Introduction
Aeromonas hydrophila, a water-loving bacterium, has a wide range of pathogenicity and can infect various animals, causing septicemia or local infections such as skin ulcers. During hot summers, it can cause large-scale outbreaks of diseases in aquatic animals, especially fish. Once infected, fish can suffer from high mortality rates, causing unavoidable losses for aquaculturists.1 To prevent such occurrences, numerous chemical drugs and antibiotics have been widely used in aquaculture. However, the misuse of antibiotics can lead to drug resistance and drug residues in organisms, which not only have a negative impact on the treatment of fish diseases but also pose significant harm to human health.
The Wuchang bream (Megalobrama amblycephala) is a freshwater fish known for its substantial production volume.2 However, the aquaculture environment often contains bacteria, viruses, and exogenous pathogenic factors,3 leading to massive outbreaks of diseases in Wuchang bream. Emodin, a kind of Chinese herbal medicine, has the advantages of pure nature, no residue and low toxicity. Many beneficial functions have been reported for emodin, such as anti-bacterial and anti-inflammatory, antioxidation and scavenging free radicals, and regulating immunity. Previous experiments conducted by our research group have shown that emodin has good in vitro antibacterial ability against A. hydrophila; and as a drug additive, emodin can resist the infection of A. hydrophila in Wuchang bream. However, it is not known whether it can achieve the purpose of resisting bacterial infection by improving the antioxidant function of fish.
The antioxidant defense system in animal organisms is a complex system that has evolved. It includes enzymatic and non-enzymatic antioxidant systems.4 The enzymatic antioxidant system primarily comprises enzymes such as Superoxide Dismutase (SOD), Catalase (CAT), Glutathione-S-Transferase (GST), Glutathione Peroxidase (GPx), and Glutathione Reductase (GR). The non-enzymatic antioxidant system includes substances like Vitamin E, Vitamin C, Glutathione, Nitric Oxide, and Carotenoids, which act as reducing agents.5–7 This enzyme system maintains the balance between oxidative and anti-oxidative processes in the body, ensuring normal physiological responses.
Based on this, this study is designed to investigate the effects of emodin on the physiological metabolism and antioxidant ability of Wuchang bream infected with A. hydrophila. The results can provide a theoretical basis for the development of healthy aquaculture and the prevention and control of fish diseases.
Materials and methods
Experimental diets. The formulation and proximate composition of the basal diet are shown in Table 1. The basal diet was supplemented with 0 (control), 30, 100, and 150 mg emodin kg-1 diet, respectively. The emodin (the purity >99%) was provided by Feida Chemical Reagent Company with (Xi’an, China). To prepare the experimental diets, the ingredients were ground to the fine powder using a 60-mesh sieve. Then, dry ingredients were mixed and water was added (40% v w-1) to form a soft dough. The dough was then pelleted using a laboratory pellet machine and dried in a forced-air oven at room temperature. After drying, the diets were broken into smaller pieces and sieved into the proper pellet size. All diets were stored at -20 ℃ until used.
Fish husbandry. Fish were obtained from a fish farm of the Fresh-water Fisheries Research Center, Chinese Academy of Fishery Sciences. Prior to experiments, fish were fed the basal diet for 15 days for acclimation. Fish were hand-fed with the test diets three times (08: 00, 12: 00 and 16: 00) per day until apparent satiation for 14 days. During the experiment period, fish were retained under a nature photoperiod. The temperature was controlled at 26 ± 1°C over the experimental period, pH 7.2-7.8, ammonia nitrogen<0.05 mg L-1, and dissolved oxygen>6 mg L-1.
Challenge test.
Preparation of bacteria. Gram negative A. hydrophila was originally isolated from the infected fingerling M. amblycephala. The A. hydrophila used in this experiment was provided by the Fish Disease Laboratory of Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences. Under aseptic conditions, a strain of aquatic A. hydrophila stored at -80℃ was inoculated onto LB solid agar plates and cultured for 20 hours at 28℃ in a constant temperature incubator. A single colony was then selected and inoculated into 5 mL of LB liquid medium and cultured overnight at 28℃ on a constant temperature shaker at 180 rpm min-1. The bacterial suspension was diluted using sterile LB liquid medium in a 10-fold series
The LC50 of 7 days was determined by intraperitoneal injection of 48 fish with graded concentrations of A. hydrophila (106,107,108,109 and 1010 CFU mL-1) at 24℃, yielding LC50 of 7 days is 5×106 CFU mL-1. Based on the experimental results, the concentration of 1×106 CFU mL-1 was used for the infection test.
Experiment design. All fish (average weight, 50.4 ± 2.35 g) were divided into 15 aquarium with the density of 25 fish per tank (three replicates each treatment) as Group I: uninfected fish fed basal control diet (negative control, NC), Group II: infected fish fed basal control diet (positive control, PC), Group III: infected fish fed the diet containing 30 mg emodin kg-1 (30), Group IV: infected fish fed the diet 100 mg emodin kg-1 (100), and Group V: infected fish fed the diet 150 mg emodin kg-1 (150).
Sample collection. On day 0, 1, 4, 7, and 14 of the experiment, three fish were randomly selected from each tank and anesthetized with 100 mg L-1 MS-222 (tricaine methanesulfonate, Sigma, USA). Blood was collected by the caudal venipuncture using 1 mL heparinized syringes. The blood was centrifuged (3500×g, 10 min, 4 ℃) to obtain plasma and stored at - 80 ℃ until the analysis.8 Then, 3 fish were rapidly dissected for collecting liver. The liver was stored at - 80 ◦C until molecular, immunological and antioxidant index analysis (Seval and Emin, 2020).
