Introduction
Nuclear power is one of the most important sources of electricity generation globally, accounting for approximately 14% of global electricity. It does not produce carbon emissions or other gases that affect climate change and can provide a stable supply of electricity. Therefore, nuclear power has become one of the primary sources of global electricity supply, with 448 nuclear reactors currently in operation.1,2 Water is an indispensable component in the operation of nuclear power, as the steam-water cycle during nuclear power operation requires a significant amount of water. The temperature at the nuclear power plant’s water discharge is about 7-10°C higher than at the intake.3,4 Consequently, nuclear power plants are generally located by the sea or in inland areas rich in water resources. Inevitably, the thermal discharge can have certain impacts on aquatic ecosystems, including water, sediment, and marine life. Fishery resources are an essential component of natural resources, serving not only as a significant source of food for humans but also providing employment, economic benefits, and social welfare for those engaged in fishing activities.5 Water temperature directly affects the body temperature of most fish species. Therefore, the impact of thermal discharge on fish has been a focal point of research. Current studies on the effects of thermal discharge on fish primarily concentrate on areas such as temperature tolerance,6,7 growth, and development,8 spatial distribution,9 habitats and physiological ecology.8 Research findings indicate that the impact of thermal discharge on fish varies. Current studies generally expose fish to lethal or sublethal temperatures under thermal shock conditions. However, in the actual operation of nuclear power plants, the temperature of thermal discharge is influenced by hydrological conditions such as tides in different regions, resulting in variations in temperature over time within mixing zones. Therefore, the temperature settings of current studies are idealized, overlooking the effects of hydrological conditions like tides on the duration of exposure in different elevated temperature mixing zones, leading to discrepancies with real-world scenarios. Research in this area is lacking, and it is necessary to conduct further studies to address this gap.
The Trachinotus ovatus belongs to the order Perciformes, family Carangidae, and genus Trachinotus. It is a warm-water pelagic fish predominantly found in the tropical and temperate regions of the southern Chinese seas.9 The Nibea albiflora is part of the order Perciformes, family Sciaenidae, and genus Nibea. It is a demersal fish found in shallow seas, primarily distributed in China’s temperate and subtropical nearshore waters.10 Both fish species are important targets for fishing and aquaculture, possessing significant economic value. Coastal waters serve as crucial areas for spawning, fattening, and foraging.
This study employed T. ovatus and N. albiflora as experimental subjects and devised a methodology predicated on the probabilistic frequency of temperature increment. Indoor experiments were conducted to emulate the operating conditions of thermal discharge from two nuclear power plants in Zhejiang and Guangdong provinces, China, across different seasons. The thermal effects of thermal discharge on the T. ovatus and N. albiflora were investigated. The findings contribute foundational data and technical insights for the construction of a more scientific impact assessment indicator system for thermal discharge.
Materials and Methods
Experimental Materials
Experiments on T. ovatus and N. albiflora were conducted in May 2022 at the South China Sea Fisheries Research Institute’s experimental base in Shenzhen, Guangdong, and at the East China Sea Fisheries Research Institute’s experimental base in Ninghai, Zhejiang, both under the Chinese Academy of Fishery Sciences. The two respective experimental bases provided the experimental fish. The T. ovatus had an average body length of (6.57±0.19) cm and an average body mass of (8.93±0.62) g; the N. albiflora had an average body length of (5.79±0.38) cm, and an average body mass of the experimental seawater was sourced from the vicinity of the two experimental bases, and was used after sedimentation, sand filtration, and disinfection, with water quality indicators being simultaneously tested. At the Shenzhen experimental base, the pH was 8.02. Salinity was 31.34, COD content was 1.23 mg·L-1, ammonia nitrogen content was 0.021 mg·L-1, nitrate nitrogen content was 0.005 mg·L-1, nitrite nitrogen content was 0.198 mg·L-1, and reactive phosphate content was 0.020 mg·L-1. The test results indicated that the inorganic nitrogen (ammonia nitrogen + nitrate nitrogen + nitrite nitrogen) at the Shenzhen experimental base met the Class II standards (0.30 mg·L-1)of the “Seawater Quality Standards”(GB 3097-1997); reactive phosphate also complied with the Class II standards (0.030 mg·L-1) of the “Seawater Quality Standards” (GB 3097-1997). At the Ninghai experimental base, the pH was 8.12. Salinity was 23.56, COD content was 0.54 mg·L-1, ammonia nitrogen content was 0.020 mg·L-1, nitrate nitrogen content was 0.457 mg·L-1, nitrite nitrogen content was 0.016 mg·L-1, and reactive phosphate content was 0.041 mg·L-1. The test results showed that the inorganic nitrogen (ammonia nitrogen + nitrate nitrogen + nitrite nitrogen) at the Ninghai experimental base met the Class III standards (0.50 mg/L) of the “Seawater Quality Standards” (GB 3097-1997); reactive phosphate also complied with the Class III standards (0.045 mg/L) of the “Seawater Quality Standards”(GB 3097-1997).
