Introduction
Pseudomonas aeruginosa is a dangerous Gram-negative opportunistic pathogen that can lead to severe acute and chronic infections, often resulting in high morbidity and mortality rates of up to 40%.1,2 P. aeruginosa is a versatile bacterium that combines various organic compounds to acquire nutrients. It is commonly found in diverse environments like soil, water, vegetation, human skin, and oral mucosa.3,4 P. aeruginosa is a significant concern for immunocompromised individuals, as it is a common cause of sepsis in neutropenic cancer patients, pneumonia, and respiratory failure.5–7 2017, the World Health Organization identified P. aeruginosa as a critical priority pathogen for new antibiotic research and development.8,9 This designation stems from its genetic background and antibiotic resistance profile. P. aeruginosa has intrinsic resistance to many antibacterial drugs and can rapidly develop antibiotic resistance through chromosomal mutations or horizontal gene acquisition.10–13 Recent studies estimate that antibiotic-resistant P. aeruginosa caused 1.27 million deaths in 2019, highlighting its global health impact.14
P. aeruginosa develops resistance and can acquire antibiotic tolerance through forming biofilms, which are complex bacterial clusters that adhere to surfaces and are embedded in a self-produced matrix.13 Biofilms are complex bacterial communities surrounded by extracellular polymeric substances (EPS), protecting against environmental stresses and enhancing antibiotic resistance.15–19 Bacteria trapped in biofilms can withstand up to 1000 times more antibiotics than free-living bacteria, making it challenging to treat antibiotic-resistant infections.20–22 The rise of multidrug-resistant P. aeruginosa strains poses a global public health threat. These strains limit treatment options, leading to increased morbidity and mortality.12 As a result, alternative antimicrobial strategies are needed to combat P. aeruginosa infections effectively. Recently, several therapeutic approaches have been developed to control P. aeruginosa infections. These include antibiotic treatment,23 enhancing drug penetration into bacterial cell membranes,24 and inhibiting biofilm formation using plant extracts.25–30 In addition, other methods to control P. aeruginosa infections involve using host-specific phages and infection modes that depend on the interaction between viral proteins and the host bacterial surface. Many studies have reported the significant control of bacteriophages against antibiotic-resistant bacterial strains such as E. coli, P. atrosepticum, A. baumannii, S. aureus, S. typhimurium, and K. pneumoniae.31–38
Given the limitations of conventional antibiotics in treating biofilm-mediated and drug-resistant infections, alternative approaches are urgently needed. Bacteriophage therapy has emerged as a promising option.39–43 Phages offer several advantages over antibiotics, including specificity for target bacteria, the ability to evolve alongside bacterial resistance, and self-replication within the host.44–48 Moreover, some phages have demonstrated the ability to inhibit biofilm formation. For example, Nordstrom et al.49 suggested that phage therapy could be a promising alternative for eliminating biofilms formed by various clinical strains of P. aeruginosa isolated from cystic fibrosis patients. Phages LUZ19 and LUZ24 were effective in controlling P. aeruginosa infections.49–51
In this study, we isolated and characterized a novel P. aeruginosa bacteriophage, KG853. We investigated its morphology, growth kinetics, stability, host range, and genome characteristics. Additionally, we assessed its effectiveness in biofilm inhibition. This comprehensive characterization aims to evaluate KG853’s potential as a therapeutic agent against P. aeruginosa infections and contribute to the growing knowledge of phage-based control strategies for foodborne pathogens.
Materials and Methods
Bacterial cultures and preparations
Pseudomonas aeruginosa ATCC 27853 and other reference strains from ATCC (American Type Culture Collection) and NCTC (National Collection of Type Cultures) were used in this study. These included V. parahaemolyticus NCTC 10884, V. parahaemolyticus NCTC 10885, V. parahaemolyticus ATCC 17802, V. vulnificus NCTC 11218, V. vulnificus ATCC 29307, Salmonella enterica subsp. enterica serovar Typhimurium ATCC 14028, S. enterica subsp. enterica serovar Enteritidis ATCC 13076, S. enterica subsp. enterica serovar Enteritidis ATCC 49223, and Escherichia coli ATCC 25922. All strains were cultured in Tryptic Soy Broth (TSB) and on Tryptic Soy Agar (TSA) at 37°C with shaking at 120 rpm for 24 hours.
