INTRODUCTION
Nanomaterials (NMs) with at least one dimension of 100 nm or less were classified into three types based on their dimensions, including zero-dimensional NMs (e.g., nanoparticles), 1-dimensional NMs (e.g., nanowires, nanorods, and nanotubes), and 2-dimensional NMs (e.g., nanolayers).1,2 NMs have gained tremendous attention owing to their beneficial applications in electronics, life science, environmental protection, energy, national defense, and the chemical industry.3 In recent years, with the improvement of production technology of NMs and the increased demand of the downstream industry, the market for NMs has shown an increasing trend in terms of global market value.4 However, the advancement of the NM industry has been found to be correlated with the occurrence of environmental pollution, consequently leading to potential risks to public health.
ZnO nanoparticles (ZnONPs), a type of zero-dimensional NMs, are typical versatile metal oxide nanoparticles with unique features, such as low cost and chemical stability. They are widely utilized in drug delivery, food packaging, pigments, fire retardants, and nutritional supplements.5 The dissolved Zn ions mostly contributed to the overall toxicity of ZnONPs.6 These particles release Zn ions into animal cells, which are eliminated from the body via gills or kidneys once they surpass the required levels for fish maintenance. Whereas the release of Zn ions from the gill and kidney is limited. Excessive accumulation of Zn ions or nano-form may cause damage to tissues.7
1-dimensional carbon nanostructured materials, commonly known as carbon nanotubes (CNTs), are among the most celebrated entities in the field of nanotechnology. They have been applied in diverse fields, such as semiconductor chips, fuel cells, and lasers, due to their extraordinary mechanical and electrical properties arising from their nanoscopic dimensions.3 Previous studies have shown that CNTs were associated with toxic effects, such as growth, development, reproduction, and immune response in several experimental animals.8,9 In the natural environment, organisms are likely exposed to mixtures of discharged pollutants, which may interact with each other and change the potential effects. The adverse effects of the mixtures have aroused great attention among researchers7,10,11. It has been reported that the destiny of other contaminants could be impacted by CNTs possessing extensive surface areas.12 Several studies have reported the effects of CNTs on the uptake, accumulation, tissue distribution, or toxicity of contaminants, such as phenanthrene, copper, benzo[a]pyrene, triphenyltin, and terbium.13–15 However, data on the effects of exposure mixtures of CNTs and other NMs are still limited. Several studies have investigated the combined effect of ZnONPs and carbon NMs, which mainly focused on the impacts on bioaccumulation and behavior. The ecotoxicity of the combination of ZnONPs and carbon NMs could be changed, which was observed in several research. For example, Shim et al. (2016) suggested that the photocatalytic degradation activity and the mechanical strength of ZnONPs could be significantly enhanced when ZnONPs and carbon materials were mixed.16 However, Sayadi et al. (2022) reported that multi-layer graphenes (MLGs) decreased the histopathological effects of the ZnONPs on both gills and intestinal tissues and fish behavioral effects.17 Ye et al. (2018) showed the same results with ZnONPs and graphene oxide nanoparticles (GONPs) mixture exposure to zebrafish.10 Thus, the effects of ZnONPs and carbon materials on fish remains unclear.
Histopathology could provide a reference for clinical diagnosis by observing the changes in the tissue structure and morphology. It is a strong tool for toxicity assessment.2 Common carp (Cyprinus carpio), one of the most crucial fish in central Asia, is a good laboratory model for toxicological research.18 To our knowledge, a holistic analysis of the ecotoxicological effects of ZnONPs in combination with CNTs has not been investigated in the liver tissue of fish. In the present study, we intend to explore the adverse effects of ZnONPs in combination with CNTs on the pathological changes and apoptosis in the liver of common carp after four weeks of exposure. This information will supply toxicological data for risk assessment and management strategies.
