bacterial Community Diversity
According to the PCoA analysis, it was found that the bacterial community composition of the aquaculture water samples fed with nematodes (Group 1) and red worms (Group 3) was relatively similar. In contrast, those fed with shrimp meat (Group 2), brine shrimp (Group 4), and water flea (Group 5) showed a closer resemblance (Figure 2). The bacterial community composition in the aquaculture water samples fed with compound feed (Group A) exhibited significant differences. Samples with similar bacterial community compositions are distributed in distinct regions, indicating substantial differences.
Composition and Structural Characteristics of Bacterial Communities
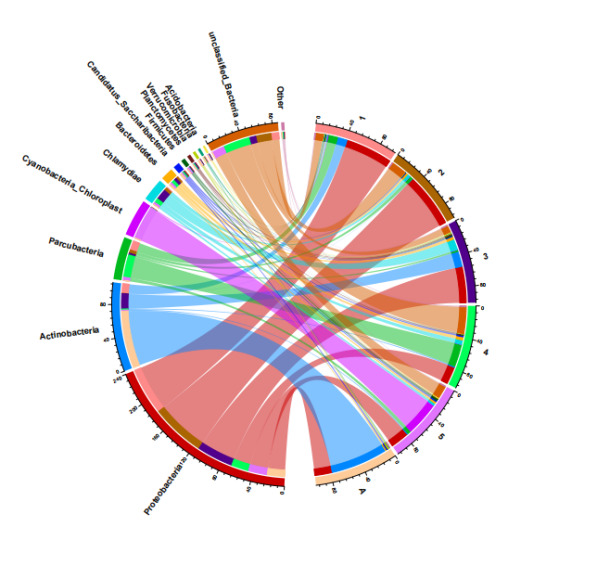
According to the 16S rRNA sequencing results, the species abundance information of samples from different feed aquaculture water bodies was statistically divided into 13 phyla with the dominant phyla mainly belonging to Proteobacteria, Actinobacteria, Parcubacteria, and Cyanobacteria. The distribution of these dominant phyla varies in water samples from different feed aquaculture conditions (Figure 3). Among them, the first dominant phylum in aquaculture water fed with nematodes (Group 1), shrimp meat (Group 2), and red worms (Group 3) as feed is Proteobacteria, with relative abundances of 59%, 68[A] RNA processing and modification;[B] Chromatin structure and dynamics;[C] Energy production and conversion;[D] Cell cycle control, cell division, chromosome partitioning;[E] Amino acid transport and metabolism;[F] Nucleotide transport and metabolism;[G] Carbohydrate transport and metabolism;[H] Coenzyme transport and metabolism;[I] Lipid transport and metabolism;[J] Translation, ribosomal structure and biogenesis;[K] Transcription;[L] Replication, recombination and repair;[M] Cell wall/membrane/envelope biogenesis;[N] Cell motility;[O] Post-translational modification, protein turnover, and chaperones;[P] Inorganic ion transport and metabolism;[Q] Secondary metabolites biosynthesis, transport, and catabolism;[R] General function prediction only;[S] Function unknown;[T] Signal transduction mechanisms;[U] Intracellular trafficking, secretion, and vesicular transport;[V] Defense mechanisms;[W] Extracellular structures;[Y] Nuclear structure;[Z] Cytoskeleton%, and 45%, respectively; the first dominant phylum in aquaculture water fed with Brine shrimp (Group 4) as feed is Parcubacteria, with a relative abundance of 28%; the first dominant phylum in aquaculture water fed with water flea (Group 5) as feed is Cyanobacteria, with a relative abundance of 23%; the first dominant phylum in aquaculture water fed with compound feed (Group A) as feed is Actinobacteria, with a relative abundance of 71%. The top ten phyla based on relative abundance in each water body are Proteobacteria, Actinobacteria, Parcubacteria, Cyanobacteria, Chloroplast, Chlamydiae, Bacteroidetes, Candidatus, Saccharibacteria, Firmicutes, Planctomycetes, Verrucomicrobia (Figure 4).
PICRUST Function Prediction Analysis
By comparing 25 functional proteins in the COGs database, significant differences (P<0.05) were found in 24 functional microbial gene metabolic pathways among different feed-cultivated L. caeruleus water samples, as shown in Figure 5. Seven functional proteins encoding [C, E, F, G, I, P, Q] in the bacterial community of aquaculture water fed with compound feed (Group A) were significantly upregulated (P<0.05), all of which were associated with metabolism. Specifically, it involves the enhancement of functions related to [C] energy production and conversion, [E] amino acid transport and metabolism, [F] nucleotide transport and metabolism, [G] carbohydrate transport and metabolism, [H] coenzyme transport and metabolism, [I] lipid transport and metabolism, [P] inorganic ion transport and metabolism, and [Q] enhancement of functions related to energy production and conversion, as well as secondary metabolite biosynthesis, transport, and degradation [C, E, F, G, H, I, P, Q]. Eight functional proteins encoding [J, K, M, N, O, T, U, V] in the bacterial community of aquaculture water fed with water fleas (Group 5) were significantly upregulated (P<0.05). It involves the enhancement of functions related to [J] translation, ribosomal structure, and biogenesis, [K] transcription, [L] replication, recombination, and repair, [M] cell wall/membrane/envelope biogenesis, and [N] improvement of cell motility functions. The bacterial community functions in aquaculture water fed with red worms (Group 3) and nematodes (Group 1) showed higher similarity. The bacterial community function of the aquaculture water fed with shrimp meat (Group 2) was significantly lower than that of the other groups (P<0.05).