Plasma biochemical analysis. Serum alanine transaminase (ALT), aspartate transaminase (AST) and alkaline phosphatase (AKP) activities were measured by the IFCC method. The total protein (TP) content was determined by direct assay and the glucose (Glu) level was measured using the photocolorimetric method. All these kits were bought from Shenzhen Mindray Bio-Medical Electronics Co., Ltd. The measurements were conducted in a Mindray Auto Bio-chemical Analyzer (BS-400, Mindray, P.R. China).
The quantitative Real-time PCR (qRT-PCR) analysis. Primers for Cu/Zn-SOD、CAT、GPx、TNF-α and IL-1β were designed using primer premier 5.0, based on the mRNA sequences obtained from the Wuchang bream genome database of the Key Laboratory of Freshwater Fisheries and Germplasm Resources Utilization, Ministry of Agriculture, China. (As shown in Table 2). The PCR primers were produced by Shanghai General Biotechnology, Co., Ltd, China. Firstly, total RNA was extracted by RNAiso Plus (TaKaRa, Japan) and then measured by Nanodrop 2000 (Thermo Fisher Scientific, USA). The OD 260/280 of total RNA is 1.8-2.0. The RNA in each sample was diluted to 500 ng/ml, and the following quantitative analysis was carried out for 2 μg of the total RNA with a Two Steps SYBR® PrimeScript® Plus RT-PCR Kit (TaKaRa, Dalian). The qRT-PCR was performed in 20 μl reaction volumes under the following thermal profile: 1 cycle at 50 °C for 2 min, 95 °C for 3 min; 40 amplification cycles at 95 °C for 15 s, 60 °C for 1 min and conducted by ABI 7500 real-time PCR system, and the 2-△△CT method was used for analysis.
Statistical analysis. All data are presented as means ± S.E. (standard error of the mean). Data were logarithmically transformed before subjection to one-way analysis of variance (ANOVA) using SPSS 19.0. When the overall treatment effect was found to be significantly different, the LSD multiple range tests was conducted to compare the means between the levels of emodin treatment. The level of significant difference was set at P < 0.05.
Results
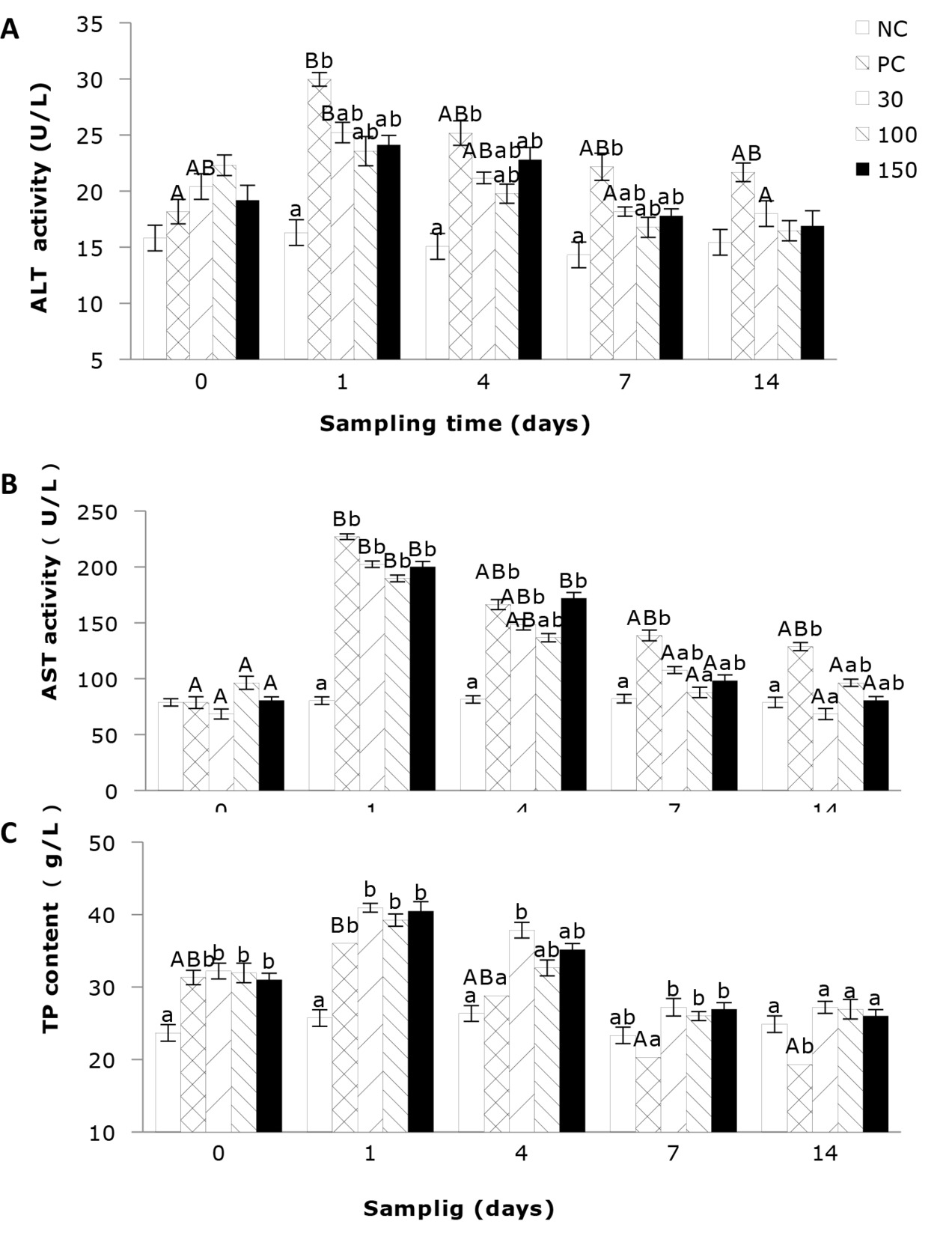
The effects of emodin on the activities of ALT and AST and the TP content in the plasma of infected Wuchang bream
As shown in Figure 1A, the plasma activity of ALT of Wuchang bream infected with A. hydrophila was significantly affected by the sampling time. The results indicate that the ALT activity in the negative control group remained stable throughout the entire experimental period and was relatively low. In contrast, the ALT activities in the other four groups showed a trend of initial increase and then decreasing. Additionally, plasma ALT activity was significantly affected by the emodin supplementation in the diet. After 4 days’ feeding, the ALT activity in all experimental groups showed a decrease compared to day 1, but the differences between the groups were similar to those on day 1. After 7 days’ feeding, the ALT activity in the positive control group remained significantly higher (P<0.05) than that in the negative control group, while the other groups showed no significant differences.
