Introduction
Salmonella spp. is a zoonotic disease caused by Salmonella spp. and encompasses more than 2600 serotypes.1,2 It is a Gram-negative, rod-shaped, facultatively anaerobic, and motile microorganism known for causing conditions such as salmonellosis, septicemia, typhoid fever, and fowl typhoid.3–5 Salmonella spp. belongs to the Enterobacteriaceae family and spreads through contaminated water, food, and the environment. Additionally, it is a commensal microorganism in the intestinal tracts of many animals, including pets, livestock, and poultry.6–8
Biofilms are clusters of microorganisms that attach to a surface and produce an extracellular matrix to anchor the cells and shield the colony from various threats, including environmental dangers, host immune responses, and antimicrobial agents.9–11 Salmonella spp. has developed surface adherence and biofilm formation as key survival tactics in challenging environments, posing a significant risk for transmission through the food supply chain.12
The current treatment for chicken salmonellosis primarily relies on antibiotic therapy. Prolonged, high-dose, and inappropriate use of antibiotics can lead to the emergence of multidrug-resistant Salmonella spp., disruption of the intestinal microbiota balance, compromised immune function, and animal toxicity due to excessive antibiotic exposure, antibiotic residues in animal products, and environmental ecosystem degradation.13–15
The World Health Organization lists Salmonella spp. as one of the top four causes of diarrhea globally.16–18 The Centers for Disease Control and Prevention estimates that 1.35 million people contract Salmonella spp. annually, resulting in 420 deaths. Salmonella spp. is considered the most common foodborne organism in imported foods from Africa to the European Union. A large proportion (72.4%) of the foodborne salmonellosis outbreaks were caused by Salmonella enterica serovar Enteritidis.19
Therefore, finding a safe and environmentally friendly treatment method is extremely necessary. Phage therapy has been proposed as an alternative to conventional treatment methods, as bacteriophages can be used to target specific bacteria while leaving beneficial bacteria unharmed.20–23 Phages are highly efficient and specific viruses that infect bacteria as a natural antibacterial agent. In phage therapy, phages target specific multidrug-resistant bacteria without affecting the intestinal microorganisms.22,24,25
Furthermore, phages infect bacteria without altering the intestinal flora and can replicate by infecting the host bacteria, reducing the need for medications. Phages employ a bactericidal mechanism that is completely different from that of antibiotics. They are not constrained by microbial antibiotic resistance, have a short research cycle, low cost, and are easy to update.22,26
In this research, we identified and studied a new bacteriophage, VT223, for its potential in controlling Salmonella enteritidis. We assessed its bacteriolytic activity, ability to combat biofilms, and examined its biological characteristics. Additionally, we conducted a comprehensive analysis of VT223’s genome sequence and compared it with other Salmonella phages such as PIZ_SAE, fmb-p1, SGPC, SHWT1, T102, vB_SenS-EnJE1, and ZCSE9. This information will be valuable for developing phage-based control strategies against various foodborne pathogens.
Materials and Methods
Bacterial cultures and preparations
The bacterial strains utilized in this study were obtained from the American Type Culture Collection (ATCC) and the National Collection of Type Cultures (NCTC). These included Salmonella enterica subsp. enterica serovar Enteritidis ATCC 49223, Salmonella enterica subsp. enterica serovar Typhimurium ATCC 14028, Salmonella enterica subsp. enterica serovar Enteritidis ATCC 13076, Vibrio parahaemolyticus ATCC 17802, Vibrio parahaemolyticus NCTC 10884, Vibrio parahaemolyticus NCTC 10885, Vibrio vulnificus NCTC 11218, Vibrio vulnificus ATCC 29307, Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27853. These strains were cultured in Tryptic Soy Broth (TSB; Himedia, India) and Tryptic Soy Agar (TSB agar; Himedia, India) at 37°C with shaking at 120 rpm for 24 hours.