Isolation and purification of phage
Bacteriophages were isolated from prawn pond water samples collected in Kien Giang province, Vietnam, using P. aeruginosa ATCC 27853 as the host bacterium. Water samples were enriched in TSB medium for one hour, followed by centrifugation at 12,000 rpm for 10 minutes at 4°C. The supernatant (1 mL) was added to 5 mL of P. aeruginosa ATCC 27853 bacterial suspension and incubated overnight. Chloroform (1%) was added, and the mixture was centrifuged at 12,000 rpm for 10 minutes at 4°C. The supernatant was collected as crude phage preparation.
Determination of host range
The host range of isolated phages was determined using the spot test method on double-layer agar plates.52 Bacterial strains were cultured in a TSB liquid medium. An aliquot (100 µL) of each bacterial culture was added to 5 mL of semi-solid medium (56°C) and poured onto TSA plates. Phage suspension (2 µL) was spotted on the bacterial lawn. The experiment was performed in triplicate. Plates were observed for the formation of clear zones (plaques), and results were recorded as positive (+) or negative (−) for plaque formation.
Biofilm inhibition assay
Biofilm reduction assays were conducted in 96-well plates.53 Bacterial strains were cultured overnight in a TSB medium with shaking at 120 rpm. Bacterial suspension (100 µL) was added to each well of a 96-well plate and incubated for 24 hours at 32°C. After incubation, the wells were washed three times with 0.9% NaCl. Phage suspension (100 µL) was added to each well and incubated at 32°C for 4 hours. The wells were then washed twice with 200 µL of 0.9% NaCl. Crystal violet (0.1%, 200 µL) was added to each well and incubated for 20 minutes at room temperature. After removing the crystal violet solution, the wells were washed thrice with 200 μL of 0.9% NaCl. Acetic acid (30%, 200 μL) was added to each well to dissolve the crystal violet. Biofilm formation was quantified by measuring the absorbance at 590 nm (OD590).54 The experiment was repeated three times to ensure reliable results.
Transmission electron microscopy (TEM)
Phage morphology was examined using transmission electron microscopy (TEM) following the method described by Taha et al.55 Briefly, 10 µL of phage suspension (109 PFU/mL) was placed on a carbon-coated copper grid and stained with 2.5% uranyl acetate for 10 minutes. The sample was examined using a JEOL 1230 transmission electron microscope (Tokyo, Japan) at various magnifications.
Phage adsorption assay
To determine the time required for phage attachment to bacteria, a mixture of bacteria and phage was prepared and incubated at 32°C. At various time points (0, 1, 2, 5, 10, 15, 20, and 30 minutes), 100 µL aliquots were removed and centrifuged at 12,000 rpm for 5 minutes. The supernatant was analyzed using the double-layer agar method to determine the number of free bacteriophages.56 The experiment was repeated three times to ensure reliable results.
One-step growth curve
A one-step growth curve was generated using a previously described method with minor modifications.57 P. aeruginosa (5 mL) was cultured at 37°C with shaking (120 rpm). An aliquot (1 mL) of the culture was transferred to a falcon tube containing 4 mL of TSB medium and incubated with shaking (120 rpm) until the bacterial count reached OD600=0.4 (approximately 3 hours). Bacteriophage (2 μL) was added to the bacterial culture, and samples were taken every 10 minutes. The phage titer was determined using the double-layer agar technique. The burst size was calculated as the ratio of the final count of liberated phage particles to the initial count of infected bacterial cells during the latent period. The experiment was conducted in triplicate to ensure the reliability of the results.
pH and temperature stability
For thermal stability testing, 10 μL of phage suspension was incubated at different temperatures (−20°C, 4°C, 37°C, 50°C, 60°C, and 70°C) for one hour. After incubation, the titer of each phage sample was determined using the double-agar layer method.51 To investigate the effect of pH on phage stability, the phage was mixed with buffers of different pH values (pH 2, 3, 4, 5, 6, 7, 8, 9, and 10) and incubated for one hour. After incubation, the titer of each phage sample was determined using the double-agar layer method.51 The experiment was repeated three times to ensure reliable results.
