Introduction
Gymnodiptychus dybowskii (Kessler, 1874), belonging to Cypriniformes, Cyprinidae, Schizothoracids, Gymnodiptychus Herzenstein,1 is a typical rare and unique cold-water fish in China, widely distributed in the Ili River in Xinjiang, and in the north slope of Tianshan Mountains.2,3 In 2004, 2019, and 2022, it was listed as the Xinjiang Uygur Autonomous Region’s Level I key protection aquatic wildlife.4
In recent years, studies on G. dybowskii have focused on genetic diversity,5,6 individual biology, 7–9 population distribution10,11 and phylogeny.12,13 Related scholars14–16 studied the embryonic development of G. dybowskii in the Kaidu River and Ili River of Xinjiang. Due to the aggravation of overfishing, water conservancy construction, and other factors, the population resources of G. dybowskii and other Indigenous fish are increasingly depleted, and the population is extremely scarce.17 Since G. dybowskii is distributed in different basins, there may be great heterogeneity and obvious evolutionary characteristics. Therefore, it is urgent to carry out relevant studies on germplasm conservation, proliferation, and breeding of G. dybowskii, especially on artificial breeding of different geographical populations, to provide technical support for the large-scale production of fish larvae. It is of great significance for the conservation and cultivation of this rare fish. By observing and studying the embryonic development of G. dybowskii in Manas River and comparing it with other fishes of Schizothoracinae, this study aims to find out the typical characteristics of its embryonic development at various stages, strengthen the conservation strategies and breeding and release practices of this unique indigenous species in the Manas River, and ensure the sustainable utilization of resources of this rare species and the maintenance of ecological balance.
Materials and Methods
Sample collection
From May to July 2021, the parents of G. dybowskii with strong physique and no body surface injuries will be selected for artificial breeding at the fish breeding station of Kenswart Water Conservancy Project in Manas River, Xinjiang. According to Wang18 and Wu et al.,3 male secondary sex characteristics were obvious, the head and male fin “beads” were obvious, and the reproductive pores were slightly protruding. The abdomen of the female fish is enlarged, soft, and elastic, and the reproductive pores are red and prominent. The parental information of G. dybowskii is shown in Table 1.
Artificial insemination and incubation
Dry insemination was carried out with a male-to-female ratio of 1:2 ~3 when the parents of G. dybowskii were mature. After complete insemination, the fertilized eggs were rinsed with water for 10 ~ 20 min until the egg membrane absorbed water and expanded, and hatching began. The water temperature was kept above 8.00 mg/L, and it was 15.6 ~ 17.7 ℃ (16.23 ± 0.52) ℃.
The incubation facility is a self-made 0.40 m × 0.30 m × 0.10 m incubator frame, sewn with 40-mesh screen silk and placed in a 4.00 m × 0.80 m × 0.50 m flume. The fertilized eggs are laid on the screen in the hatching box with a density of 2 ~ 4 grains /cm2, and they are stirred gently once a day for 5 ~ 6 h to maintain sufficient dissolved oxygen and prevent accumulation and death. Dead eggs are picked and removed twice in the morning and evening to prevent mildew.
Artificial insemination and incubation
During embryonic development, 10-15 fertilized eggs were randomly selected with a straw every 1 h and placed in a petri dish with water for incubation. The eggs were observed under an anatomical microscope (SMZ-140 N2GG, MOTIC, Xiamen) and a camera (PowerShot A3000 IS, Canon, Xiamen). The sequence of embryo development and morphological changes at each stage were recorded. It was observed every 20-30 min before the blastocyst stage and every 1 h during the organ differentiation stage, and more than 50% of the observed individuals showed new features as the criteria for dividing developmental periods.19,20
Data analysis
Photoshop CS6 for image processing. Estimation of effective accumulated temperature at each stage of embryonic development21:
K=NT
Where K is the accumulated temperature (℃·h), N is the time required for development to a certain stage (h), and T is the average water temperature (℃) at the development stage. SPSS 18.0 and ORIGIN 9.0 were used and expressed as Mean and standard deviation (Mean ± S.D.).