Isolation and purification of bacteriophage
The bacteriophages were isolated from 310 samples of abattoir wastewater, pond mud, prawn pond water, and diseased shrimp from the Mekong Delta provinces using the double-layer agar method.27 In brief, 2.5 mL of each sample was mixed with 7.5 mL of TSB and kept overnight at 37℃ with shaking at 120 rpm. Afterward, the mixture was centrifuged at 16128 g for 10 minutes, and the resulting phage-containing supernatant was collected. Subsequently, about 200 µL of bacteria (106 CFU/mL) were cultured overnight. Once the bacteria reached the logarithmic phase, they were mixed with 5 mL of preheated semi-solid TSA medium and spread on the TSA agar plate. Approximately 2 µL of the crude filtrate of phage was added to the solidified semi-solid TSA plate and cultured at 37℃ for 24 hours. The plates were observed for transparent areas or plaques at the inoculation point. The transparent plaque was then collected from each plate and inoculated into a 1.5 mL centrifuge tube containing sodium chloride magnesium sulfate (SM) buffer (50 mM Tris–HCl, 100 mM NaCl, 10 mM MgSO4 [pH 7.5]). The solution was centrifuged at 16128 g for 10 minutes, and the supernatant was finally collected. The filtered supernatant was titrated again by the double-layer plaque assay method.27 After being incubated at 37°C for 24 hours, different plaques were selected based on size and transparency and resuspended in 100 µL SM buffer. The purification continued until homogeneously distributed plaque-containing phage isolates were obtained. The purified phage was stored in the SM buffer, mixed with 50% (v/v) glycerol, and kept in the refrigerator at -20 °C until further analysis.
Determination of host range
The host range of the phages was determined using the double-layer plate drop method with the aforementioned strains. In brief, 5 µL of the purified phage culture medium was added dropwise to a TSA plate covered with different serotypes of bacteria. After overnight incubation, the plaques were categorized as clear, turbid, or unresponsive based on their clarity.28 The experiment was repeated three times for reliable results.
Transmission electron microscopy (TEM)
Phage morphology was determined by using transmission electron microscopy. The method followed was as described by Taha et al.29 First, a sample of 10 µL of the phage at a concentration of 109 PFU/mL was stained with 2.5% uranyl acetate. The sample was then placed on a carbon-coated Cu-grid and left to dry for 10 minutes before being examined using TEM. Images of the stained phage were captured at various magnifications using TEM (JEOL 1230, Japan).
One-step growth curve
The one-step growth curve was created based on a previous report with slight modifications.30 A 5 mL of S. Enteritidis ATCC 49223 was incubated at 37°C with shaking at 120 rpm. Then, 1 mL of the culture was transferred into a flask containing 4 mL of TSB medium and incubated with the same shaking conditions until OD600=0.4 (after three hours). Subsequently, 2 μL of bacteriophage was added to the flask containing the bacterial culture, and the phage titer was measured every 10 minutes using the double-layer agar technique. The burst size was calculated as the ratio of the final count of liberated phage particles to the initial count of infected bacterial cells during the latent period.
pH and temperature stability
The thermal stability of the isolated and purified phage samples was determined by culturing them at -20°C, 4°C, 60°C, and 70°C. The phage titer was determined by the double-layer agar method for 30 minutes and 60 minutes after culture.28
The pH stability was determined by mixing the isolated and purified phage samples in a series of test tubes containing SM buffer with different pH values (2, 3, 5, 7, 7.5, 9, and 10 adjusted using NaOH or HCl solution). After culturing at 37°C for 2 hours, the phage titer was titrated by the double-layer agar method.28
Biofilm inhibition assay
Biofilm reduction was conducted in 96-well plates.31 Bacterial strain S. Enteritidis ATCC 49223 was cultured overnight in liquid TSB medium at 120 rpm. Then, 100 µL of bacterial suspension was added to the plates and incubated for 24 hours at 32°C. After incubation, the bacterial suspension was removed, and the plates were washed three times with 0.9% NaCl. Subsequently, 100 µL of phage was added to the plates and incubated at 32°C for 4 hours. The phage and bacteria mixture were then removed and washed twice with 200 µL of 0.9% NaCl. Following this, 0.1% crystal violet was added to the wells and incubated for 20 minutes at room temperature. After incubation, the crystal violet was removed, and the wells were washed three times with 200 μL of 0.9% NaCl. To dissolve the crystal violet, 30% acetic acid was added to each well. Finally, biofilm formation was evaluated by measuring OD590 nm.32
Lytic activity of phage
The lytic activity of phages against S. Enteritidis ATCC 49223 was examined in vitro. Initially, 100 μL of logarithmic-growth phase host bacterial culture (106 CFU/mL) was mixed with an equal volume of phage suspension in a 96-well microplate.33 The OD600 nm was measured at 0, 2, 4, 6, and 24 hours. The experiment was repeated three times to ensure reliable results.