Phage genome sequencing and bioinformatics analysis
Phage DNA was extracted and sequenced using the Illumina HiSeq platform at the KTEST laboratory in Ho Chi Minh City, Vietnam. Open reading frames (ORFs) were predicted using BLAST v2.13.0 against the NCBI nt virus GenBank database. Genome organization was visualized using Geneious software, and comparative genomic analysis was performed using Mauve.
Statistical analysis
Data were processed and analyzed using Microsoft Excel 2019 and Minitab 18 software. Experimental results were analyzed by one-way ANOVA. Differences were considered significant at p<0.05.
Results
Host specificity
The host range assessment of phage KG853 against several pathogenic bacterial strains (Figure 1) revealed that KG853, in addition to lysing its primary host P. aeruginosa ATCC 27853, also infected V. parahaemolyticus NCTC 10885 and S. typhimurium ATCC 14028, as evidenced by plaque formation. These results demonstrate that phage KG853 has a broad host range, indicating its potential for phage therapy applications.
Biofilm inhibition assay
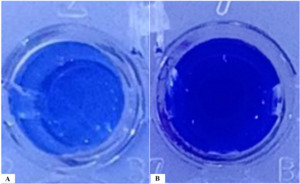
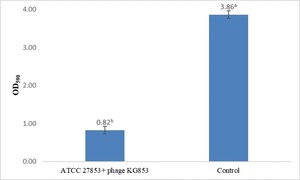
Biofilm staining results showed that phage KG853 significantly reduced biofilm formation compared to the control (p<0.05) after a 4-hour incubation period (Figure 2). When 24-hour biofilms of P. aeruginosa ATCC 27853 were treated with bacteriophages, the OD590 decreased from 3.86 to 0.82, representing a 78.7% reduction (Figure 3). This demonstrates that the biofilm was effectively eliminated by phage treatment.
Transmission electron microscopy (TEM)
The plaque morphology of KG853 on P. aeruginosa ATCC 27853 was characterized by small, transparent plaques (0.5 mm in diameter) surrounded by opaque outer borders (Figure 4A). TEM images of KG853 revealed a phage with a head and tail structure, with the head diameter measuring 60.99 nm (Figure 4B). Based on its icosahedral shape and the presence of a head and tail structure, the phage is classified within the Bruynoghevirus genus. The virions also feature short, non-contractile tails measuring about 20×8 nm in size, aligning KG853 with the Steigviridae family according to the International Committee on Taxonomy of Viruses (ICTV) guidelines.58
Phage adsorption assay
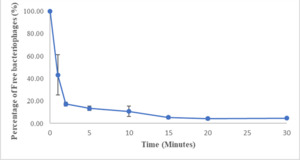
The adsorption assay results indicated that most bacteriophages rapidly attached to host cells, with over 50% attaching within one minute and over 90% attaching within 15 minutes (Figure 5).
One-step growth curve
The one-step growth curve analysis revealed that the replication cycle of KG853 in host cells lasts approximately 50 minutes (Figure 6). The initial 30 minutes represent the latent phase of KG853 replication, during which phages complete adsorption and genome injection. The active replication phase occurs between 30 and 50 minutes, during which new phages are assembled and released from host cells. The highest number of phages was observed 50 minutes post-infection, with a burst size of 136 virions per infected cell.