As shown in Figure 1B, the plasma AST activity of the negative control group was relatively stable and comparatively low, while the AST activities in the other four groups were significantly influenced by feeding time, exhibiting a trend of initial increase followed by a decrease. Among them, supplementation of 30 and 100 mg emodin kg-1 diet can significantly reduce the plasma AST activity in A. hydrophila-infected fish, allowing it to return to normal levels in the shortest time. Additionally, the plasma AST activity in Wuchang bream was significantly affected by the supplementation of emodin. At 1 day of feeding, the AST activity in the positive control group and the 30, 100, and 150 mg/kg groups was significantly higher (P < 0.05) than in the negative control group. After 4 days’ of feeding, the AST activity in the 100 mg/kg group showed no significant difference (P > 0.05) compared to the negative control group, but the positive control group and the 30 and 150 mg/kg groups remained significantly higher than the negative control group. After 7 days’ feeding, the differences in AST activity between the 30, 100, and 150 mg/kg groups and the negative control group were not significant (P > 0.05), but the positive control group remained significantly higher (P < 0.05) than the negative control group and the 100 mg/kg group. After 14 days’ feeding, only the positive control group showed higher AST activity than the negative control group.
In Figure 1C, the total protein (TP) content of the negative control group remains relatively stable and at a lower level. The positive control group was significantly affected by feeding time (P < 0.05), while the impact on the other groups was not significant. Additionally, emodin significantly influenced the plasma TP content in Wuchang bream. At the beginning of the experiment, the TP content in the positive control group and 30, 100, and 150 mg/kg groups rapidly increased and was significantly higher (P < 0.05) than in the negative control group, although there were no significant differences among the four groups. After 1 day of feeding, the TP content in all experimental groups continued to rise, with the positive control group and the 30, 100, and 150 mg/kg groups still significantly higher (P < 0.05) than the negative control group. After 4 days’ feeding, the 30 mg/kg group remained significantly higher (P < 0.05) than the negative control group and the positive control group, while the 100 and 150 mg/kg groups showed no significant differences (P > 0.05) compared to the negative control and positive control groups. After 7 and 14 days’ feeding, the TP content in the positive control group was the lowest and significantly (P < 0.05) lower than the other four groups.
The effects of emodin on the levels of COR, T3, and T4 in the plasma of infected Wuchang Bream
As shown in Figure 2A, the content variation of cortisol (COR) in the negative control group remained stable and at a lower level. The supplementation of emodin significantly affected the plasma COR content in the Wuchang bream. At the beginning of the experiment, the plasma COR level in the infected fish rapidly increased and was significantly higher than that in the negative control group (P < 0.05). After 1 day of feeding, the COR level in the positive control group continued to rise and was significantly higher than that in the negative control group (P < 0.05). After 4 days’ feeding, the COR level in the positive control group remained significantly higher than that in the negative control group (P < 0.05).
As per Figure 2B, the thyroxine (T3) levels in Wuchang bream infected by Aeromonas hydrophila were significantly influenced by the supplementation of emodin in the diet, while the impact of feeding time was not significant (P > 0.05). At the beginning of the experiment, infection with A. hydrophila led to a rapid and significant (P < 0.05) increase in plasma T3 levels compared to the negative control group. After 1 day of feeding, the T3 levels in all experimental groups increased, with the positive control group showing a significant (P < 0.05) elevation compared to the negative control group. Subsequently, the T3 levels in all groups began to decrease. After 4 days’ feeding, the T3 levels in the positive control group remained significantly (P < 0.05) higher than those in the negative control group, with no significant differences observed among the other groups (P > 0.05). After 7 days of feeding, the T3 levels in the positive control group were significantly (P < 0.05) higher than those in the 100 mg/kg group, while no significant differences were observed among the other groups. Subsequently, the T3 levels in all groups tended to stabilize.