Experimental Methods
Acclimation
Initially, specimens of T. ovatus and N. albiflora were cultured separately in aquaculture pools at two experimental sites. According to the surface seawater temperature conditions outside the thermal discharge mixing zones of two coastal nuclear power plants in Zhejiang and Guangdong provinces during the four seasons of spring, summer, autumn, and winter, four groups of acclimation temperatures were set. Specifically, the surface seawater temperatures near the Guangdong nuclear power plant were 21.5℃ (average spring temperature), 27.5℃ (average summer temperature), 25.5℃ (average autumn temperature), and 17.0℃ (average winter temperature); near the Zhejiang nuclear power plant, the temperatures were 14.5℃ (average spring temperature), 25.0℃ (average summer temperature), 22.5℃ (average autumn temperature), and 10.0℃ (average winter temperature). The water temperature was adjusted to each acclimation temperature at 1℃·d-1 using a heating and cooling unit. The acclimation period lasted for 7 days, during which artificial feed was provided once in the morning and evening (09:00 and 17:00), with uneaten feed and feces removed, and one-third of the seawater changed after 30 minutes of feeding. An oxygen pump was continuously used throughout the acclimation process to maintain dissolved oxygen levels above 6.0 mg·L-1 under natural lighting with a light cycle of 12 hours light (L) and 12 hours dark (D). After acclimation, healthy and similarly sized individuals were selected for the experiments.
Experiment on the Impact of Thermal Shock on Fish Survival Rates
The definition of probabilistic frequency of temperature increment is the proportion of time a specific temperature increase persists within 24 hours. Based on the thermal discharge conditions and hydrological characteristics of the sea areas near two nuclear power plants in Zhejiang and Guangdong provinces, the temperature increase amplitudes were set at 0.5℃, 1.0℃, 2.0℃, 4.0℃, and 8.5℃. The proportions of the time this temperature increment persisted within 24 hours were set at 25%, 50%, 75%, and 100%, respectively.
The experiment assessing the effects of thermal shock on fish survival was conducted in 100 L glass tanks filled with 80 L of water. Initially, the glass tanks were submerged in larger aquaculture pools to equalize water levels, and a heating and cooling unit was used to control the water temperature. The experiment was divided into elevated and control temperature groups, with three replicates for each temperature setting. Temperature increments were regulated to within ±0.5°C using heating rods. Upon reaching the designated rise amplitude, 30 fish were introduced into each tank. The heating was stopped when the experiment reached the predetermined duration, with a total experimental time of 24 hours. For ease of description, this is called the elevated temperature-duration probability experimental group. Before the experiment, the test organisms were fasted for 24 hours. During the experiment, an oxygen pump was used to aerate the water, maintaining dissolved oxygen levels above 6.0 mg·L-1. Water temperature was monitored using a multiparameter water quality analyzer (YSI 650 MDS), and observations and records of the test fish’s survival, stress, and mortality were made. The definition of fish mortality was the cessation of gill cover movement or no response to touch. Natural lighting was used throughout the experiment, with a light cycle of 12 hours light (L) and 12 hours dark (D). It was ensured that the mortality rate in the control group was ≤10% by the end of the experiment; otherwise, the experiment would be conducted again.
Data Processing and Analysis
The statistical results are presented as mean ± standard deviation (Mean±SD). Data were statistically analyzed using IBM SPSS Statistics 22.0 (IBM Inc., USA). Initially, the experimental data underwent normality (Kolmogorov-Smirnov test) and homogeneity of variance testing (Levene test).A two-factor analysis of variance (one-way ANOVA) was used to analyze the effects of elevated temperature amplitudes and duration probabilities on survival rates and their interaction, with a significance level set at 0.05.
