Introduction
Despite the significant economic importance of many decapod crustaceans, overcoming reproductive barriers in artificial breeding remains a challenging issue for numerous species.1 The key premise for the development of crustacean ovary is the synthesis and accumulation of yolk, which is a process of consuming nutrition and energy, although the potential mechanism of the maturation of crustacean ovary is not clear, there is evidence that this process is affected by the combination of environmental factors and the endocrine regulatory system of crustacean itself.2,3 As an important environmental condition, temperature has a significant impact on a series of physiological activities of crustaceans. Taylor4 found that temperature variations significantly affect the respiratory rate, acid-base balance, and oxygen delivery by hemolymph in crustaceans. With the increase in temperature, the molting interval of Acanthomysis robusta is significantly shortened, the growth rate is significantly increased, and the time to reach sexual maturity is considerably advanced.5
Shrimps belong to animals with variable temperatures, and their metabolic rate change under the influence of ambient temperature. The research of Lin et al6 shows that temperature directly affects the metabolism rate of Litopenaeus vannamei, within the appropriate temperature range, the higher the temperature, the faster the metabolism rate and growth rate of the shrimp. When the temperature exceeds the optimal range, metabolic intensity surpasses normal levels, leading to energy being diverted to stress responses. This reduces the energy available for growth and hinders gonadal development. When the temperature falls below the optimal range, shrimp experience reduced digestive enzyme activity and decreased metabolic intensity. This inadequate energy supply hampers shrimp growth and slows gonadal development. Generally, shrimps have a relatively complete reproductive regulation system, which is regulated by neuropeptides, hormones, neurotransmitters and other hormones. These hormone levels are adjusted with temperature changes.7 Temperature also influences the activity of digestive enzymes in shrimp. At typical culture temperatures below 30℃, enzyme activity decreases as the temperature drops. This reduction impairs the shrimp’s ability to digest and absorb nutrients, subsequently affecting gonadal development.8
Exopalaemon carinicauda, belongs to Decapoda, Palaemonidae and Palaemon, is mainly distributed in the coastal waters of China and the western part of the Korean Peninsula. It is a very important economic shrimp in the coastal areas of China. Its annual output can reach more than 50000 tons and the output value exceeds 500 million US dollars in China.9 It has the characteristics of strong reproductive capacity, but its parent shrimp ovary development is not synchronous, which makes it difficult to carry out large-scale artificial breeding. In practical production, selecting mature female shrimp often requires substantial human and material resources, posing challenges to the sustainable development of E. carinicauda commercial culture.10 At present, the large-scale seedling cultivation technology of E. carinicauda has not been effectively established. E. carinicauda is relatively easy to cultivate due to its strong adaptability to temperature and salinity. However, the impact of environmental factors on its growth and metabolism is often overlooked, hindering in-depth research on its physiology and biochemistry and limiting improvements in breeding efficiency, both in theoretical research and practical production. Therefore, this study intends to explore the effect of temperature on the growth and gonadal development of E. carinicauda by configuring different temperature gradients, to provide a reference for determining the appropriate temperature conditions for the artificial culture of E. carinicauda and the ripening of parent shrimp.
Materials and Methods
Experimental animals
The E. carinicauda used in the experiment was obtained from the offspring of the overwintering parent shrimp breeding population in the Key Laboratory of Marine Biotechnology, Jiangsu Ocean University. The breeding population was raised in the recirculating mariculture system, 20 female egg-carrying shrimp were randomly selected, and each egg-carrying shrimp was separately and stocked in a plastic water tank (38 × 26 × 20 cm) for incubation. During incubation, water temperature was kept at 25℃ and salinity at 24 ppt. After hatching, the female shrimp were removed to prevent the female shrimp from preying on the larvae. During the breeding of the larvae, artificially cultured Nannochloropsis and Artemia salina nauplii were fed. After the size of larvae reached 1 cm, they were transferred to different circulating water culture tanks, the larvae in each culture tank were a family, the individual size reached 4.75 ± 0.26 cm after 3 months of cultivation.