Phage genome sequencing and bioinformatics analysis
The phage nucleotide extraction was performed from a lysate sample with a QIAamp Viral RNA mini kit according to the manufacturer’s instructions (QIAGEN, Germany) and sequenced by next-generation technology with the Illumina MiniSeq system. Metaspades (v3.15.5) with optimized parameters for de novo assembly of DNA sequences BLAST v2.13.0 on the Genbank virus database (updated 06/06/2023) to identify contigs originating from phages. A total of 343,969 reads were utilized for the phage genome assembly. The raw reads were assembled with Unicycler v 0.4.8, and the resulting contigs were annotated with PATRIC using a specialized database for bacteriophage annotation. All tools were run with default parameters unless otherwise specified. ORFs (open reading frames) were found in the bacteriophage genome by searching with GeneMarkS (http://exon.gatech.edu/GeneMark/genemarks.cgi) and ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The putative functions of the ORFs were examined for homologs using BLASTP (Database refseq_protein). A circular map of the bacteriophage genome was generated using CGView (https://proksee.ca/).
Results
Isolation and morphological characteristics of phage VT223
In this study, we isolated phage VT223 from prawn pond water and used S. Enteritidis ATCC 49223 as a host strain. Figure 1A shows that this phage forms transparent circular plaques on an S. Enteritidis bacterial background, typical of a lytic bacteriophage. TEM analysis of purified VT223 particles revealed a structure comprising an icosahedral head with a diameter of 70.72 nm and a non-contractile tail of 119.76 nm (Figure 1B). Based on morphological features, phage VT223 belongs to the Jerseyvirus according to the guidelines of the International Committee on Taxonomy of Viruses.34
Host specificity
Phage VT223 was tested against a panel of ten bacterial strains, and it was found to be effective against three strains of Salmonella: Salmonella enteritidis ATCC 49223, Salmonella enteritidis ATCC 13076, and Salmonella typhimurium ATCC 14028 (Table 1). These results indicate that phage VT223 has a specific infectivity towards Salmonella spp. bacteria.
One-step growth curve
The one-step growth curve of phage VT223 on Salmonella enterica subsp. enterica serovar Enteritidis ATCC 49223 bacteria exhibited a latent period of 30 minutes and a burst period of 100 minutes, resulting in an average burst size of 446 PFUs/cell (Figure 2).
Thermal and pH stability
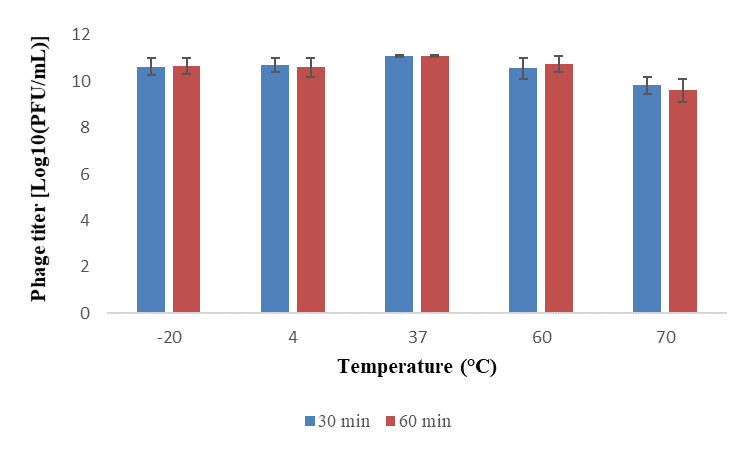
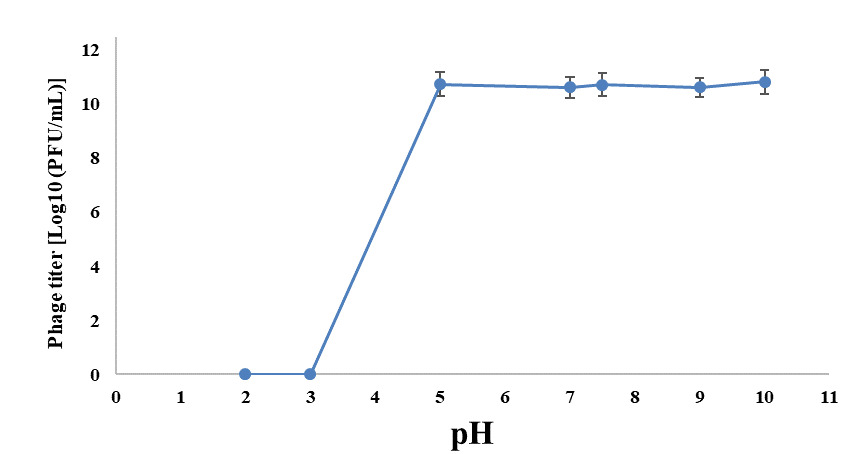
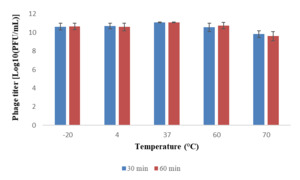
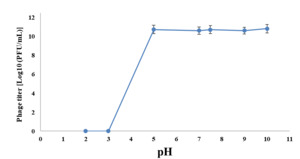
Phage VT223 demonstrated high infectivity even after exposure to extreme temperatures of -20℃ or 60℃ for 30 and 60 minutes, respectively. However, its sensitivity increased at higher temperatures. The phage’s stability decreased gradually at temperatures above 60℃, and its activity decreased with longer incubation times at the same temperature (Figure 3). The isolated and purified phage VT223 was tested for stability at different pH levels (2, 3, 5, 7, 9, and 10, with pH 7.5 as the control). After two hours, the results showed that the phage remained active at pH 5-10 but lost activity at pH 2-4 (Figure 4). Phage VT223 illustrated better stability in alkaline conditions and sensitivity to acidic or strongly basic environments.