Results
The Impact of Probabilistic frequency of Temperature increment on the Survival Rate of T. ovatus Across Different Seasons
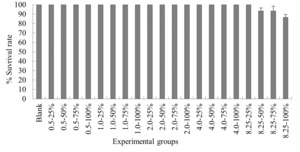
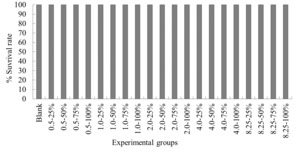
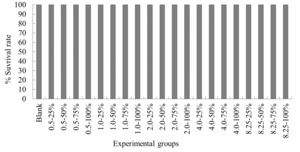
The experimental results on the impact of probabilistic frequency of temperature increment on the survival rate of T. ovatus across different seasons showed that under spring temperature conditions, no mortality was observed in the control group or any of the experimental groups of T. ovatus (Figure 1). No mortality occurred in the control group of T. ovatus in summer temperature conditions. However, in the experimental groups with 8.5ºC-50%, 8.5ºC-75%, and 8.5ºC-100% elevated temperature-duration probability, mortality was observed in T. ovatus, with average survival rates of (93.3±3.3) %, (93.3±5.1) %, and (86.7±2.9) % respectively. Among these, the 8.5ºC-100% elevated temperature-duration probability group exhibited the highest mortality rate in T. ovatus, and there was a significant difference compared to the 8.5ºC-50% and 8.5ºC-75% elevated temperature-duration probability groups. Under autumn and winter temperature conditions, no mortality was observed in the control group or any of the experimental groups of T. ovatus (Figures 3-4).
The Impact of Probabilistic frequency of Temperature increment on the Survival Rate of N. albiflora Across Different Seasons
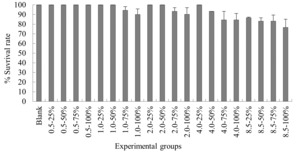
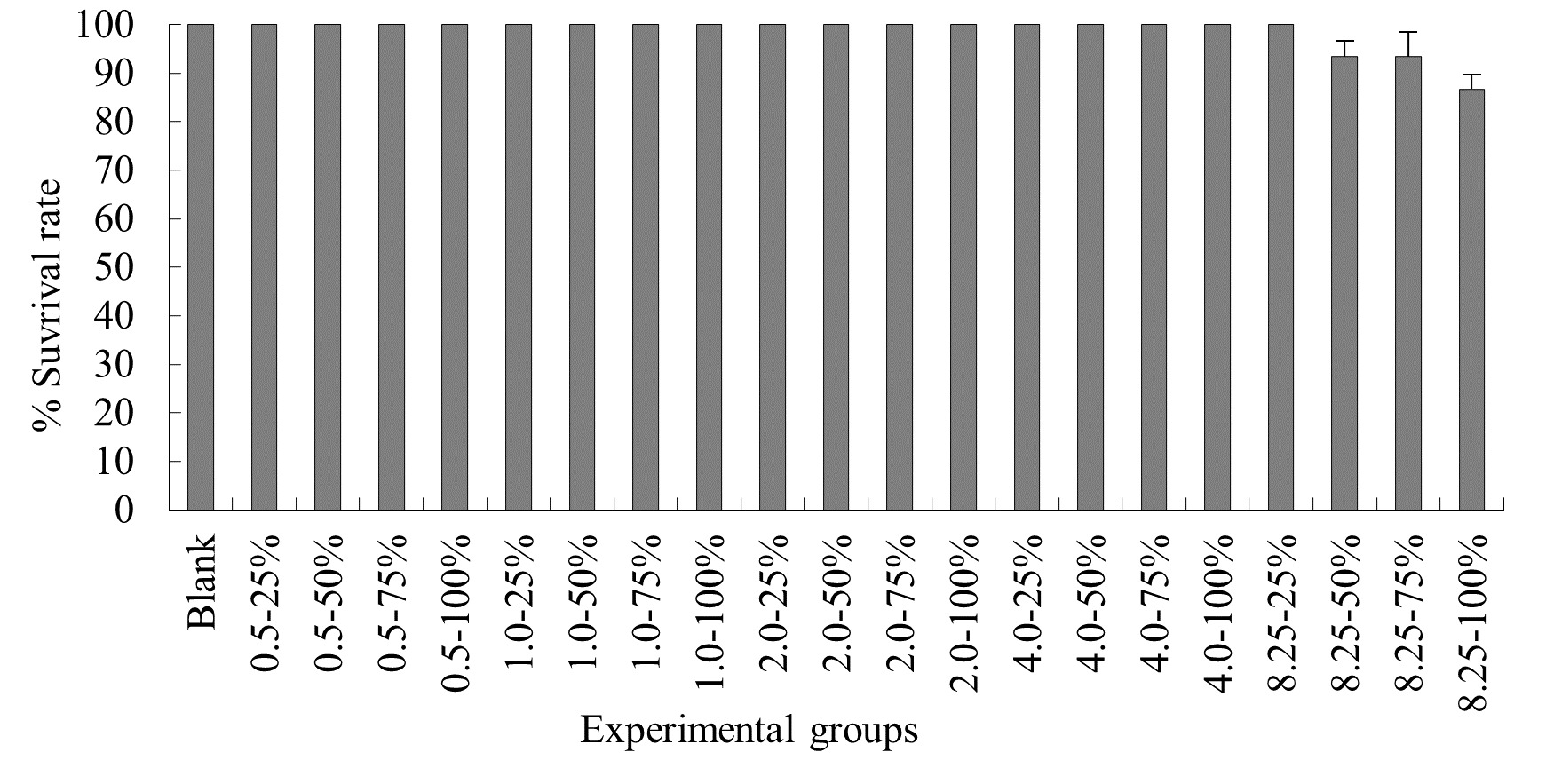
The experimental results on the impact of probabilistic frequency of temperature increment on the survival rate of N. albiflora across different seasons showed that under spring temperature conditions, certain mortality was observed in the experimental groups with 1.0ºC-100%, 2.0ºC-75%, 2.0ºC-100%, 4.0ºC-75%, 4.0ºC-100%, 8.5ºC-75%, and 8.5ºC-100% elevated temperature-duration probability, with average survival rates of (94.4±3.8)%, (94.4±3.3)%, (94.4±2.9)%, (93.3±5.1)%, (88.9±2.3)%, (85.7±3.3)%, and (83.3±3.8)% respectively. No mortality occurred in the control group or the other elevated temperature-duration probability experimental groups of N. albiflora. Overall, as the experimental temperature and duration probability increased, the mortality rate of N. albiflora showed an upward trend, with the highest mortality rate observed in the 8.25ºC-100% duration probability treatment group. Significant differences in survival rates were found between the 4.0ºC-75%, 4.0ºC-100% and 8.5ºC-75%, 8.5ºC-100% elevated temperature-duration probability experimental groups (Figure 5).
Under summer temperature conditions, no mortality was observed in the control group, the various 0.5ºC rise duration probability experimental groups, and the 1.0ºC-25% elevated temperature-duration probability experimental group of N. albiflora. Mortality occurred in all other experimental groups, with average survival rates for 1.0ºC-50%, 1.0ºC-75%, 1.0ºC-100%, 2.0ºC-25%, 2.0ºC-50%, 2.0ºC-75%, 2.0ºC-100%, 4.0ºC-25%, 4.0ºC-50%, 4.0ºC-75%, 4.0ºC-100%, 8.5ºC-25%, 8.5ºC-50%, 8.5ºC-75%, and 8.5ºC-100% being (90.0±3.3)%, (90.0±5.8)%, (90.0±3.3)%, (90.0±5.8)%, (90.0±3.3)%, (84.4±5.0)%, (84.4±8.8)%, (90.0±6.7)%, (80.0±6.7)%, (70.0±3.3)%, (70.0±3.8)%, (83.3±3.3)%, (70.0±1.9)%, (70.0±3.3)%, and (61.1±6.9)% respectively. Overall, as the experimental temperature and duration probability increased, the mortality rate of N. albiflora generally showed an upward trend, with the highest mortality rate observed in the 8.25ºC-100% duration probability treatment group, where the average mortality rate was 35%. Significant differences in survival rates were found between 2.0ºC-75% and 2.0ºC-100% compared to 2.0ºC-25% and 2.0ºC-50%; 4.0ºC-75% and 4.0ºC-100% compared to 4.0ºC-25% and 4.0ºC-50%; and 8.5ºC-50% and 8.5ºC-75% compared to 8.5ºC-25% and 8.5ºC-100% elevated temperature-duration probability experimental groups (Figure 6).