Experimental design
In this experiment, five temperature gradients were set, which were 16℃, 20℃, 24℃, 28℃ and 32℃ respectively, and the water temperature was controlled with a special constant temperature heating rod for aquaculture. The seawater was prepared with seawater crystals and pure water, the seawater was used after continuous aeration for 24 hours. Each test group was equipped with 3 parallel tanks, and the specification of aquaculture tank was 55 cm (length) × 40 cm (width) × 30 cm (height). 30 shrimp were placed in each cage, and the test period was 30 days.
Daily management of breeding test
During the trial, keep the salinity was stable at 24 ppt, and the light cycle was 8L:16D, black shading cloth was used to control the light cycle, pH was 7.8-8.1, and continuously aerated to make the dissolved oxygen in the water body>5 mg/L. Formulated feed was fed twice a day (8:00, 17:00), and the feeding amount was 3% of the body weight of E. carinicauda. Plastic siphon was used to remove the excreta and any uneaten feed. Any dead shrimp was immediately removed. The residual feed was removed 1.5 hours after feeding, and the feeding amount was appropriately increased or decreased according to the feeding situation of E. carinicauda, and the water changed by 20% every day.
Sampling and determination of relevant indicators
During the experiment, the number of molting times was recorded according to the number of head and breastplate molted everyday. After the experiment, the survival mantissa and ovarian maturation mantissa of each group were counted, and 10 shrimp were randomly selected from each group. Sterile surgical blade and tweezers were used to dissect and take out the ovary and hepatopancreas, weighed the body mass, ovary mass, and hepatopancreas mass, and then quickly frozen them in liquid nitrogen. After the sample was thawed, it still maintained the temperature of 2-8℃, 9 times the volume of PBS (pH7.4) was added according to the weight to volume ratio of 1g:9ml, and centrifuged for 20 min at 2500 r/min after full homogenization under 4℃, collected the supernatant and store it for testing. A micro-sampler was employed to puncture the pericardial cavity of E. carinicauda, and hemolymph was extracted, the volume ratio of anticoagulant (Sodium citrate 50 mmol/L, EDTA-2NA 10 mmol/L, pH 7.3) and hemolymph is 1:1. After mixing, centrifuged at 4℃, 3500 r/min for 10 min to remove the blood cell precipitation, extracted the supernatant and store it in the environment of 4℃ for determination within 24 hours.
The concentrations of total protein (TP), total cholesterol (T-CHO), triglyceride (TG) and estradiol (E2) in the ovary were determined using the kit of Nanjing Jiancheng Bioengineering Institute and according to the instructions. The concentration of vitellinogen (Vg) in hepatopancreas, the activity of lipase (LPS) and trypsin (Trypsin) and the concentration of gonadotropin-releasing hormone (GnRH) in hemolymph were determined. The concentration of TP was determined by Coomassie brilliant blue method. The concentration of T-CHO was determined by COD-PAP method. The concentration of TG was determined by GPO-PAP enzyme method. The concentration of E2, GnRH and Vg was determined by enzyme-linked immunocompetitive method. Enzyme-linked immunocompetitive method can be used for the quantitative determination of antigen and Hapten, and also for the determination of antibodies. Taking the determination of antigens as an example, specific antibodies were adsorbed on a solid phase carrier, and added the antigen to be tested and a certain amount of known enzyme labeled antigen. They compete to bind with solid phase antibody, after washing and separation, the enzyme-labeled antigen that binds to the solid phase was negatively correlated with the content of the tested antigen.11,12
Gender identification and ovarian development staging of shrimp
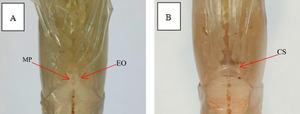
E. carinicauda is dioecious. In male shrimp, there is a pointed small protrusion in the center of the foot base in the fifth step, which is called the male protrusion. In female shrimp, the distance between the feet in the fourth and fifth steps is relatively wide, smooth, and flat, without an open seminal receptacle. The morphological differences between male and female shrimp are shown in Figure 1.