Discussion
The feeding environment affects the microorganisms that fish come into contact with, which in turn affects the survival and growth of fish through host-microbial interactions.5 Fish live in aquatic environments and can obtain bacteria and microorganisms from the water that affect their health and community composition.6
The composition of feed plays a decisive role in the growth of juvenile fish, especially the significant differences between feeding live feed and compound feed. This situation has been confirmed in the growth experiments of sea bass7 and juvenile fish of coilia.8 The results of this study showed that the compound feed group exhibited the highest specific growth rate and mass gain rate, while the water flea group and brine shrimp group showed lower rankings with significant differences. The diversity of bacterial community was higher in the water flea group and brine shrimp group, while the compound feed group had the lowest diversity. According to the original observation, the mouth cleft of the 3-month-old electric yellow cichlid juveniles gradually enlarged. The compound feed was more suitable for their feeding caliber, and the higher fat content in the compound feed contributed to faster juvenile growth, resulting in accelerated growth of the young fish. However, the individual size of live feed such as brine shrimp and water fleas are relatively small, and the growth rate of juvenile fish is slower due to the expenditure of some energy during search and predation. In production, water fleas feed on chlorella vulgaris, while brine shrimp need to feed on ciliate protozoa for nutritional enhancement before being used as feed for juvenile fish. Moreover, due to the small size of water fleas and brine shrimp, some residues still remain in the aquaculture water during daily water changes, participating in the metabolic cycle system of the aquaculture water, resulting in higher bacterial richness and diversity.
Analysis of Dominant Bacterial Communities and Their Functions in Aquaculture Water with Different Feeds
The temperature, chlorophyll and dissolved oxygen in water bodies are important environmental factors that affect the structure of bacterial communities (Langenheder et al., 2014). The results of this study show that, at the level of phylum classification, there are similarities and differences in the main dominant bacterial communities of several aquaculture water bodies with different feeds. The first dominant bacterial community in aquaculture water fed with nematodes, shrimp meat, and red worms is Proteobacteria, which is also the main dominant bacterial community in other experimental groups of aquaculture water. The reason for this is related to the relatively large proportion of Proteobacteria in the molecular or phenotypic classification of prokaryotes.9 In the artificial aquaculture water environment of various organisms such as sea cucumber (Li et al.,10; Dou et al., 2010; Ren et al.,11) and grouper,12 it exists as a dominant bacterial community. Proteobacteria can form symbiotic relationships with animals in aquaculture water bodies and play an important role in nitrogen, carbon, and sulfur cycling in the water environment.13,14 The dominant bacterial community in the aquaculture water body fed with water fleas in this experiment is Cyanobacteria. The reason for this can be attributed to the fact that water fleas feed on algae, and the water contains a higher concentration of algae. The physical and chemical factors of water provide inorganic salts and organic nutrients for the growth and reproduction of microorganisms, and the structural characteristics of bacterial communities are often influenced by the concentration of physical and chemical factors in their environment (Nishiyama et al., 2009; Lina et al., 2018). Cyanobacteria are important components of marine ecosystems and primary productivity. They can produce O2 by assimilating CO2, fix nitrogen under aerobic conditions, and reduce sulfur through anaerobic respiration in the dark (Jie et al., 2000). The first dominant bacterial community in the aquaculture water of the experimental group fed with compound feed is Actinobacteria. The Beta diversity diagram and collinearity analysis diagram at the phylum level show significant differences in the bacterial composition of the compound feed experimental group compared to other experimental groups. Actinobacteria can promote the decomposition of organic matter in the aquatic environment. During the aquaculture process, feeding electric yellow cichlid juveniles with compound organic feed results in undigested feed and feces not being fully decomposed in the water, leading to deposition and an increase in Actinobacteria content. In the daily feeding process, composite feed is a type of bait that is easier to obtain and preserve than fresh bait, but it is also more likely to cause the accumulation of ammonia nitrogen in the breeding environment. Therefore, when feeding compound feed, it is necessary to observe the activity, feed intake, and excretion of electric yellow cichlids, keep the bottom of the pond clean, observe changes in water quality indicators, and regularly add nitrifying bacteria.
Overall, this study employed high-throughput sequencing technology to analyze the microorganisms in the circulating water of electric yellow cichlids cultivated with six types of feeds. The diversity and abundance of bacterial communities in each aquaculture water body were revealed, and their bacterial communities’ structural and functional differences were compared. The correlation between bacterial community variations and different feed-based aquaculture environments was obtained. This provides valuable insights for the healthy cultivation of electric yellow cichlids.
Acknowledgments
The National Marine Genetic Resource Center supported this study.
Authors’ Contribution
Conceptualization: Chengxu Ha (Equal), Yunzhong Wang (Equal). Data curation: Chengxu Ha (Equal), Yi Ren (Equal). Formal Analysis: Chengxu Ha (Equal), Cuihua Yang (Equal). Investigation: Chengxu Ha (Equal), Cuihua Yang (Equal). Methodology: Chengxu Ha (Equal), Cuihua Yang (Equal). Project administration: Chengxu Ha (Equal), Chengxu Ha (Equal), Yunzhong Wang (Equal), Peng Jin (Equal), Yi Ren (Equal). Resources: Chengxu Ha (Equal), Yunzhong Wang (Equal), Peng Jin (Equal). Software: Chengxu Ha (Equal), Yi Ren (Equal). Writing – original draft: Chengxu Ha (Lead). Writing – review & editing: Chengxu Ha (Lead). Funding acquisition: Yunzhong Wang (Equal).
Competing of Interest – COPE
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Conduct Approval – IACUC
All procedures were conducted in accordance with the “Guiding Principles in the Care and Use of Animals” (China).
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.