Under autumn temperature conditions, mortality was observed in the experimental groups with elevated temperature-duration probabilities of 1.0ºC-75%, 1.0ºC-100%, 2.0ºC-75%, 2.0ºC-100%, 4.0ºC-50%, 4.0ºC-75%, 4.0ºC-100%, 8.5ºC-25%, 8.5ºC-50%, 8.5ºC-75%, and 8.5ºC-100%, with average survival rates of (94.4±3.8)%, (90.0±5.8)%, (93.3±3.8)%, (90.3±6.7)%, (93.3±0.0)%, (84.4±8.8)%, (84.4±6.7)%, (86.7±0.6)%, (83.3±3.3)%, (83.3±6.1)%, and (76.7±8.4)% respectively. No mortality occurred in the control group or the other elevated temperature-duration probability experimental groups of N. albiflora. Overall, as the experimental temperature and duration probability increased, the mortality rate of N. albiflora showed an upward trend, with the highest mortality rate observed in the 8.5ºC-100% duration probability treatment group. Significant differences in survival rates were noted between 1.0ºC-75% and 1.0ºC-100%; 2.0ºC-75% and 2.0ºC-100%; 4.0ºC-50% and 4.0ºC-75%, 4.0ºC-100%; and 8.5ºC-50%, 8.5ºC-75% compared to 8.5ºC-25%, 8.5ºC-100% elevated temperature-duration probability experimental groups (Figure 7).
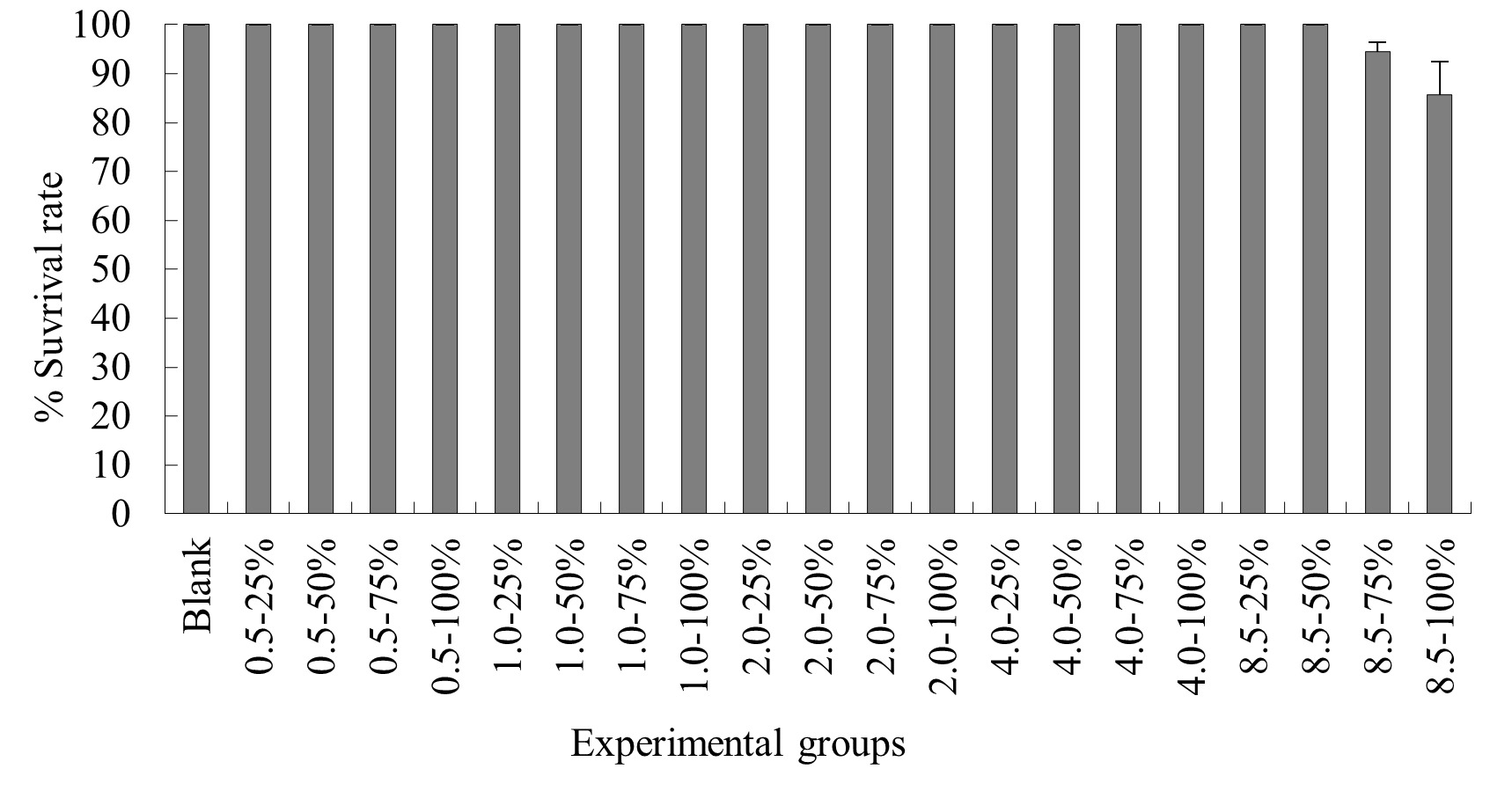
Under winter temperature conditions, mortality was observed only in the N. albiflora experimental groups with 8.5ºC-75% and 8.5ºC-100% elevated temperature-duration probabilities, with average survival rates of (94.4±1.9) % and (85.6±6.9) % respectively. No mortality occurred in the control group or the other experimental groups of N. albiflora, with the highest mortality rate observed in the 8.5ºC-100% treatment group. Significant differences in survival rates were found between the 8.5ºC-75% and 8.5ºC-100% elevated temperature-duration probability experimental groups (Figure 8).
The Impact of Probabilistic frequency of Temperature increment on the Survival Rate of T. ovatus Across Different Seasons