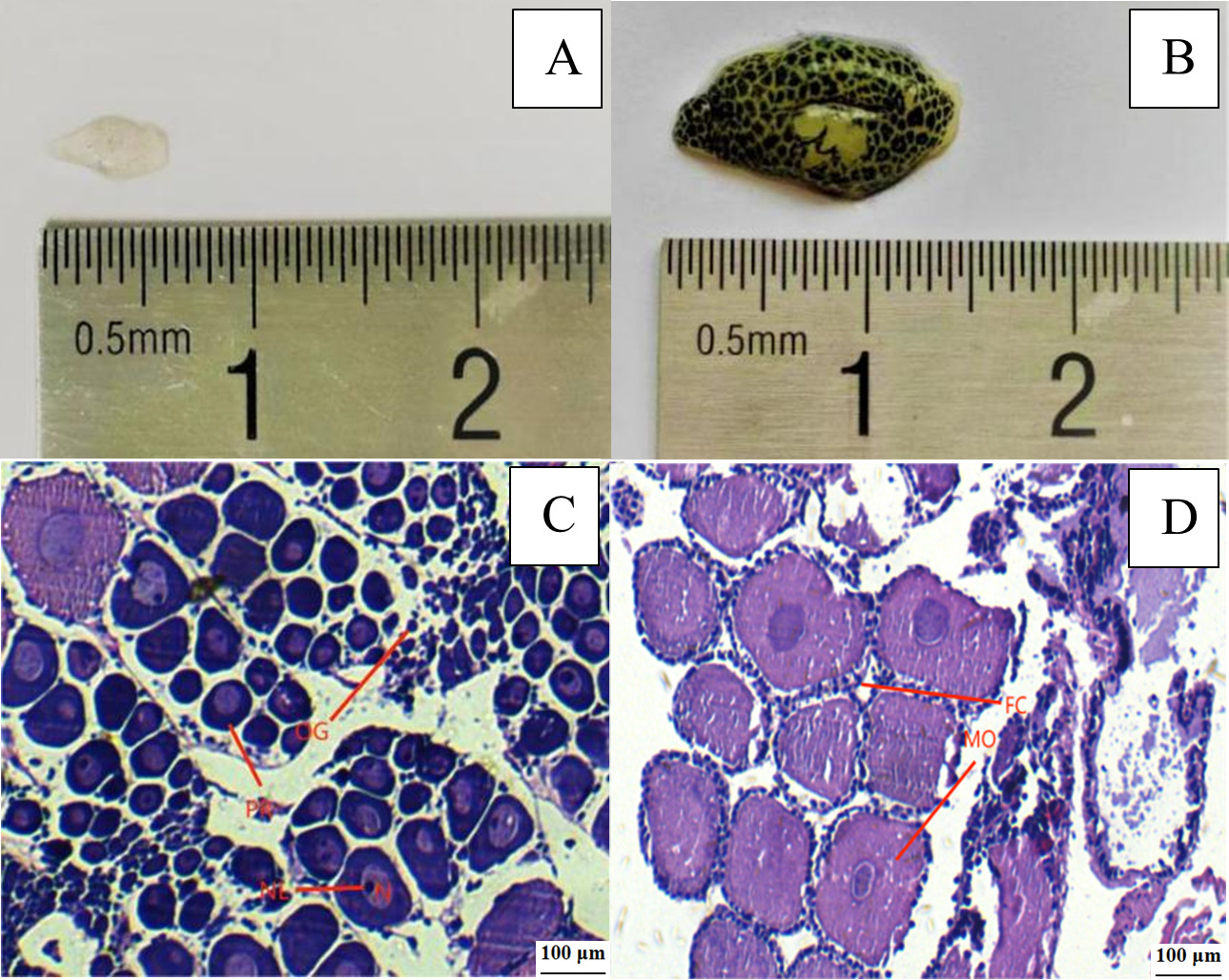
According to Wang’s classification standard 13, the ovary development of E. carinicauda can be divided into five stages, i. e. stage I, II, III, IV and V. In stage I, the ovaries were completely transparent and located under the heart, visible after dissection. In stage II, the ovaries were translucent and visible through the exoskeleton. In stage III, the ovaries were gray, and ovarian membranes were covered with small black dots. In stage IV, the ovaries were yellow. Uncovered the transparent head breastplate of the shrimp, the oocytes were wrapped in the ovarian membranes which were covered with dark green or brown dots. In stage V, the mature stage, the ovaries were bright yellow, and the ovarian membranes were covered with dark green or tan spots. The undeveloped (stage I) and mature ovaries (stage V) are shown in Figure 2. In stage I of ovarian development, many Oogonium were distributed in the center of the ovary. The periphery of Oogonium is the oocyte in the prophase of yolk synthesis. In the stage V of ovarian development (mature stage), section observation shows that mature oocytes were mainly present in the ovaries, with follicular cells distributed around them.