MATERIALS AND METHODS
FISH REARING CONDITION
Juveniles of common carp were obtained from a commercial fish farm (Henan province, China). The average length of the fish was 13.78 ± 1.52 cm and the mean weight was 23.3 ± 5 g. Fish were reared at 22 ± 2 ℃ under a 12 h light/12 h dark cycle for 14 days and fed a commercial basal diet at a rate of 2% biomass. The commercial basal diet contains moisture (≤12%), crude protein (≥30%), crude fiber (≤10%), crude fat (≥4%), and crude ash (≤12%). Mean values of water parameters were maintained including dissolved oxygen ≥80%, pH=7.3±0.15, nitrates <20 mg L-1, nitrites <0.1 mg L-1, ammonia <0.01 mg L-1, and total hardness=130.3 ± 8.1 mg L-1 CaCO3. To maintain the concentration of ZnONPs and MWCNTs, half of the exposure solutions were changed daily.
CHARACTERIZATION OF NMs AND DISPERSION PREPARATION
MWCNTs and ZnONPs were purchased from Nanjing XFNANO Materials Tech Co., Ltd (Jiangsu, China). The average diameter of ZnONPs (XF106) was 30-80 nm. The average diameter and length of MWCNTs (XFM 70) were 4-6 nm, and 0.5-2 μm respectively. Stock dispersion solution for ZnONPs dispersion at a concentration of 7.5 g L-1 was prepared in deionized H2O as described by Lee et al.4 ZnONPs were further characterized to reconfirm their shape and size by transmission electron microscopy (TEM) (FEI Talos F200S, FEI Co., USA).19 Measurement of the size of 150-200 nanoparticles was performed using nano-measure software 1.2.00. Preparation of MWCNTs was performed according to our previous study.19
EXPOSURE ASSAY
A blank control group, a ZnONPs group (50 mg L-1 ZnONPs single exposure), and two combined exposure groups including LSC-ZnONPs (50 mg L-1 ZnONPs and 0.25 mg L-1 MWCNTs) and HSC-ZnONPs group (50 mg L-1 ZnONPs and 2.5 mg L-1 MWCNTs) were set. Each group had three replicate tanks filled with 60 L water and contained 20 fish. Throughout the experimental period of four weeks, 50% of the total water amount in each tank was replaced daily. The fish were daily fed twice with a commercial basal diet at a rate of 2% biomass. The exposure concentrations of ZnONPs in this study were chosen based on the previous study which indicated that the 14d-LC50 value was 50 mg L-1 in zebrafish.4 The dose of MWCNTs was chosen based on our previous toxicity studies.19
HISTOLOGICAL ASSESSMENT
For analysis of the pathological changes, the livers from each group were surgically removed after four-weeks of exposure. The selected tissues were fixed by immersion in 10% paraformaldehyde for 24 h. Then, serial tissue sections (6 µm) were sliced from paraffin-embedded samples. Then, H&E staining was performed and photographed using the Pannoramic Scan (3DHISTECH Kft, Budapest, Hungary).
ULTRASTRUCTURAL OBSERVATIONS
For examining the ultrastructural changes, the liver samples were collected and washed with phosphate buffered saline. Then, the samples were pre-fixed with glutaraldehyde solution, fixed with OsO4, dehydrated with ethanol, cleared in acetone, embedded, sectioned, double stained with uranyl acetate and lead citrate, and then examined with TEM (Hitachi H-7650, Hitachi Co., Japan).
TERMINAL DEOXYNUCLEOTIDYL TRANSFERASE dUTP NICK END LABELING (TUNEL) ANALYSIS
For apoptosis analysis, TUNEL staining was performed following the manufacturer’s instructions (Roche, Mannheim, Germany). Briefly, paraffin sections were dewaxed in xylene, hydrated in continuous gradient alcohol, incubated with protease K, stained with TdT and dUTP mixture, and restained with DAPI. The whole slide digitalization was analyzed using the Pannoramic Scan (3DHISTECH Kft, Budapest, Hungary) and Indica labs HALO software. Apoptotic rate (AR) was calculated as follows: AR (%) = (number of positive staining cells/total number of cells) × 100.
qRT-PCR ANALYSIS
qRT-PCR was used to investigate apoptosis at the mRNA level. The mRNA level of caspase3, Bcl2-associated X (Bax), and X-box binding protein 1 (XBP1) gene were assessed using the StepOneTM Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The β-actin gene was used as the internal control. The specific primers are shown in Table 1. Each sample was run in triplicate for analysis, and relative mRNA expression levels were calculated according to the comparative Ct (2-△△Ct) method.20
DATA ANALYSIS
All data were analyzed using SPSS version 18.0 software. Differences of apoptotic rate and relative mRNA expression levels between groups were assessed using student’s t-test and one-way analysis of variance (ANOVA) method. The differences in results were considered statistically significant when p<0.05.