Biofilm inhibition assay
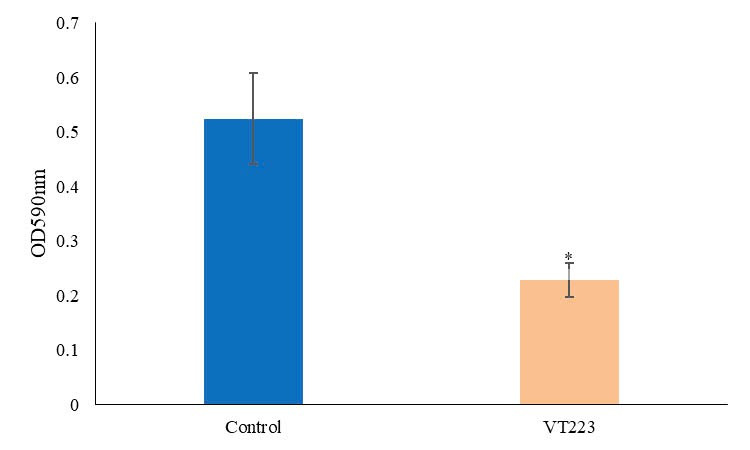
Biofilm of S. Enteritidis ATCC 49223 was grown in 96-well microplates. In the biofilm inhibition assay, phage VT223 was mixed with S. Enteritidis ATCC 49223 before incubation. The optical density (OD) values of S. Enteritidis were measured at a wavelength of 590 nm using a spectrophotometer. The results, depicted in Figure 5, indicate that phage VT223 effectively inhibited the growth of S. Enteritidis bacteria. After four hours of incubation, the OD value decreased significantly from 0.52 to 0.22, representing a 57.7% reduction. This inhibition was statistically significant compared to the control group (p<0.01).
Lytic activity of phage
The results presented in Table 2 demonstrate the lytic ability of phage VT223 on S. Enteritidis ATCC 49223 across five different time points. Phage VT223 exhibited a significant in vitro lytic capacity (P<0.05) after 0, 2, 4, 6, and 24 hours of incubation at 37°C. In the control group at 0 hours, the OD value was 0.68. Following inoculation with phage VT223, the OD value decreased to 0.27. Similarly, at 2, 4, 6, and 24 hours, the OD values were 0.75, 0.82, 0.89, and 1.56, respectively, which decreased to 0.26, 0.35, 0.56, and 0.92 after the addition of phage (Table 2).