In Figure 2C, the negative control group exhibited relatively lower and nonsignificant changes in thyroxine (T4) levels at different time points. The remaining groups were significantly influenced by feeding time, with the supplementation of 100 mg emodin per kg of diet rapidly bringing the plasma T4 levels to normal within the shortest time. Additionally, plasma T4 levels were significantly affected by the supplementation of emodin in the diet. After 1 day’ feeding, the plasma T4 levels in the positive control group and the 30, 100, and 150 mg/kg emodin groups rapidly increased and were significantly (P < 0.05) higher than those in the negative control group. After 4 days’ feeding, compared to the previous sampling, the T4 levels in all experimental groups, except the negative control group, showed a decrease. However, the positive control group remained significantly (P < 0.05) higher than the negative control group, while there were no significant differences (P > 0.05) among the other groups. After 7 days’ feeding, the plasma T4 levels in the 30, 100, and 150 mg/kg emodin groups all decreased to levels with no significant differences (P > 0.05) compared to the negative control group but were significantly (P < 0.05) lower than those in the positive control group.
The effects of emodin on plasma AKP activity, Glu, and TG Levels in Wuchang bream after infection
The influence of emodin on plasma AKP activity in Wuchang bream following intraperitoneal infection with A. hydrophila is depicted in Figure 3A. The results indicate that the plasma AKP activity in the positive control group was significantly affected by feeding time (P < 0.05). Supplementation of 30, 100, and 150 mg emodin per kg of diet can significantly inhibit the reduction in plasma AKP activity in fish after infection. Furthermore, the supplementation of emodin in the diet also affected the plasma AKP activity. At the start of the experiment and on the 1st and 4th day of feeding, the influence of emodin supplementation on plasma AKP activity in each group is not significant (P > 0.05). However, at 7 and 14 days of feeding, the AKP activity in the positive control group was significantly lower than that in the 100 mg/kg group (P < 0.05), with no significant differences observed among the other groups (P > 0.05).
The impact of emodin on Glu content in fish infected with A. hydrophila is illustrated in Figure 3B. The results indicated that at the beginning of the experiment, there was no significant difference in Glu content among the groups, and the levels were relatively low. After 1 day’ feeding, the Glu content in the positive control group and the 30, 100, and 150 mg/kg emodin groups increased significantly (P < 0.05), surpassing that of the negative control group. After 4 days ’ feeding, there was no significant change in Glu content among the groups compared to the previous sampling (P > 0.05), and the Glu content in the infected groups remained significantly higher than that in the negative control group. By 7 days’ feeding, the Glu content in the 30, 100, and 150 mg/kg emodin groups showed a decrease, with the 100 and 150 mg/kg groups significantly lower than the positive control group (P < 0.05).