RESULTS
CHARACTERIZATION FINDINGS OF MWCNTs AND ZnONPs
The TEM image of ZnONPs indicated the monomodal distribution (Figure 1A). The total average diameter of ZnONPs used in the study was 56.89 nm (Figure 1B). ZnONPs were observed with a diameter range of 30-84 nm. MWCNTs were characterized as described in our previous study.19
HISTOPATHOLOGICAL AND ULTRASTRUCTURAL CHANGES
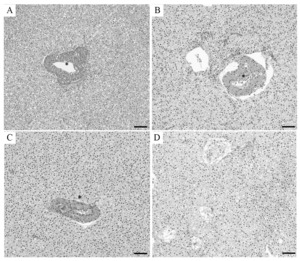
No death was found in all experimental groups. Figure 2A illustrates a normal hepatic portal vein area with intact endothelial and large interlobular vascular lumen. The hepatocytes were close to the hepatic interlobular vascular in the control groups (Figure 2A). In the ZnONPs group, endothelial denudation, eosinophilic deposits, and luminal stenosis were identified in the hepatic interlobular vascular. The portal vein was detached from the surrounding hepatocytes (Figure 2B). The same histological changes were observed in the liver of fish in the LSC-ZnONPs group. However, the fish showed a smaller gap between the hepatic portal vein and the surrounding hepatocytes compared with that in the ZnONPs group (Figure 2C). The hepatic interlobular vascular was severely damaged in the liver of the HSC-ZnONPs group (Figure 2D).
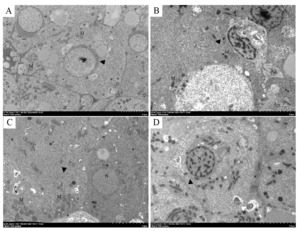
The ultrastructural changes in the liver tissue were also observed. In the control group, normal hepatocytes with parallel-arranged endoplasmic reticulum (ER) and ellipsoidal mitochondria were observed (Figure 3A). Compared with the control group, the exposure groups experienced notable alterations, which were demonstrated by the disruption of ER arrangement and the enlargement of ER cistern. Mitochondria crista dissolution and fracture were also present (Figures 3B, 3C, 3D). Among the exposure groups, fewer changes of nuclei were found in the LSC-ZnONPs group (Figures 3C). Moreover, autophagosomes containing the clusters of MWCNTs were identified in the HSC-ZnONPs group (Figures 3D).
APOPTOSIS OF HEPATOCYTES
TUNEL assay and qRT-PCR were used to determine the impact of exposure of ZnONPs individually, and ZnONPs and MWCNTs mixture on apoptosis. As shown in Fig 4A, no signal with green fluorescence was found in the liver of the control group, suggesting the absence of apoptosis. However, DAPI-TUNEL double-labeled nuclei were detected in groups exposed to ZnONPs individually, and ZnONPs and MWCNTs mixture. Additionally, the number of DAPI-TUNEL double-labeled nuclei increased significantly in exposure groups when compared with the control group (p<0.05) (Fig 4B, 4C, 4D, 4E). The percentage of TUNEL-positive nuclei was reduced by 52% in the LSC-ZnONPs group and increased by 33% in the HSC-ZnONPs group, respectively, when compared with the ZnONPs group (Fig 4E). These results indicated that the addition of low level of MWCNTs attenuated the apoptosis.
qPCR assay also indicated the increase of expression of apoptosis-related genes including caspase3, Bax, and XBP-1 in the exposure groups (Fig 5A, 5B, 5C). The addition of MWCNTs significantly down-regulated the expression levels of caspase 3 and Bax gene, compared with the ZnONPs group. Moreover, the expression levels of caspase 3 and Bax gene in the HSC-ZnONPs group were higher than those in the LSC-ZnONPs group (Fig 5A, 5B). Nevertheless, the addition of a low concentration of MWCNTs up-regulated the expression levels of the XBP-1 gene, but the addition of a high concentration down-regulated the expression levels of the XBP-1 gene when compared with the ZnONPs group (p<0.05) (Fig 5C).