Results
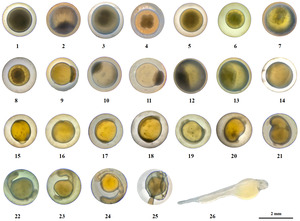
The embryonic development process of G. dybowskii was divided into 7 stages, namely fertilization, cleavage, blastula, gastrula, neurula embryo, organ formation, and membrane hatching, with a total of 26 periods (Table 2).
Morphological characteristics of mature eggs
The eggs of G. dybowskii were heavy, large, round, yellowish, with an egg diameter of (2.32 ± 0.21) mm (Figure 1-1). They are slightly viscous and swell when exposed to water. After fertilization, the viscosity of the egg was enhanced, and the viscosity was gradually weakened and the eggs dispersed. At about 0.71 h after fertilization, the height of the polar eminence of the blastoderm animal was about 1/4 of the yolk (Figure 1-2).
Cleavage stage
At about 1.14 h after fertilization, a division groove appeared in the raised blastoderm, and two similar cells divided, that is, the 2-cell period (Figure 1-3). Embryo development is accelerated, and another division groove perpendicular to the first division groove appears, dividing cells into balls, that is, the 4-cell period (Figure 1-4). Then, the two divisions divide in parallel once, forming 2 columns of 4 in each column, an 8-cell period (Figure 1-5). Continue to divide, the cell size is uneven and irregular arrangement, that is, 16-cell period (Figure 1-6). At the 32-cell period, the individual differences of the mitotic bulb became larger (Figure 1-7). During the 64-cell period, the 6th division, the division time was different, and the spatial distribution and shape characteristics of blastomes were different (Figure 1-8). The division continues, with increased mitotic balls and elevated accumulation above the yolk, resulting in a Multi-cell period (Figure 1-9).
Blastula stage
About 10-15 h after fertilization, the cells continue to divide, the number increases, and the blastoderm eminence is obvious, which is the early blastula period (Figure 1-10). The height of the blastocyst decreases, which is the mid-blastula period (Figure 1-11). As the blastocyst expands to the edge, the blastoderm shrinks and appears as a “cap” over the yolk sac, i.e., the late-blastula period (Figure 1-12).
Gastrula stage
About 20 to 30 h after fertilization, the blastocyst cells are wrapped to the bottom of the plant to 1/3 of the yolk height, and the cell edge is curled into a blastocyst ring, which is the early gastrula period (Figure 1-13). Cells on one side of the ring rise to form a blastoshield, the mid-gastrula period (Figure 1-14). The front end of the blastoderm expands and further extends to form a larger yolk plug, which is the late-gastrula period (Figure 1-15).
Neurula embryo stage
About 35-48 h after fertilization, the subderm is covered with yolk 5/6, the plant has very little yolk, the “horseshoe” brain vesicle is formed, and the whole embryo body lies on the yolk sac, which is the neurula embryo period (Figure 1-16). Egg yolk is enclosed under the germ layer, and the blastopore is closed, which is the blastopore closing period (Figure 1-17).
Organ formation stage
About 56.52 h after fertilization, 2 ~ 4 pairs of body segments were observed in the middle of the embryo body, which gradually increased, that is, the metameres appearance period (Figure 1-18). At 68.20 h, the length of the embryo body was extended with the increase of myotome, and the outline of the eye sac was formed, that is, the eye sac formation period (Figure 1-19). 76.67 h, the yolk sphere was depressed, the tail separated from the yolk sphere, and entered the caudal bud appearance period (Figure 1-20). At 81.17 h, irregular contractions of the back myotome and tail swaying occurred at a frequency of about 7-9 times /min and then gradually accelerated, that is, the muscular effect period (Figure 1-21). At 82.53 h, the eyes were visible, the embryonic body twisted frequently, and the movement mode was whole body movement, which was transferred from the head to the tail, that is, the lens appearance period (Figure 1-22). At 83.70 h, a pair of “stone” -like particles appeared in the ear sac of the embryonic body, which was the otoliths formation period (Figure 1-23). At 88.73 h, there were ventricular vacuoles at the head of the embryo body and yolk sac, with heart beating and transparent blood, that is, the heart beating period (Figure 1-24).