As shown in Figure 3C, the plasma TG levels in the negative control group remained relatively stable and low. In contrast, the TG levels in the other four groups exhibited a trend of initial decline followed by an increase, with the positive control group significantly influenced by the feeding time (P < 0.05). Additionally, the supplementation of emodin in the diet significantly affected plasma TG levels. After 1 day’ feeding, the plasma TG levels in the positive control group and the 30, 100, and 150 mg/kg emodin groups were all significantly lower than those in the negative control group (P < 0.05). However, by 4 days’ feeding, the TG levels in these four groups increased, with the positive control group still significantly lower than the negative control group and the 100 mg/kg group (P < 0.05), while no significant differences were observed compared to the other two groups (P > 0.05).
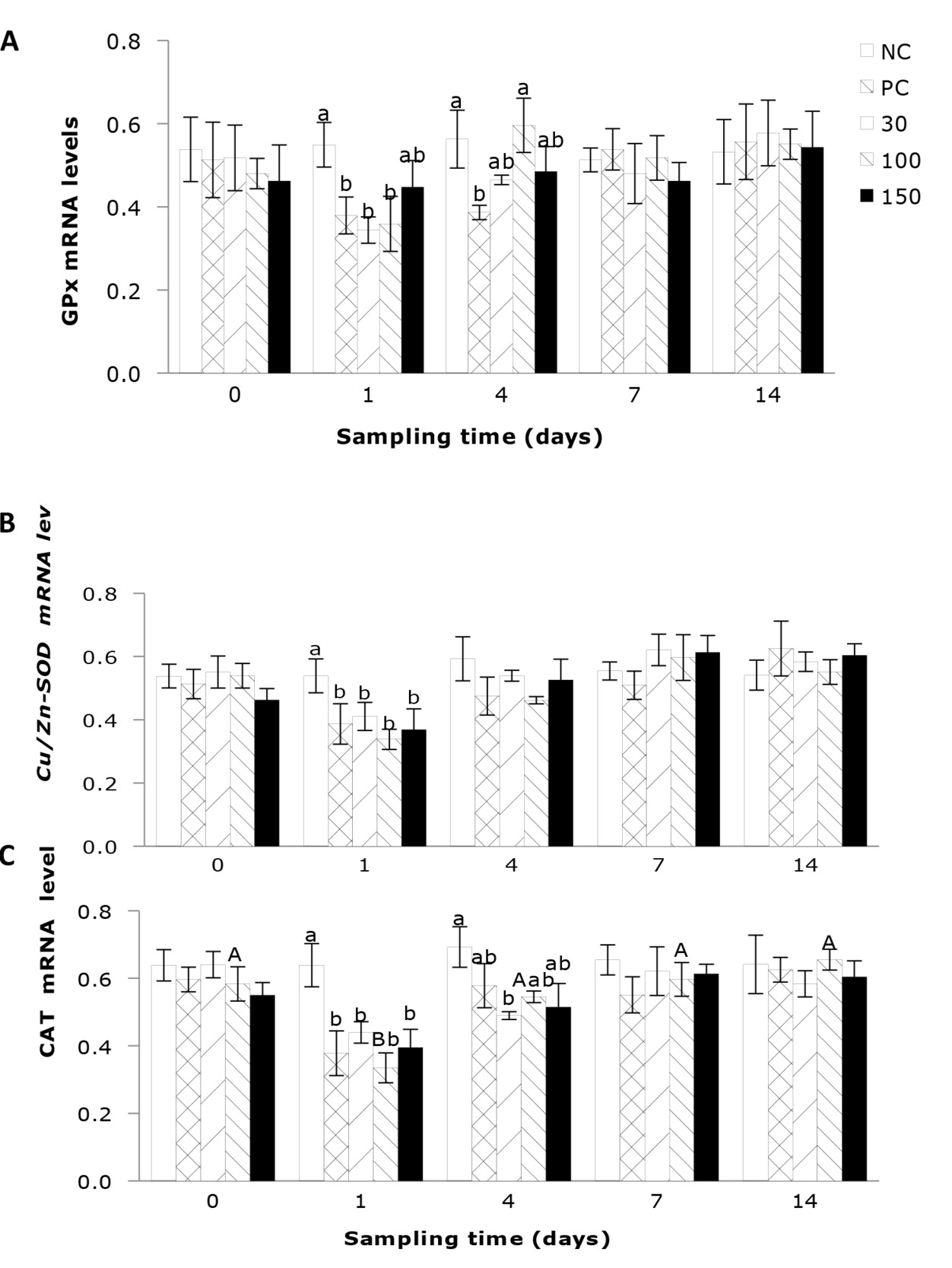
The Influence of emodin on the expressions of antioxidant genes, TNF-α, and IL-1β in Wuchang bream after infection
The impact of emodin on the liver GPx expression in fish infection with A. hydrophila is shown in Figure 4A. The results indicate that the liver GPx expression in infected fish was significantly influenced by the supplementation of emodin. After 1 day’ feeding, the liver GPx expression in the positive control group and the 30, 100 mg/kg emodin groups was significantly lower than that in the negative control group (P < 0.05). By 4 days’ feeding, the liver GPx expression in the positive control group was significantly lower than that in the negative control group and the 100 mg/kg emodin group (P < 0.05).
As shown in Figure 4B, the hepatic Cu/Zn-SOD gene expression in fish post-infection was not significantly influenced by the feeding duration across all groups (P > 0.05). Furthermore, at day 1 of feeding, the infected groups exhibited a significant reduction (P < 0.05) in the hepatic Cu/Zn-SOD compared to the negative control group. The influence of emodin on the hepatic CAT gene expression in fish after infection is depicted in Figure 4C. Emodin had a significant effect on the hepatic CAT gene expression in fish. After 1 day’ feeding, the CAT gene expression in the positive control group and the 30, 100, and 150 mg/kg emodin groups was significantly lower than that in the negative control group (P < 0.05). By 4 days’ feeding, the hepatic CAT gene expression in the 30 mg/kg group was significantly lower than that in the negative control group, while there were no significant differences among the other groups (P > 0.05).
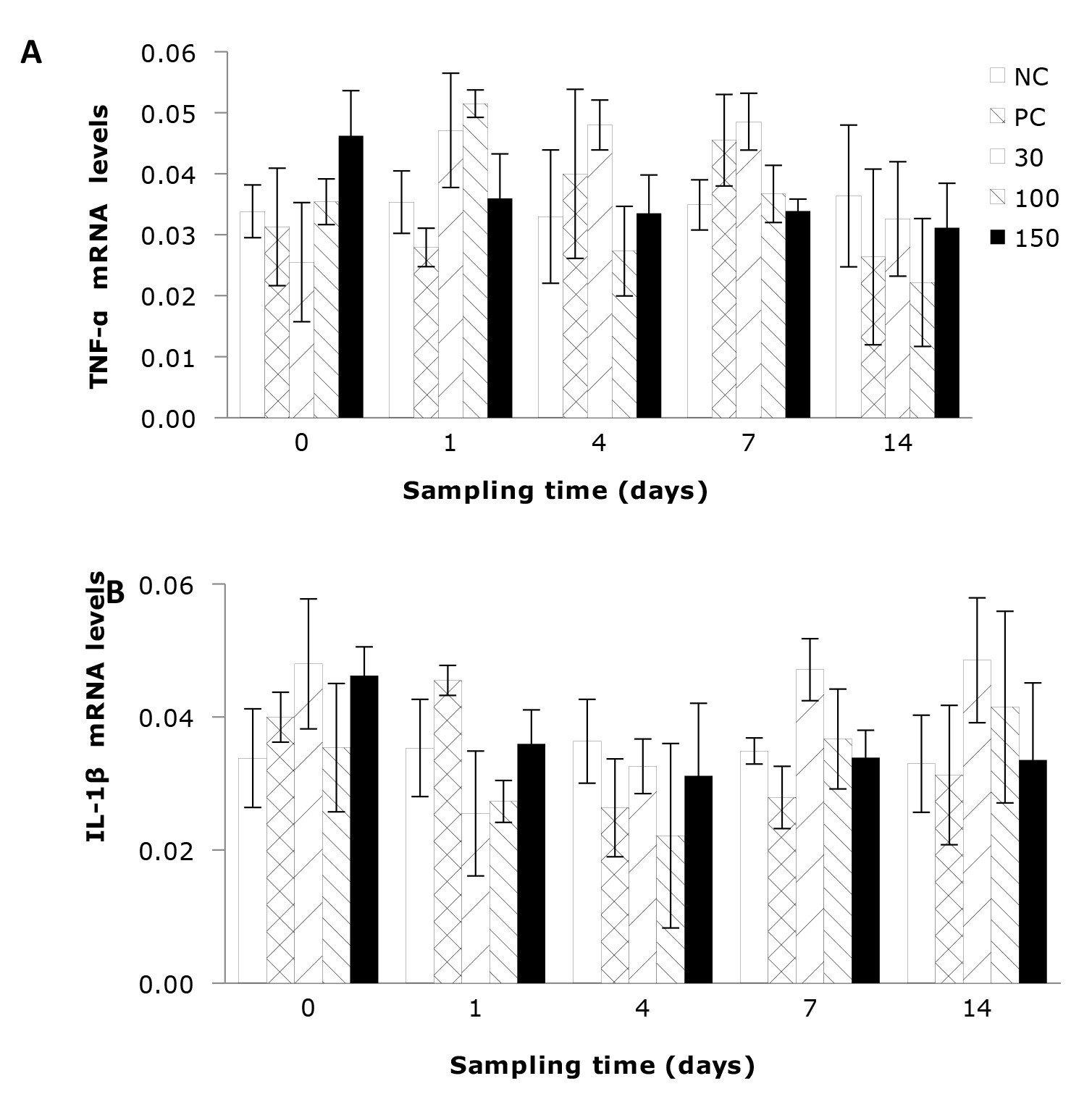
The impact of emodin on the relative expression levels of hepatic TNF-α and IL-1β gene expressions in fish after infection is illustrated in Figures 5A, B. The results indicated that the gene expressions of hepatic TNF-α and IL-1β in infected fish were not significantly influenced by the dietary supplementation of emodin or the feeding duration (P > 0.05).
Discussion
A. hydrophila is a commonly found bacterium in aquaculture environments.9 Some strains of A. hydrophila can also cause diseases in humans.10 Apart from affecting humans, A. hydrophila can induce hemorrhagic septicemia and ulcers in freshwater fish, leading to fish mortality.11,12 Fish exhibit a series of physiological responses following infection with A. hydrophila. Total protein (TP) is involved in maintaining normal osmotic pressure, constant pH, and associated with the transport of lipids, bilirubin, cholesterol, and phosphate, indirectly reflecting the level of non-specific immune response in the organism.13 Some researchers propose that the content of blood proteins increases under acute stress, such as transportation, ammonia nitrogen exposure, or pathogenic bacterial infection, and decreases under chronic stress.14,15 Studies have shown that rainbow trout (Oncorhynchus mykiss) fed with feed containing 0.1% oligosaccharides during transportation