Data Analysis
Survival rate (SR), specific growth rate (SGR), molting frequency rate (MF), gonadosomatic index (GSI) and ovarian maturation rate were calculated according to the following formula.
SR (%)=MN×100SGR (%/d)=(lnWt−lnW0)t×100MF (times/tail/d)=MmNs×t×100GSI (%)=WLW×100ovarian maturation rate (%)=NfN×100
In the formula, M is the survival number (tail) of E. carinicauda in each group at the end of the test, and N is the number (tail) of E. carinicauda in each group at the beginning of the test; Wt is the average body mass at the end of the test (g), W0 is the average body mass at the beginning of the test (g); Mm is the number of molting shrimp (times) during the test, and Ns is the number of molting shrimp (tail); T is the test period (d); WL is the ovarian mass (g), and W is the body mass (g) of E. carinicauda; Nf is the mantissa (tail) of ovarian development and maturity at the end of the test, and N is the mantissa (tail) released at the beginning of the test.
SPSS 23.0 was used for one-way ANOVA analysis of the test data, the impact of possible covariates on the analysis results was excluded through covariance analysis, SNK test was used to analyze the significance of the differences between groups, P<0.05 indicated that the differences were significant, all data were expressed in mean ± standard deviation (Mean ± SD), and the chart was drawn using Origin 2017.
Results
Effects of different temperatures on survival, growth and molting of E. carinicauda
The effects of different temperatures on the survival, growth and molting of E. carinicauda are shown in Table 1. The survival rate of E. carinicauda reached the highest value of 84.44 ± 11.71% under environmental conditions with a temperature of 20℃. Between 20℃ and 32℃, the survival rate of E. carinicauda gradually decreased with the increase of temperature, and decreased to the lowest value at a temperature of 32℃, which was 61.11 ± 5.09%.
Within the experimental temperature range, the final body weight and specific growth rate of E. carinicauda showed a trend of first increasing and then decreasing with the increase of temperature (P<0.05). It had the worst growth performance at 16℃, with the final body weight and specific growth rate being 1.97 ± 0.10 g and 1.10 ± 0.13 %·d-1, respectively, and then increased with the increase of temperature. With the fastest growth rate at 28℃, the final body weight and specific growth rate reached 2.31 ± 0.06 g and 1.58 ± 0.10 %·d-1, respectively, which were significantly higher than those in the temperature 16℃ group (P<0.05). Under the temperature of 32℃, the growth performance of E. carinicauda decreased slightly, and the final body weight and specific growth rate were slightly lower than that of the temperature 28℃, with no significant difference (P>0.05).
The changing trend of the molting rate of E. carinicauda under different temperature conditions was consistent with the final body weight and specific growth rate. As the temperature increases, the molting rate first increases and then decreases (P<0.05). The lowest molting rate was 3.81 ± 0.36 times·(tail·d)-1 at 16℃, and the highest value was 4.74 ± 0.56 times·(tail·d)-1 at 28℃, there was no significant difference between the groups (P>0.05).