Genome features and annotation of phage VT223
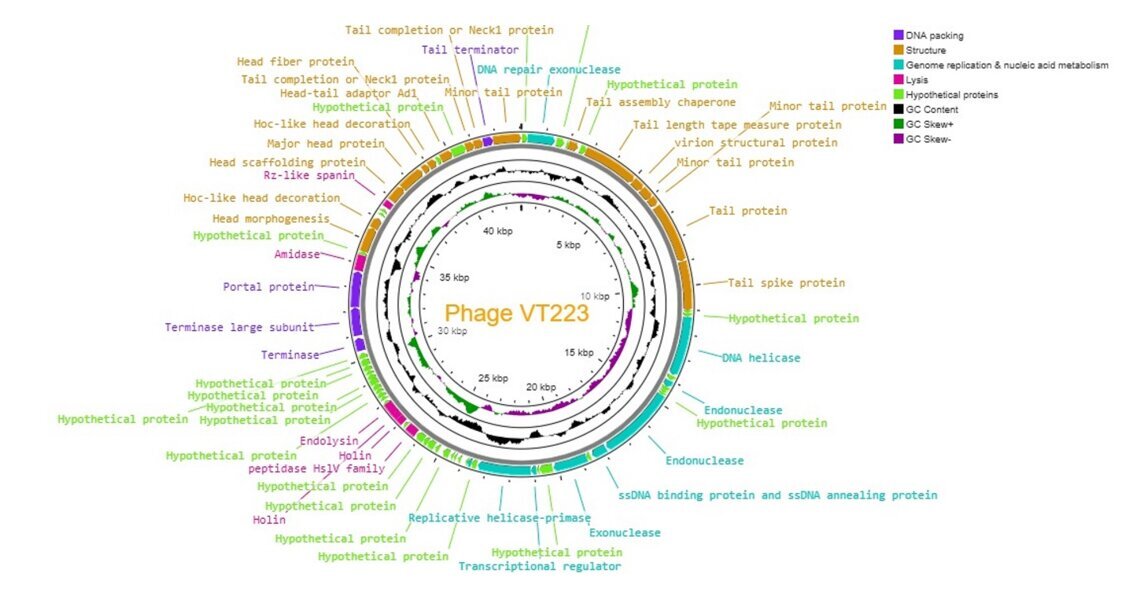
Phage VT223 has a complete genome consisting of a 43,062 bp circular dsDNA molecule with a GC content of 49.6%. It encodes 77 putative open reading frames (ORFs), with 33 ORFs having known functions and 44 ORFs with uncharacterized functions. Among them, 33 ORFs (42.86%) have known functions, while 44 ORFs have uncharacterized functions after BLASTp alignment (Table 3). Based on their function, we grouped the 77 ORFs into four groups based on different functions: structural protein group, genome replication and metabolism-related group, bacteria lysis group, and DNA packing groups. The structural protein group contained minor tail proteins (ORF36, ORF45, ORF46), tail spike protein (ORF48), tail assembly chaperone (ORF41), tail length tape measure protein (ORF43), tail protein (ORF47), tail completion or Neck1 proteins (ORF33, ORF34), head morphogenesis (ORF21), head scaffolding protein (ORF26), head-tail adaptor Ad1 (ORF31), major head protein (ORF27), hoc-like head decoration (ORF22, ORF29), and virion structural protein (ORF28). The DNA packing groups included tail terminator (ORF35), portal protein (ORF18), and terminase (ORF16). The Genome replication and metabolism-related group included DNA repair exonuclease (ORF38), DNA helicase (ORF51), ssDNA binding protein and ssDNA annealing protein (ORF58), replicative helicase-primase (ORF64), exonuclease (ORF60), and transcriptional regulator (ORF63). The bacteria lysis group included Rz-like spanin (ORF25), amidase (ORF19), endolysin (ORF04), holin (ORF02, ORF03), and peptidase Hs1V family (ORF76). The annotation information of VT223 was uploaded to GenBank with the accession number: PP280813. The whole genome is visualized in Figure 6. The genome contains no tRNA genes, lysogenic phage-related markers, toxin genes, virulence genes, or antibiotic resistance genes, making it a potentially safe agent for controlling Salmonella spp. infection.
Comparative genomic and phylogenetic tree construction
The phage genome sequence was compared using the blastn (megablast) program in NCBI. Phage VT223 showed similarities of 78%, 78%, 76%, 76%, 78%, and 87% with Salmonella phage vB_SenS_Psm140 (PP437543.1), phage vB_SenS_Psm147 (PP437545.1), phage JD02 (OL652658.1), phage JD01 (NC_073189.1), phage vB_SalS_JNS02 (PP189839.1), and phage T102 (NC073211.1), respectively (Figure 7). According to the ICTV’s taxonomy rules, viruses are classified as different species if their genomes differ by more than 5% and as different genera if they differ by more than 30%. Therefore, phage VT223 can be identified as a new species within the Jerseyvirus compared to the closest phage T102.
Discussion
Antimicrobial resistance (AMR) has rendered the success rate of antibiotics uncertain for an increasing number of bacterial pathogens. In 2019, over 1.2 million people died directly from AMR bacterial infections. If no action is taken, it is projected that by 2050, 10 million people will succumb to drug-resistant diseases annually. Researchers widely acknowledge the potential of bacteriophages to replace antibiotics in treating bacterial diseases. In addition to their role in food safety, phages could be utilized to detect multidrug-resistant bacteria in environments like hospitals, and they could also be employed for surface decontamination.35 Therefore, isolating new and sensitive phages and studying their physiological and genomic characteristics is imperative to enhance the phage arsenal.