stress exhibited a significant increase in serum TP content.14 Huang et al.16 conducted a study on the pathological and physiological responses of the hemorrhagic ascites disease in Carassius auratus gibelio, revealing that the plasma TP content in diseased fish was significantly lower than that in the healthy fish. In addition, Harikrishnan et al.17 studied the effects of plant extracts on the physiological responses of common carp (Cyprinus carpio), demonstrating that the addition of plant extracts to the diet increased TP content in the serum, enhancing the fish’s resistance to pathogenic bacterial infections. In this experiment, it was also observed that at the beginning of the experiment, the TP content in the negative control group was significantly lower than in the other four groups. After 7 days of feeding, the plasma TP content in the positive control group was significantly lower than in the negative control and emodin groups. However, compared to the positive control group, supplementing emodin in the diet significantly increased the plasma TP content in infected fish, restoring it to normal levels. Notably, supplementing 100 mg emodin per kg diet could rapidly restore the fish’s TP content to a level similar to that of the negative control group. This could be attributed to the severe liver damage caused by A. hydrophila, as the liver is the sole site for protein synthesis. Therefore, when liver function is severely impaired, and functional liver cells are reduced, protein synthesis is significantly decreased. Emodin has a certain reparative effect on the liver. Supplementing emodin in the diet can repair the damaged liver, increase the TP content in Wuchang bream infected with A. hydrophila, promote physiological metabolism, and enhance the organism’s immune response.
Plasma ALT and AST are essential aminotransferases widely present in animal mitochondria, playing a crucial role in the body’s protein metabolism. AST is primarily found in the liver and indicates the liver’s physiological status.18 When liver cells are damaged, ALT is released into the bloodstream. The relative increase in plasma ALT activity reflects disturbances in liver function and is considered the most specific and widely used indicator of liver damage.19,20 Huang et al.16 suggested that pathogenic bacterial infections could lead to an increase in serum TP content in Chinese shrimp (Fenneropenaeus chinensis). Nakano et al.21 studied the physiological responses of rainbow trout (Oncorhynchus mykiss) under oxidative stress, revealing that the addition of astaxanthin to the diet reduced serum AST and ALT activities in rainbow trout under oxidative stress. Additionally, Rao et al.22 investigated the serum physiological responses of rohu (Labeo rohita) and demonstrated that 0.01-0.05% turmeric significantly reduced AST and ALT activities. In this experiment, at the early stage of infection, the plasma AST and ALT activities in the positive control group and the 30, 100, 150 mg/kg emodin groups were significantly higher than those in the negative control group. However, with the extension of feeding time, all three emodin groups were able to reduce the plasma AST and ALT activities in Wuchang bream infected with A. hydrophila. Nevertheless, compared to the positive control group, the reduction in plasma AST and ALT activities in the 100 mg/kg group was significantly greater than that in the 30 and 150 mg/kg groups. This indicates that emodin to a certain extent repaired liver damage, consistent with the conclusions drawn from the total protein content.