Effect of different temperatures on digestive enzyme activity of E. carinicauda
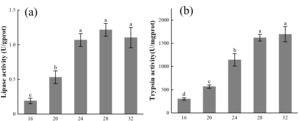
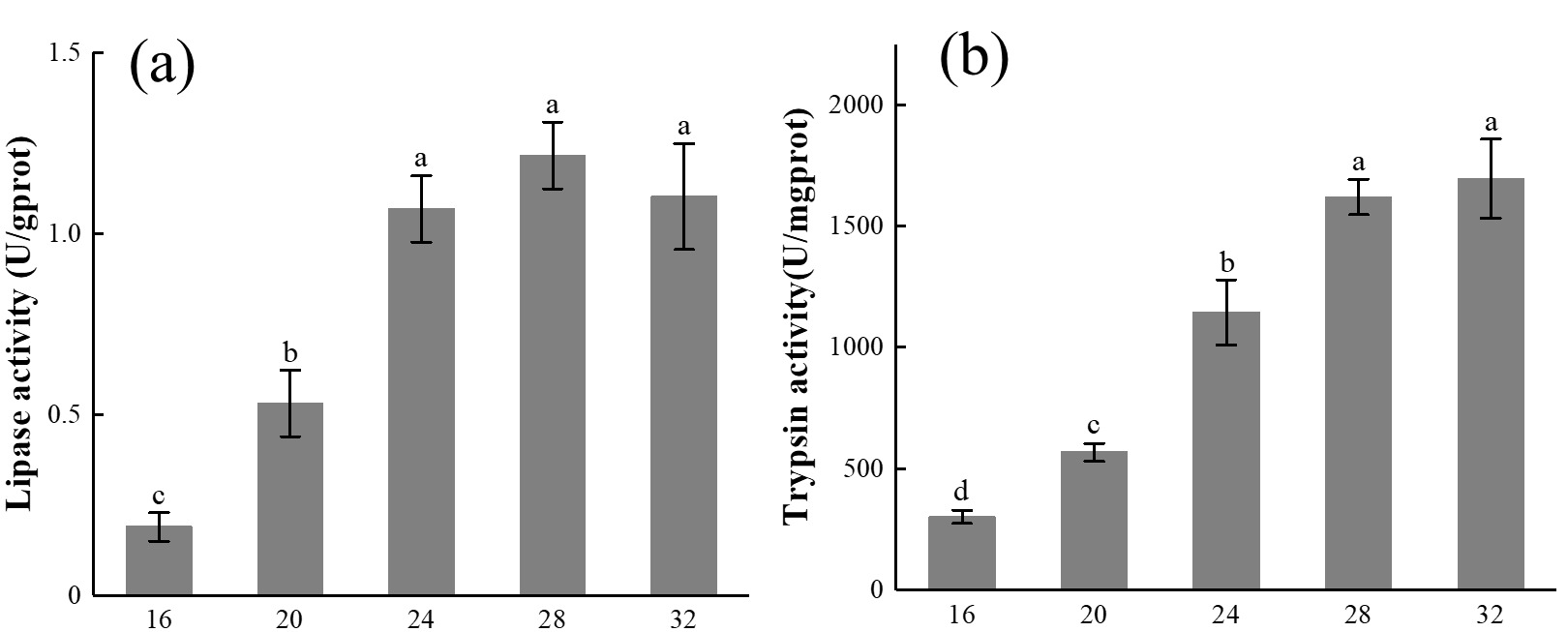
The effect of different temperatures on the lipase activity of E. carinicauda is shown in Figure 3-a. It can be seen from Figure 3-a that the lipase activity of E. carinicauda increased first and then decreased with the increase of temperature in the test temperature range (P<0.05). At 16℃, the lipase activity was the lowest 0.19 ± 0.04 U/gprot, which was significantly lower than the other four groups (P<0.05). The lipase activity reached the highest value 1.22 ± 0.09 U/gprot at 28℃, and decreased slightly at 32℃, there was no significant difference in lipase activity between 24℃, 28℃ and 32℃ groups (P>0.05).
The effect of different temperatures on the trypsin activity of E. carinicauda is shown in Figure 3-b. It can be seen from Figure 3-b that the trypsin activity increases with the increase of temperature in the test temperature range, the lowest value 300.90 ± 27.27 U/mgprot is at 16℃, and the highest value 1695.45 ± 163.42 U/mgprot is at 32℃.
Figure 3 Digestive enzyme activity of E. carinicauda at different temperatures. 30 shrimp were raised at 16℃, 20℃, 24℃, 28℃, and 32℃, determination of digestive enzyme activity after 30 days. The data is shown as Mean ± SD, different superscripts indicate significant difference between groups (P<0.05).
Effects of different temperatures on the ovarian development performance of E. carinicauda
The effect of different temperatures on gonadosomatic index of E. carinicauda is shown in Figure 4-a. It can be seen from Figure 4-a that the gonadosomatic index of E. carinicauda is lowest at 16℃, which is 4.36 ± 0.69%, and then, with the temperature rising, the gonadosomatic index gradually increases, reaching the maximum value 7.56 ± 0.46% at 32℃, which is significantly higher than the temperature 16℃, 20℃ and 24℃ groups (P<0.05), and the gonadosomatic index of the temperature 28℃ group is slightly lower than the temperature 32℃.