The two-factor analysis of variance results for the effects of elevated temperature-duration probability on the survival rates of T. ovatus and N. albiflora (Table 1) revealed that, in spring, elevated temperature had no significant effect on the survival rate of N. albiflora, whereas duration probability significantly affected it. There was an interaction between elevated temperature and duration probability. In summer and autumn, both elevated temperature and duration probability significantly influenced the survival rate of N. albiflora, with an interaction between these two factors. In winter, neither elevated temperature nor duration probability significantly affected the survival rate of N. albiflora, but there was an interaction between them. In summer, elevated temperature significantly affected the survival rate of T. ovatus, while duration probability did not have a significant effect, indicating an interaction between elevated temperature and duration probability.
Discussion
Temperature is an extremely important factor in aquatic environments. As one of the primary variables in physiological ecology, temperature is related to the metabolic processes of poikilothermic animals’ basic life history. It is one of the key factors controlling poikilotherms’ life cycle and behavioral changes through physiological and biochemical means.11 Fish lack the ability to regulate their body temperature, so their body temperature changes with the surrounding temperature, leading to changes in growth, behavior, reproduction, metabolic activities, lifespan, and survival rates.12 Generally, fish may seek optimal temperature conditions as their habitat, and significant temperature changes under optimal conditions can be considered a stress source for fish behavior.13,14 Therefore, thermal emissions from power plants, including nuclear power plants, directly impact fish.