DISCUSSION
Due to the broad applications of ZnONPs, their potential adverse effects on aquatic organisms including fish have also received broad attention.4,5,21–26 Du et al. (2016) indicated that the survival rate of zebrafish was decreased by 50% after exposure to 50 mg L-1 ZnONPs (diameter ≤100 nm) for 14 days.27 However, ZnONPs with the same concentration caused no mortality to common carp in the present study. The total average diameter of ZnONPs is 56.89 nm, along with a diameter range of 30-84 nm. Hence, the size of ZnONPs, developmental stage, exposure time, and species of fish may influence the mortality of fish. Suganthi et al. (2019) and Munir et al. (2019) reported that ZnONPs decreased the oxygen-carrying capacity of the blood cells and lymphocyte levels in tilapia and Catla catla.23,26 Dekani et al. (2019) and Xiong et al. (2011) suggested antioxidant defense system imbalance, liver injury, and hepatopancreas dysfunction in common carp and zebrafish following exposure to ZnONPs.5,22 De Campos et al. (2019) showed ZnONPs-caused deficits in antipredator behaviors and stress responses in Nile tilapia.21 Cong et al. (2017) demonstrated that ZnONPs increased both heart rate and malformation percentage in medaka.24 In the present study, hepatic interlobular vascular was observed at the microscopic level. The liver of seabream exhibited findings such as disarrangement of ERs, expansion of ERs cistern, dissolution of mitochondria cristae, and fracture at the ultramicroscopic level, which were consistent with our findings.25
Apoptosis plays an important role in the pathogenesis of diseases and is used for the prediction of tissue injury.11,28 In this study, apoptosis was observed in all exposure groups. BAX has been reported as an important molecular switch for mitochondria-mediated apoptosis. Caspase 3 is the downstream molecule for inducing cell apoptosis.29 Caspase 3 also has been reported as the executor of the exogenous apoptotic pathway.30 Previous studies also indicated that XBP1 played a critical role in the regulation of apoptosis.11,31 Wang et al. (2022) indicated that XBP1 over-expression remarkably increased the expression of BAX and caspases.11 Here, ZnONPs individually, and in combination with MWCNTs induced upregulation in the expression levels of BAX, XBP1, and caspase 3. The expression levels of BAX and caspase 3 were decreased in the combined exposure groups compared with the ZnONPs group, but XBP1 expression was enhanced in the LSC-ZnONPs group. These results suggested that ZnONPs or MWCNTs may induce apoptosis by multiple signal pathways. On one side, these nanomaterials induced apoptosis by regulating the expression of XBP1 and modulating other signal pathway molecules. On the other side, they modulated the BAX/caspase3 signal pathway, inducing exogenous apoptosis.
Recently, several studies have also explored the toxic effects of CNTs on fish, including Oncorhynchus mykiss, Channa punctatus, Poecilia reticulata, Nile tilapia, Astyanax altiparanae, Carassius auratus, and Danio rerio. CNTs induce some toxic effects, such as altering histopathology, antioxidant and enzyme activities, DNA structure, reproductive hormones, and survivability of early developmental stages of fish.3,18,32,33 Additionally, inflammation and apoptosis were found in our previous study.34 CNTs could interact with other toxic substances, leading to changes in toxicity. Some researchers suggested the additive toxic effects of CNTs on other pollutants. For instance, Qu et al. (2014) demonstrated that OH-MWCNTs significantly increased Cd accumulation and evoked more severe hepatic oxidative stress in the liver of Carassius auratus.12 Azari et al. (2020) indicated that the presence of MWCNTs enhanced the cytotoxicity of benzo α pyrene (BAP) in A549 lung cells.15 Increased mortality and decreased spawning rate were found in the Tigriopus japonicus after MWCNTs and triphenyltin combination exposure.14 In agreement with the above-mentioned results, in the present study, the addition of 2.5 mg L-1 MWCNTs enhanced the liver injuries of common carp, which was manifested by the aggravated