Pathogens serve as crucial ecological stressors, and in the event of a pathogenic infection, the hypothalamus-pituitary-adrenal (HPA) axis in fish is subjected to continuous stimulation. This leads to substantial secretion of corticotropin-releasing hormone (CRH) by the hypothalamus, triggering the pituitary gland to release a significant amount of adrenocorticotropic hormone (ACTH). ACTH, in turn, induces an increase in the secretion of cortisol (COR) in the organism.8,23,24 COR is a growth-inhibiting steroid. Prolonged elevated levels of COR can be detrimental to the animal body, promoting protein breakdown and reducing fat storage, thereby causing changes in energy metabolism, overall metabolism, and other key physiological functions. Severe effects may include reduced quantity of lymphocytes and white blood cells in fish.25 Literature indicates that COR has inhibitory effects on the resistance of rainbow trout and can also suppress the immune response in Atlantic salmon (Salmo salar), reducing phagocytosis and lymphocyte mitosis while diminishing antibody production and IgM synthesis. Additionally, research on coho salmon (Oncorhynchus kisutch) has demonstrated COR’s ability to inhibit the proliferation of immune cells such as T and B lymphocytes.26 In this study, A. hydrophila infection increased cortisol levels in Wuchang bream. However, feeding with emodin was found to suppress the elevation of cortisol levels in Wuchang bream, thereby, to some extent, preserving the organism’s immune function. The optimal supplementation was observed at 100 mg emodin per kg of diet.
The hypothalamus-pituitary-thyroid axis in fish plays a crucial role in the regulation of the neuroendocrine system 27. When fish experience bacterial infection stress, the neuroendocrine axis is stimulated, leading to the hypothalamus releasing a significant amount of thyrotropin-releasing hormone (TRH), which acts on the pituitary gland. This, in turn, results in an increased secretion of thyroid-stimulating hormone (TSH), promoting the synthesis and secretion of thyroid hormones (T3 and T4). T4, when bound to target cells in the body, reacts to regulate the metabolism of the three major nutrients. In this experiment, infection by Aeromonas hydrophila significantly elevated plasma T3 and T4 levels in Wuchang bream, causing a partial manifestation of hyperthyroidism. However, feeding with emodin quickly alleviated thyroid hyperactivity. Contrastingly, research indicated that starvation stress can lead to a decrease in serum T3 and T4 in rainbow trout, accompanied by a reduction in the liver’s 5’-monodeiodinase activity28 and a decrease in T3 nuclear receptor quantity. This is opposite to the trend observed in T3 and T4 levels in this experiment. It is likely due to the crucial role of protein in influencing thyroid hormone production. Severe starvation results in insufficient protein intake by the organism, thereby reducing thyroid hormone synthesis and impacting the metabolism of the three major nutrients in the body.
Alkaline phosphatase (AKP) is a crucial metabolic regulatory enzyme in organisms, directly involved in calcium-phosphorus metabolism and the transfer of phosphate groups. It plays a role in immunoregulation. Additionally, AKP can enhance the recognition and phagocytic abilities of invaded organisms against pathogens by altering the surface structure of the pathogen, contributing to the overall immune defense of fish. In this experiment, infection with A. hydrophila resulted in a rapid increase in AKP activity in Wuchang bream. However, supplementing 30, 100, and 150 mg of emodin per kg of diet in subsequent feedings partially reduced AKP activity. In the late stage of infection, the AKP activity in the positive control group was lower than that in the negative control group. This may be attributed to the rapid elevation of AKP activity in fish during acute stress, such as bacterial infection, while prolonged infection can lead to compromised immune function and reduced overall metabolism. The specific mechanisms involved require further investigation.
Fish in the natural aquatic ecosystems often face the invasion of pathogenic bacteria. When the concentration of pathogenic bacteria in the surrounding water exceeds a certain level, it can lead to infections in fish, affecting normal metabolism and causing a stimulus that rapidly increases cortisol (COR) levels in the serum. The secretion of hormones like COR enhances glycogen breakdown and gluconeogenesis in the liver. To meet the energy demands of this process, blood glucose levels quickly rise.29 Therefore, many experts consider Glu as an indicator of the organism’s stress response. TG is primarily used for energy storage,30 and the level of COR is positively correlated with the body’s fat metabolism. In this experiment in Wuchang bream, A. hydrophila infection led to a rapid increase in Glu content. The elevated plasma COR and thyroid hormone levels observed in this experiment, under the stress of A. hydrophila injection, further promote gluconeogenesis in the liver, resulting in increased Glu levels. The experiment also indicated a decrease in serum TG levels under A. hydrophila infection. However, compared to the positive control group, feeding emodin to diseased fish significantly reduced Glu levels only after 4 days of feeding. This may be because, in the early stages of bacterial infection, glycogen is rapidly converted to glucose in the organism, leading to elevated blood glucose concentrations. On the other hand, emodin repairs the fish liver cells, increasing hepatic gluconeogenesis,31 and accelerating glycogen breakdown. The specific mechanisms involved still require further research.