The findings of this study reveal that in different seasons, the mortality rates of T. ovatus and N. albiflora both escalated with increasing acclimation temperatures. When the temperature increase amplitude was small, the mortality rate was low or there was no mortality; however, as the temperature increase became more significant, the mortality rate noticeably increased. This is related to the temperature tolerance of fish, as different species have varying upper and lower limits of temperature tolerance. Within the optimal temperature range, a moderate temperature increase can enhance the feeding ability of marine fish, promote their sexual maturity, and accelerate growth. However, once the temperature exceeds their tolerance range, it may culminate in mortality.15,16 In this experiment, within the same season, the survival rate of T. ovatus under the same temperature increase amplitudes and duration probabilities (Figures 1-8) was significantly higher than that of N. albiflora. This is because the lethal temperatures, critical temperatures, and thermal adaptation capacities differ among fish species, closely related to the latitude, geographic distribution, and the temperature of their birth habitats. Generally, warm-water fish have a greater heat tolerance than cold-water species.17 The T. ovatus is a more typical southern fish species,9 while the N. albiflora is more typical of northern regions.10 The habitat temperature of the T. ovatus in different seasons is significantly higher than that of the N. albiflora, making the N. albiflora more sensitive to temperature increment compared to the T. ovatus. Specifically, the suitable temperature range for T. ovatus is about 25-32°C,18 while for N. albiflora, it is approximately 20-25°C.19 Therefore, in spring, autumn, and winter, the acclimation temperatures combined with different temperature increment still fall within the suitable temperature range for T. ovatus. Only in summer, when the acclimation temperature is combined with an 8.5℃ temperature increase, do some experimental groups experience lower mortality. The suitable temperature range for N. albiflora is relatively narrow; in summer and autumn, compared to T. ovatus, even a lower temperature increase combined with the acclimation temperature exceeds the suitable temperature range for N. albiflora, leading to mortality. However, in spring and winter, the water temperature, combined with different temperature increment for N. albiflora, generally remains below its suitable temperature range. Given that the experimental methodology of this study differs from typical fish tolerance tests—placing fish under thermal shock at predetermined experimental temperatures—the main cause of mortality is likely the stress response caused by sudden thermal shock under low-temperature conditions.
Fish can perceive changes in water temperature and regulate their body temperature within a narrow adaptive range through physiological and ecological behaviors to avoid harm from extreme high temperatures.20 Whether exposure to temperatures near their lethal limits results in death depends on the duration of exposure to such temperatures, a phenomenon that is more evident in the experiments with N. albiflora. The results of this study show that at lower temperature increase amplitudes, the duration of elevated temperature has a minimal impact on the survival rate of N. albiflora. However, as the temperature increase amplitude grows, the effect of elevated temperature duration on the survival rate of N. albiflora becomes more significant, indicating that the longer the duration of elevated temperature, the lower the survival rate. Increased temperatures can mobilize the rates of metabolic enzymes, ion channels, mitochondria, and other important biochemical reaction processes in the organism to compensate for the impact of temperature stress. However, prolonged exposure beyond the organism’s maximum physiological adjustment limit leads to a weakening or collapse of physiological compensation mechanisms, ultimately resulting in death.21
Previous studies assessing the impact of thermal power plant thermal discharge on aquatic organisms, such as fish, mainly focused on the tolerance of aquatic life under different temperature increase amplitudes, often overlooking the interaction between actual operating conditions and hydrological conditions over time. The results of this study indicate that in assessing the impact of thermal power plants on fish, especially for temperature-sensitive species, to enhance the accuracy of the assessment, it is not only necessary to consider the temperature increase amplitudes but also the duration of different temperature increase amplitudes as an important factor to be taken into account.
CONCLUSION
The mortality patterns of T. ovatus and N. albiflora vary across seasons and in response to temperature changes. T. ovatus shows remarkable resilience, with no mortality in spring, autumn, and winter, and only succumbing in summer. In contrast, N. albiflora experiences mortality throughout the year, with the most severe losses also in summer. Both species exhibit higher mortality rates with increasing temperatures and longer exposure times, indicating that the survival rates of these fish are influenced by both the magnitude and the duration of temperature increment. T. ovatus demonstrates a higher tolerance to temperature fluctuations than N. albiflora, suggesting a species-specific capacity to withstand environmental changes.
Authors’ Contribution
Conceptualization: Lei Li (Lead). Investigation: Lei Li (Lead). Project administration: Chenshan Shao (Lead). Supervision: Jiaying Cai (Lead). Validation: Jiaying Cai (Lead). Formal Analysis: Baojun Tang. Visualization: Baojun Tang (Lead). Writing – original draft: Baojun Tang (Lead). Data curation: Weiwei Su (Lead). Resources: Weiwei Su (Lead). Software: Weiyi Zou (Lead). Methodology: Weiyi Zou (Lead). Writing – review & editing: Mei Jiang (Lead).
Ethical Conduct Approval – IACUC
Research involving animals
Our experiments involving vertebrates were conducted within the ethical guidelines provided by our institution and national or international regulations. We confirm that all efforts were made to ameliorate animals’ suffering during the experiments.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.