The effect of different temperatures on the ovarian maturation rate of E. carinicauda is shown in Figure 4-b. It can be seen from Figure 4-b that the ovarian maturation rate of E. carinicauda continuously increases with the increase of temperature within the test temperature range. Among them, under the temperature of 16℃, the ovarian maturation rate was the lowest, 46.67 ± 5.77%, and rose to the highest level 63.33 ± 3.33% under the temperature of 32℃.
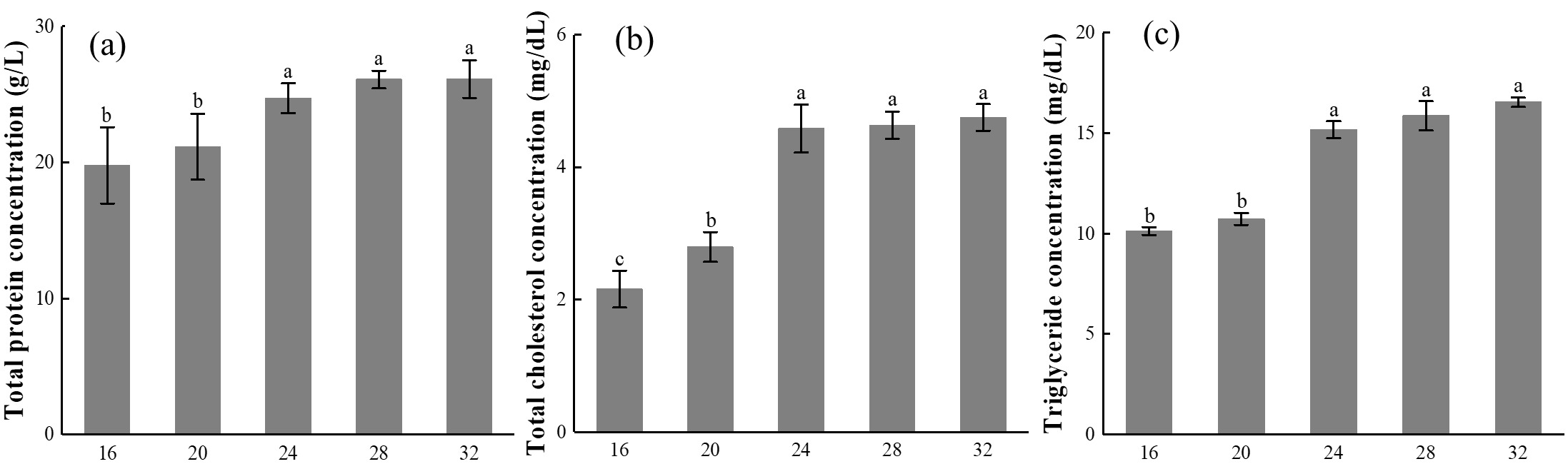
Effect of different temperatures on the concentration of main nutrients in the ovary of E. carinicauda
The effect of different temperatures on the concentration of TP in the ovary of E. carinicauda is shown in Figure 5-a. It can be seen from Figure 5-a that within the test temperature range, the TP concentration in the ovary of E. carinicauda increases with the increase in temperature, reaching the highest value 26.13 ± 1.39 g/L at 32℃.
The effect of different temperatures on the concentration of T-CHO in the ovary of E. carinicauda is shown in Figure 5-b. Under the conditions of 16℃ and 20℃, the concentration of T-CHO in the ovaries of E. carinicauda was relatively low, being 2.16 ± 0.28 mg/dL and 2.79 ± 0.23 mg/dL, respectively, when the temperature reached 24℃, the concentration of T-CHO suddenly increased to 4.58 ± 0.34 mg/dL, significantly higher than that of the temperature 16℃ and 20℃ groups (P<0.05), the concentration of T-CHO in the temperature 24℃, 28℃, and 32℃ groups was at the same level, with no significant difference (P>0.05).
The effect of different temperatures on the concentration of TG in the ovary of E. carinicauda is shown in Figure 5-c. The variation trend of TG concentration in the ovary of E. carinicauda was consistent with that of T-CHO concentration. Among them, the TG concentration in the temperature 32℃ group was the highest, reaching 16.53 ± 0.23 mg/dL, significantly higher than that in the temperature 16℃ and 20℃ groups (P<0.05).