histological changes, ultrastructural changes, and apoptosis in the HSC-ZnONPs group compared with the ZnONPs group. On the contrary, several studies have shown the decreased toxicity of pollutants in the presence of CNTs.7 Sayadi et al. (2020) reported the reduction of graphene nanosheets (GNs) on the bioavailability of ZnONPs in blackfish.7 Ye et al. (2018) revealed the antagonistic effect of graphene oxide nanoparticles (GONPs) and ZnONPs on zebrafish.10 In the present study, it was found that the presence of 0.25 mg L-1 MWCNTs deduced the ZnONPs-induced liver injuries. Hence, we proposed the notion that MWCNTs had limited capacity to alleviate the toxic effects of ZnONPs, which could be due to the interaction mechanism of MWCNTs and ZnONPs. Previous studies showed that ZnONPs induced cell damage by dissolved Zn ions and nano-form ZnONPs.10 Zn ions rather than nano-form ZnONPs are reported to play the most important role in the overall toxicity of the ZnONPs.7 In addition, MWCNTs have strong sorption capacity due to the large surface area. A previous report has suggested that the carbon black material could deliver metal ions into cells by adsorbing capability.35 Therefore, we deduced that MWCNTs delivered Zn ions into the hepatocytes. However, MWCNTs bound tightly with less Zn ions in the LSC-ZnONPs group. Zn ions were not easily desorbed from MWCNTs, so ZnONPs combined with low concentration of MWCNTs reduced the toxic effects compared with the ZnONPs group. On the contrary, the combination of MWCNTs and ZnONPs at a high level demonstrated a cumulative impact.
Autophagy is considered as a potential mechanism of NMs toxicity. Several research have revealed that nanoparticles including carbon NMs, protein conjugated nanoparticles, gold nanoparticles, fullerene-derived NMs, cadmium selenide and indium gallium phosphide quantum dots, ZnONPs, nano neodymium oxide, and rare earth oxide nanocrystals induced autophagy.36–38 Here, MWCNTs within autophagosomes were found in the liver of common carp in the HSC-ZnONPs group, indicating that cell autophagy and lysosomal dysfunction occurred after co-exposure to 50 mg L-1 ZnONPs and 2.5 mg L-1 MWCNTs. However, cell autophagy was not observed in other groups. Hence, we assume that autophagy induction may be correlated with the NMs concentration. Detailed analysis of the mechanism of the interaction of ZnONPs and MWCNTs in the exposure medium beyond the scope of this initial study. Zhang et al. (2019) demonstrated that NM-induced autophagy promoted cell death or elicit pro-survival, which depending on the cell type and structure of NMs.39 Hence, whether autophagy promotes the ZnONPs and MWCNTs mixture-induced apoptosis needs further exploration.
ACKNOWLEDGMENTS
This study was funded by research grants from National Natural Science Foundation of China (No.32202904). The authors would like to thank Nanjing XFNANO Co., Led for the nanomaterials.
AUTHORS’ CONTRIBUTION-CREDIT
Conceptualization: Yashuai Wang (Equal), Xiaochan Gao (Lead). Methodology: Yashuai Wang (Equal), Xuehan Niu (Equal), Jiayong Chen (Equal). Writing – review & editing: Xuehan Niu (Equal), Ruiyi Xu (Equal), Xiaochan Gao (Lead). Formal Analysis: Yong Huang (Equal), Xiaochan Gao (Equal). Investigation: Hongtao Ren (Equal), Xiaochan Gao (Lead). Writing – original draft: Xiaochan Gao (Equal). Resources: Xiaochan Gao (Lead). Funding acquisition: Xiaochan Gao (Lead). Supervision: Xiaochan Gao (Lead).
COMPETING OF INTEREST–COPE
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICAL CONDUCT APPROVAL – IACUC
All experiments and handling of the animals were conducted according to the research protocols approved by the Institutional Animal Care and Use Committee, Henan University of Science and Technology.
INFORMED CONSENT STATEMENT
All authors and institutions have confirmed this manuscript for publication.
DATA AVAILABILITY STATEMENT
Data is available upon reasonable request.

_the_tem_image_of_znonps._(b)_diameter_distribution_h.png)
__znonps_group_(b)__lsc-znonps_.png)

_the_tem_image_of_znonps._(b)_diameter_distribution_h.png)


__znonps_group_(b)__lsc-znonps_.png)