Thermal and pH stability
pH stability tests demonstrated that KG853 remained stable at pH values ranging from 4 to 10, while it was utterly inactivated at pH 2 and 3 (Figure 7A). Thermal stability assays showed that the phage maintained a titer of approximately 109 PFU/mL after one hour of incubation at temperatures ranging from −20°C to 50°C. The phage titer was slightly reduced to 108 PFU/mL at 60°C, and the phage was inactivated at 70°C (Figure 7B).
Genome features and annotation of phage KG853
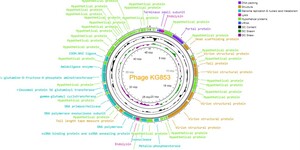
Genome sequencing and analysis identified 84 genes as coding sequences (CDS) in the KG853 genome (Figure 8). Of these, 60 CDSs (71.4%) were of unknown function (hypothetical proteins). No genes associated with KG853 toxicity were found. The remaining 24 genes were identified based on homology to known genes and encode proteins involved in phage structure, packaging, lysis, and DNA replication, such as primase/helicase, DNA polymerase, endolysin, and terminase small subunit.
KG853 possesses two distinct DNA polymerase genes: DNA polymerase and DNA polymerase exonuclease. The DNA polymerase exonuclease gene follows the primase/helicase gene and has overlapping segments. The primase/helicase and DNA polymerase genes are located nearby, consistent with their functional relationship in the DNA replication f.59,60
Two endolysin-encoding genes were identified in the KG853 genome. One is near the gene encoding the small terminase subunit, while the other is near the DNA polymerase gene. These endolysins likely play a role in bacterial cell lysis during phage infection and virion release.61
The small terminase subunit of KG853 is located in the 9,500-10,000 bp region, preceding the endolysin gene and partially overlapping. The terminase complex, comprising small and large subunits, is crucial for dsDNA packaging in the tailed phages.62,63
Three tRNA genes were identified, all clustered in the genome’s 9,000-9,500 bp region. The specific function of these tRNA genes in KG853 remains to be determined.
Comparative genomic and phylogenetic analysis
Comparative genome analysis using the NCBI blast (megablast) program revealed that phage KG853 shares high sequence similarity with several other Pseudomonas phages (Figure 9). The overall sequence identity was 99% with Pseudomonas phage PaP3 (NC_004466.2), 99% with phage Epa1 (MT108723.1), 96% with phage vB_PaeP_p2-10_Or1 (NC_019813.1), 99% with phage HZ2201 (OQ427617.1), and 99% with phage ZCPS1 (ON156559.1).
Discussion
Bacteriophages have emerged as a promising alternative to traditional antibiotics in treating bacterial infections, particularly considering the growing threat of antibiotic-resistant bacteria in aquaculture, agriculture, and healthcare. The ease of isolation, low development costs, and effectiveness against drug-resistant bacteria make bacteriophages an attractive option for combating infections.64 However, before phages can be considered for clinical application, thorough characterization, and genomic analysis are essential to ensure their safety and efficacy.
In this study, we isolated and characterized phage KG853, demonstrating potent lytic activity against P. aeruginosa, a notorious pathogen known for its multidrug resistance. The rapid adsorption kinetics of KG853, with over 90% of phages attaching to host cells within 15 minutes, coupled with its high burst size of 136 PFU/cell after a 50-minute replication cycle, suggest that this phage could be highly effective in controlling P. aeruginosa populations. These characteristics are crucial for developing phage-based therapies, as they inform optimal dosing strategies and help predict treatment outcomes.
The stability of KG853 under various environmental conditions is another crucial factor in its potential therapeutic application. Our results show that KG853 maintains its stability over a wide range of pH (4-10) and temperatures (-20°C to 50°C). This broad stability profile indicates that KG853 could potentially survive in diverse physiological environments, enhancing its therapeutic potential. However, its inactivation at extreme pH levels (2-3) and high temperatures (≥70°C) should be considered when developing formulation and storage protocols.