Membrane hatching stage
About 106.70 h after fertilization, the larval fish exhaled the membrane and entered the hatching period (Figure 1-25). Before the emergence of the membrane, the embryonic body rolls or rotates faster and impinges on the egg membrane. The egg membrane becomes soft and thin, breaks near the tail, free into the water and swings, then twists violently, the break becomes larger, and the embryonic body completely emerges from the membrane (Figure 1-26). The newly emerging membranous larva is light yellow, with distinct body segments and no body pigment formation. The yolk sac is spherical at the front and club-like at the back, close to the abdomen of the larvae. The hemoglobin on yolk was visible to the naked eye, but the pigment of eyeball, body pigment, pectoral fin and gill were not. Larvae lie on the bottom side for most of the time, and move up or up intermittently.
According to morphological characteristics, the embryonic development process of G. dybowskii can be divided into 7 stages: fertilization, cleavage, blastula, gastrula, neurula embryo, organ formation and membrane hatching, with a total of 26 periods. When the water temperature was 15.6 ~ 17.7℃, the period from fertilized egg to hatching lasted 142.55 h, and the accumulated temperature was 2376.72 ℃·h. The cleavage period lasted 9.41 h, and the accumulated temperature was 150.92 ℃·h. The blastula period lasted 9.16 h, and the accumulated temperature was 143.90 ℃·h. The gastrula period lasted 23.57 hours and the accumulated temperature was 375.74 ℃·h. The duration of neurula embryo period was 7.28 h, and the accumulated temperature was 116.73 ℃·h. The duration of organ differentiation was 38.6 h, and the accumulated temperature was 636.02 ℃·h.
Discussion
Similarities and differences in egg cell color with other species
Schizothoracinae generally inhabit plateau waters, and are commonly found in the slow flow of wide valley channels or the rapids of canyon channels,22 and have strong adaptability to extreme plateau environments in terms of morphology and ecology. In this study, the eggs of G. dybowskii were observed to be yellowish in color, similar to those of S. pseudaksaiensis,23 S. wangchiachii,24 S. prenanti15 and S. davidi25; Like Schizothorax species, Triplophysa is a genus of Cypriniformes that live at high altitudes. The egg color of T. yarkandensis,26 T. angeli27 and T. leekeri28 were light yellow. This common trait may be the result of evolutionary adaptations to clear water and specific light conditions on the plateau, helping to protect fertilized eggs from predators while allowing embryos to develop normally in extreme environments. It can be seen that the color of fertilized eggs of fish, as a part of evolution, reflects the adaptability of species to the environment, and this evolutionary trend reflects the adaptability of the evolutionary characteristics of fertilized eggs of cypriniformes fishes in different ecological niches, thus ensuring the continuity of species in the extreme environment of the plateau.
Changes in the fish’s living environment and food composition lead to changes in egg color.29 The color of fertilized eggs of G. dybowskii from Manas River (faint yellow), Kaidu River (orange or light yellow)14 and Kunes River (bright yellow)15,16 was compared, and it was found that the color of eggs produced by the parents of three different geographical populations was different. Wild G. dybowskii captured in Kaidu River was directly artificially inseminated and observed, while the parent fish were transferred to the breeding station for domestication and artificial oxytocin in Kunes River. Related scholars30–33 also found in the study of embryonic development of S. biddulphi and Aspiorhynchus laticeps, that the change of egg color was affected by living environment and food.