Effects of different temperatures on the concentrations of vitellogenin and sex hormones in E. carinicauda
The effect of different temperatures on the concentration of Vg in E. carinicauda is shown in Figure 6-a. Within the experimental temperature range, the Vg in the hepatopancreatic tissue of E. carinicauda presents a trend of first increasing, then decreasing, and then increasing with the increase in temperature (P<0.05). The concentration of Vg in the 20℃ group was the highest, at 2.67 ± 0.14 μg/mL, temperature 28℃ group had the lowest concentration, which is 1.94 ± 0.21 μg/mL, significantly lower than the temperature of 16℃ and 20℃ groups (P<0.05).
The effect of different temperatures on the concentration of GnRH in E. carinicauda is shown in Figure 6-b. At temperatures of 24℃ and 28℃, the concentration of GnRH in the hemolymph of E. carinicauda was relatively high and relatively close, being 81.82 ± 4.03 ng/L and 82.67 ± 3.22 ng/L, respectively, the difference between the two groups was not significant (P>0.05), but both were significantly higher than the temperature of 16℃, 20℃, and 32℃ (P<0.05).
The effect of different temperatures on the concentration of E2 in E. carinicauda is shown in Figure 6-c. There was no significant difference in the concentration of E2 in the ovarian tissue of E. carinicauda, among the temperature 20℃, 24℃, and 28℃ groups (P>0.05), the highest concentration of E2 in the temperature 28℃ group was 26.62 ± 1.49 ng/L, which was significantly higher than that in the temperature 16℃ and 32℃ groups (P<0.05).
Discussion
Effects of different temperatures on the survival and growth of crustaceans
In general, the population size and survival rate of crustaceans peak at temperatures near their natural habitats, which is the result of natural selection. However, the survival of artificially cultured crustaceans is influenced by the species’ own tolerance to temperature and the temperature of previous domestication.14,15 Environmental fluctuations associated with seasonal climate change are important for triggering physiological and behavioral adjustments in aquatic organisms. Temperature is considered to be one of the most important environmental factors affecting organisms, the biological effects of these factors are complex and widespread,16–18 and have significant effects on many physiological processes of crustaceans. For example, the oxygen consumption rate, food intake, and growth rate of Paralithodes camtschaticus increase with temperature, but the mortality rate significantly increases under high-temperature conditions.19 Under high-temperature conditions, the oxygen consumption rate of adult Cyclorapsus punctatus increases rapidly, while the oxygen consumption rate of juvenile crabs shows a slow upward trend.20 The survival rate and growth rate of Macrobrachium amazonicum at 28℃ were significantly higher than those of the temperature 30 and 32℃ groups.21 It can be seen that different species and developmental stages can lead to differences in their appropriate temperature environment.
The results of this study show that the survival rate of E. carinicauda is higher at temperatures ranging from 16 to 28℃, and significantly decreases at temperatures ranging from 32℃. This indicates that 16-28℃ is the suitable survival temperature for E. carinicauda, and high temperature is not conducive to the survival of E. carinicauda, which is similar to the previous research results. This study shows that the molting rate and specific growth rate of E. carinicauda gradually increase with the increase of temperature in the temperature range of 16 to 28℃, reaching the highest value at 28℃. When the temperature is 32℃, the molting rate and specific growth rate decrease, which may be due to the abnormal metabolic rate caused by excessive temperature, which consumes energy to resist temperature stress and leads to growth inhibition. At the same time, we have also observed that under high temperature conditions, white shrimp with ridged tail may have difficulty molting, or even fail to molt, leading to death. Based on the above analysis, we believe that 28℃ is the suitable temperature for the survival and growth of E. carinicauda.
Effects of different temperatures on the gonadal development of crustaceans
Sea water temperature has a significant impact on the growth and reproductive patterns of variable temperature on marine animals.22,23 The relationship between ovarian development and temperature provides important information for the life history of marine animals. Studies have shown that biological individuals in higher-temperature environments achieve sexual maturity with smaller body sizes than individuals in lower-temperature environments.23,24 This phenomenon is known as the temperature size rule.25 From the perspective of biological adaptability, this phenomenon is explained as a compensatory mechanism for reproductive difficulties at low temperatures,26 as larger females typically have strong reproductive capacity. Higher temperatures can promote the early development and maturation of the ovaries of marine organisms, while low temperatures can delay the development of the ovaries of marine organisms, the ovaries mature only when they are large.