Morphological TEM analysis revealed that KG853 belongs to the Bruynoghevirus genus within the Steigviridae family. This classification provides valuable insights into the phage’s biology and potential behavior, as members of this family are known for their ability to infect and lyse various Gram-negative bacteria, including Pseudomonas species.58
One of the most significant findings of this study is KG853’s ability to reduce P. aeruginosa biofilms. Biofilms are notoriously difficult to treat with conventional antibiotics due to their complex structure and increased resistance.15–18 The observed 78.7% reduction in 24-hour biofilms after treatment with KG853 is particularly promising, as it suggests that this phage could be effective against established infections. This anti-biofilm activity, combined with KG853’s lytic capabilities, makes it a strong candidate for treating chronic P. aeruginosa infections, particularly in settings where biofilm formation is a major concern, such as in cystic fibrosis patients or on medical devices.
Genomic analysis of KG853 revealed 84 coding sequences, with 24 genes identified as having known functions related to phage structure, DNA replication, packaging, and host cell lysis. The absence of lysogenic or virulence genes in the KG853 genome is a positive indicator for its potential use in phage therapy, as it suggests a lower risk of unintended consequences such as horizontal gene transfer of virulence factors.65
The presence of multiple DNA polymerase genes and endolysin-encoding genes in the KG853 genome may contribute to its efficient replication and lytic activity. While their specific function remains unclear, identifying tRNA genes could enhance the phage’s ability to adapt to different host environments.66
Comparative genomic analysis revealed high sequence similarity between KG853 and several other Pseudomonas phages, including PaP3, Epa1, vB_PaeP_p2-10_Or1, HZ2201, and ZCPS1. This similarity suggests that KG853 is part of a well-established group of Pseudomonas-infecting phages, which could provide additional insights into its behavior and potential applications through comparative studies.
While our results are promising, it is essential to acknowledge the limitations of this study. In vitro experiments, while informative, may only partially predict the behavior of KG853 in complex biological systems. Further research, including in vivo studies in animal models, will be crucial to evaluate the safety and efficacy of KG853 as a potential therapeutic agent.
In conclusion, bacteriophage KG853 demonstrates several characteristics that make it a promising candidate for phage therapy against P. aeruginosa infections. Its broad host range, rapid adsorption, high burst size, stability under various conditions, and ability to reduce biofilms all contribute to its potential as an antimicrobial agent. The genomic analysis further supports its therapeutic potential while offering insights into its biology and evolution. Future studies should focus on in vivo efficacy, safety assessments, and the development of delivery strategies to fully realize the potential of KG853 in combating multidrug-resistant P. aeruginosa infections.
Acknowledgments
The authors thank the Department of Research Affairs, the Institute of Food and Biotechnology, and Can Tho University for their valuable support. This study is fully funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106.05-2019.335.
Authors’ Contribution
Conceptualization: Truong Thi Bich Van (Lead). Methodology: Truong Thi Bich Van (Equal), Tran Thi Lieu (Equal). Writing – review & editing: Truong Thi Bich Van (Equal), Le Hoang Bao Ngoc (Equal). Funding acquisition: Truong Thi Bich Van (Lead). Resources: Truong Thi Bich Van (Lead). Supervision: Truong Thi Bich Van (Lead). Formal Analysis: Nguyen Thi Loan Anh (Equal), Tran Thi Lieu (Equal), Le Viet Dung (Equal). Investigation: Nguyen Thi Loan Anh (Equal), Tran Thi Lieu (Equal), Le Viet Dung (Equal). Writing – original draft: Nguyen Thi Loan Anh (Equal), Vo Van Thanh (Equal).
Competing of Interest
No competing interests were disclosed.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.


_and_transmission_electron_microscopy_image_(b)_of_phage_kg853.png)

_and_thermal_(b)_stability_of_phage_kg853.png)




_and_transmission_electron_microscopy_image_(b)_of_phage_kg853.png)


_and_thermal_(b)_stability_of_phage_kg853.png)