Similarities and differences in embryonic development with other species
Fish embryo development is not only an important research topic in animal genetics and developmental biology, but also a key link in aquatic breeding.34 The embryonic development of G. dybowskii went through the stages of blastoderm formation, cleavage, blastula, gastrula, neurula embryo, organ formation and membrane hatching, which followed the law of embryonic development of freshwater bony fish.35 During the development of G. dybowskii, the muscular effect period appeared before the lens appearance period, which was opposite to that of S. irregularis,36 S. eurystomus,37 and S. dolichonema.38 Moreover, in the late stage of embryonic development, the membrane emerges first from the tail of G. dybowskii, which is similar to D. maculates39 and S. pseudaksaiensis.23 However, S. prenanti15 and S. davidi25 did not show similar characteristics in film release mode, which is related to different fish species.40
Effect of water temperature on embryonic development
Temperature is one of the key factors affecting fish reproduction. Too low water temperature prolongates the incubation period, which affects the hatching rate, and too high water temperature increases the malformation rate.41–44 Compared with the Manas River, Kaidu River14 and Kunes River,15,16 the development time of G. dybowskii was similar in Manas River and Kunes River, while the development time of G. dybowskii was longer (240 h 49 min) in Kaidu River. The key stages and developmental characteristics of the blastoderm of the three geographic populations are similar, but the developmental duration and accumulated temperature are different, which may be caused by different hatching water temperatures. The artificial incubation water temperature of G. dybowskii in Kunes River was high, which was always maintained at 12-15℃, lower than the incubation water temperature in this study, while the lowest incubation water temperature of G. dybowskii in Kaidu River was 9-13℃, resulting in differences in the embryonic development time and development stage at membrane emergence of different geographical populations.
Schizothoracinae is a cold-water fish inhabiting high altitude and low temperature water environment, and its breeding water temperature is relatively low.[@350835;36,37,39,45 Compared with the embryonic development of other Schizothoracinae, such as A. laticeps,31 Diptychus maculates,39 S. biddulphi,45 S. irregularis,36 and S. eurystomus,37 it can be seen that the incubation temperature of the embryos does not exceed 22 ℃. This is because their habitat determines their reproduction and development and the water temperature is relatively low. Low temperature can delay embryonic development of fish eggs, reduce energy consumption, effectively resist strong changes in external temperature, and thus improve the survival rate of embryos.46
In this study, there was no significantly different between the embryonic development water temperature of G. dybowskii (16.23±0.52℃) and that of A. laticeps (16.27±0.41 ℃),29 but it was lower than that required for the development of S. biddulphi (19 ~ 19.2 ℃) and S. pseudaksaiensis (19 ~ 21 ℃).23,45 This is because G. dybowskii mainly distributed in the three tributaries of the upper reaches of the Ili River, and S. pseudaksaiensis lived in the middle and lower reaches of the basin.47 Although S. biddulphi is located in the middle and upper reaches of the Tarim River basin,48 this may be due to the fact that the average water temperature in the Tarim River basin is higher than that in the Ili River.1,49–51 It can be seen that the characteristic differences of fish embryo development are closely related to the ecological environment of the water they inhabit, and these characteristics are also a manifestation of their adaptation to the ecological environment of the water.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 31360635), the Tianshan Talent Training Project of Xinjiang (2023TSYCCX0128), the Corps Science and Technology Bureau Project (2017DB003, 2022DB019).
Authors’ Contribution
Conceptualization: Chengxin Wang; Methodology: Jiangling Li, Linghui Hu; Formal analysis and investigation: Yong Song, Jiangling Li; Writing - original draft preparation: Chengxin Wang; Supervision: Yong Song, Gulden Serekbol; Writing - review and editing: Shengao Chen.
Competing of Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
All experimental protocols were approved by the Science and Technology Ethics Committee of Tarim University (approval code: TDD-KYXF 20200426, approval date: 26 April 2020) and adhered to animal welfare laws, guidelines and policies.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.