Oxygen is an essential component for aerobic organisms in oxidative metabolic processes, providing the fundamental energy source for aerobic life forms. In normal physiological metabolism, oxygen is consumed to generate various metabolic byproducts. These byproducts, along with peroxide molecules, collectively referred to as reactive oxygen species (ROS), are continuously produced and cleared in a balanced manner in the body under normal conditions. The organism maintains these reactive substances at low, beneficial, and non-harmful levels, thereby achieving a metabolic equilibrium in its oxidation and antioxidation systems.32 However, in aquaculture, fish often face challenges such as high temperatures, crowding stress, poor water quality, and the impact of external pathogens and viruses. These unfavorable environmental factors can disrupt the balance between the fish and the aquatic environment. Additionally, the environment contains numerous stressors, and fish to varying degrees, experience stimulation from stressors that induce the production of large quantities of ROS. These substances can stimulate alterations in the organism’s antioxidative defense systems, leading to oxidative stress and causing oxidative damage to macromolecular cells.25
Within a certain range, organisms can alter the production of corresponding proteins by regulating the expression levels of relevant genes. Enzymes, being primarily composed of proteins, fall under this regulatory mechanism. Antioxidant enzymes in fish are predominantly found in the liver. SOD is currently the only enzyme discovered that utilizes superoxide anions (O2-) as substrates, catalyzing their conversion into hydrogen peroxide (H2O2) and oxygen (O2). Among the three metal enzymes, Cu/Zn-SOD is particularly crucial in defending the organism against oxidative damage, exhibiting the highest expression level. CAT acts as a hydrogen peroxide scavenger, catalyzing the breakdown of hydrogen peroxide into water and oxygen molecules, preventing lipid peroxidation of cell membranes. GPx is a widely distributed enzyme in organisms that catalyzes the breakdown of peroxides, generating glutathione disulfide (GSSG) from glutathione (GSH). Simultaneously, it promotes the decomposition of hydrogen peroxide, reducing toxic peroxides to non-toxic hydroxyl compounds, thereby protecting cell membranes and functions from damage. It serves as a critical indicator of an organism’s antioxidant capacity, reflecting its strength or weakness in resisting oxidative stress. Similar to the activities of these three enzymes, there is a corresponding connection between their gene expression levels. Therefore, studying gene expression of Cu/Zn-SOD, CAT, and GPx in representative tissues provides insight into the organism’s antioxidant capabilities.
In this experiment, gene expressions of Cu/Zn-SOD, CAT, and GPx exhibited a consistent trend. In the early stages of A. hydrophila infection, the gene expression levels of Cu/Zn-SOD, CAT, and GPx were significantly lower than those in the negative control group. However, with an increase in the feeding time, the gene expression levels of Cu/Zn-SOD, CAT, and GPx in the groups supplemented with 30, 100, and 150 mg emodin per kg diet gradually recovered until reaching normal levels. In contrast, the gene expression levels of these enzymes in the positive control group remained lower than those in the negative control group. This indicated that dietary supplementation of emodin can partially repair liver damage and restore hepatic antioxidant capacity. This inference aligns with earlier indicators such as total protein and cortisol levels. This may be attributed to the synergistic action of SOD, CAT, and GPx. Under normal physiological conditions, there is a certain level of reactive ROS in the organism. H2O2 can reduce Cu2+ in SOD, rendering it inactive. CAT and GPx eliminate H2O2, providing a protective effect on SOD. Additionally, O2- can deactivate CAT and GPx, while SOD can eliminate O2-. In the context of bacterial infection, liver damage occurs, leading to a reduction in the secretion of SOD, CAT, GPx, and other enzymes, exposing the organism to lipid peroxidation damage. Therefore, these three enzymes collectively form the organism’s antioxidant defense system.
Conclusion
In conclusion, supplementing 100 emodin mg kg-1 can impact the physiological metabolism of Wuchang bream by influencing plasma AST and ALT activities, hormones such as COR, T3, and T4, as well as Glu levels. This supplementation facilitates a quicker recovery of the physiological responses in infected Wuchang bream to normal levels. Moreover, the 100 emodin mg kg-1 can maintain the dynamic equilibrium between oxidation and antioxidation via regulating the expression of antioxidant genes in the liver.
Acknowledgment
This research was funded by the Natural Science Foundation of Shandong Province (ZR2022MC003), the Modern Agriculture Industrial Technology System of MOF and MARA (CARS-45) and Shandong Province modern agricultural industrial technology system (SDAIT-13-05). The authors gratefully acknowledge the scientific research personnel of the Fish Nutrition and Processing Department, the Freshwater Fisheries Research Institute of Shandong Province, Jinan City, China, for their assistance during the sampling period. We are also grateful to the management and workers of the fish farm of the Freshwater Fisheries Research Institute of Shandong Province for all their assistance during the entire trial period.
Authors’ Contribution
Conceptualization: Yuanyuan Zhang (Equal), Wen-wen Huang (Equal). Investigation: Yuanyuan Zhang (Equal), Hong Lu (Equal), Han Ke (Equal), Hui-zhong Cheng (Equal), Li-ping Song (Equal). Formal Analysis: Yuanyuan Zhang (Equal), Han Ke (Equal), Hong-yan Tian (Equal). Writing – original draft: Yuanyuan Zhang (Lead). Writing – review & editing: Yong-an Zhu (Equal), Wen-wen Huang (Equal).
Competing of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Conduct Approval
All procedures were carried out according to the Institutional Animal Care and Use Committee Guide in Freshwater Fisheries Research Institute of Shandong Province.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All data generated or used during the study appear in the submitted article.