The results of this study show that in the temperature range of 16 to 32℃, the gonadal index and ovarian maturation rate of E. carinicauda increase with the increase in temperature, but the difference between the 28℃ group and the 32℃ group is not significant, the concentration of TP, T-CHO, and TG in the ovaries also have the same trend, indicating that higher temperature conditions can promote the accumulation of nutrients in the ovaries of E. carinicauda, leading to development and maturation, similar to the above research viewpoint. There is also an opinion that the ovarian development speed of crustaceans depends on the accumulation of temperature, that is, effective accumulated temperature, within a certain period, the higher the temperature, the more effective accumulated temperature, and the faster the ovarian development speed of crustaceans.27,28 Digestive enzyme activity reflects the ability of organisms to absorb and assimilate nutrients, which plays an important role in the sexual maturation process that requires the accumulation of nutrients.29 Protein and lipids as the most important nutritional reserves support the development of crustacean ovaries.30,31 The results showed that the activities of lipase and trypsin in E. carinicauda were higher at temperatures of 28 and 32℃, significantly higher than those in other groups, indicating that 28 to 32℃ was conducive to the digestion of food and the absorption of nutrients by E. carinicauda. In shrimp, Vg is commonly synthesized by the ovaries and hepatopancreas, which undergo endogenous gene expression, while Vg synthesized by the hepatopancreas is transported to the ovaries through hemolymph.32,33 In this study, under higher temperature conditions, the concentration of Vg in the hepatopancreas of E. carinicauda was relatively low, it is speculated that higher temperature conditions greatly promoted the physiological process of transporting Vg from the hemolymph to the ovary. Sexual hormones play an important role in the molting, growth, and reproduction of crustaceans.34–36 Estradiol is the main estrogen in the female body. Mainly secreted by the ovaries periodically, it is closely related to the development and maintenance of the female reproductive system, the development and maturation of follicles, and the ovulation process.37 Gonadotropin-releasing hormone is a peptide hormone secreted by nerve cells, which can strictly control the central nervous system and regulate the physiological secretion of males and females.38The results of this study indicate that the concentration of estradiol in the ovary and the concentration of gonadotropin-releasing hormone in the hemolymph of E. carinicauda increase with the increase of temperature in the range of 16 to 28℃. Therefore, we speculate that 28℃ is the most conducive to the synthesis and secretion of sexual hormones in E. carinicauda, which is beneficial to its ovarian development.
Conclusion
Based on growth status, digestive enzyme activity, ovarian development and nutrient accumulation, as well as sex hormone levels, and comprehensive evaluation of the above indicators, the physiological and biochemical status of E. carinicauda is optimal at a temperature of 28℃. Therefore, the most suitable water temperature for the growth and reproduction of E. carinicauda is 28℃.
Acknowledgments
Thanks to Shenzhen AlphaFeed Co., Ltd and Jiangsu Ocean University for their support.
This work was supported by the “JBGS” Project of Seed Industry Revitalization in Jiangsu Province (JBGS(2021)124), the Policy Guidance Program of Science and Technology Bureau of Lianyungang (GH2203), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22-3386).
Authors’ Contribution
Conceptualization: Zhenhui Zhao (Lead), Zhiyu Pang (Lead), Deng Deng (Supporting), Jianhong Li (Supporting), Ning Wang (Supporting), Huan Gao (Supporting), Binlun Yan (Supporting), Kangyu Deng (Supporting). Investigation: Zhenhui Zhao (Lead), Zhiyu Pang (Lead), Huan Gao (Supporting), Binlun Yan (Supporting). Writing – original draft: Zhenhui Zhao (Lead), Zhiyu Pang (Lead). Writing – review & editing: Zhenhui Zhao (Equal), Zhiyu Pang (Equal), Deng Deng (Equal), Jianhong Li (Equal), Ning Wang (Equal), Huan Gao (Equal), Binlun Yan (Equal), Kangyu Deng (Equal).
Competing of Interest – COPE
The authors of this manuscript declare no conflicts of interest.
Ethical Conduct Approval – IACUC
No ethical clearance was required for this study.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.