Transmission electron microscopy showed that bacteriophage VT223 is classified within the Jerseyvirus by the International Committee on Taxonomy of Viruses.34 Bacteriophages of the Jerseyvirus are characterized by their non-contractile tailed structure and icosahedral head. The plaque morphology of phage VT223 appears transparent and round when observed against a background of S. Enteritidis bacteria, with a typical size of approximately 3 mm, which is common for lytic phages. Plaques play a significant role in various aspects of phage research. They can exhibit different development patterns based on host, phage, and adhesion conditions. Plaques are easily visible in laboratory settings, making them a convenient tool for studying phage populations in structured bacterial communities. Additionally, plaques are commonly used for isolating, purifying, and classifying phage strains. They also provide valuable insights into the biological properties of phages, including growth curves, temperature sensitivity, and pH effects.36 The morphology of plaques is closely related to key phage characteristics such as the one-step growth curve, adsorption rate, lysis time, and diffusion rate in specific environments.37
The one-step growth curve provides valuable information about the bacteriophage’s activities. In this research, phage VT223 had a latent period of 30 minutes and reached a stable phase at 100 minutes, producing 446 PFU per host cell. The latent period of bacteriophages refers to the time from their entry into the host bacterium to lysis and release of progeny, while burst size indicates the number of newly released bacteriophages by a single host bacterium. The incubation time and the number of phages formed after one life cycle of bacteriophages are important indicators of their ability to combat bacteria.38 This study demonstrates that a shorter latency time correlates with a stronger ability to eliminate bacteria.39
Bacteriophages’ thermal and pH stability are crucial for their effectiveness in biocontrol applications. To assess the viability of VT223 in different environmental conditions, we conducted tests to determine its stability at various temperatures and pH levels. The results showed that VT223 remained highly infectious when incubated at temperatures ranging from -20ºC to 60ºC for 30 and 60 minutes, respectively. However, its activity decreased significantly at higher temperatures. The phage’s stability decreased gradually at temperatures above 60ºC, and its activity declined with longer incubation times and higher temperatures. Regarding pH stability, VT223 showed good activity at pH levels between 5 and 10, but its activity was significantly reduced at pH levels of 2 to 4. At pH 2, the phage was almost completely inhibited. This study’s findings are consistent with previous research indicating that bacteriophages are generally more resistant to alkalis than acids.40,41 For example, the vB_SalP_LDW16 phage was found to be stable at temperatures between 40-60ºC but became less active at temperatures above 60ºC. Its activity significantly decreased at pH levels below 5 and was completely inactive at pH 2.42 On the other hand, the KSL-1 phage is sensitive to temperatures above 80ºC but remains stable in a wide alkaline pH range.43 Similarly, the swi3 phage is stable at temperatures below 50ºC and within a pH range of 6-8,44 while the HY01 phage remains stable at temperatures below 65ºC and within a pH range of 4-11.45 In contrast, the VP06 phage is inactivated at 60ºC but remains stable at pH levels between 7-11 and is inactivated at pH 2-3.46
The stability of bacteriophages is crucial for their effectiveness in various applications.47 Factors such as pH, temperature, and encapsulation can impact their stability and activity. For example, phage VT223 may become inactivated at low pH, but encapsulation can help it survive in the gastrointestinal tract for therapeutic purposes.48 Storage at temperatures below freezing point may affect phage titer, necessitating the use of glycerol for preservation.49 Understanding the physiological conditions and stability of bacteriophages is essential for their successful use in combating bacterial infections. Further research is needed to optimize storage and handling practices to ensure the efficacy of bacteriophages in various applications.49
Phages may offer a valuable solution for managing invasive infections, especially those involving biofilm formation. They have shown potential in combating biofilms, even in cases where their lytic activity is not effective against planktonic bacteria. Phages can penetrate bacterial biofilms effectively, targeting cells within the dense structure and breaking down the extracellular polymeric substances (EPS) that hold the biofilm together.50,51 Studies have demonstrated that the effectiveness of phages in reducing biofilms (by 1 to 6 log) depends on various factors such as the age of the biofilm, the specific phage used, and the duration of treatment.52,53 The treatment with phage LPST153 has been shown to inhibit biofilm formation by 35-45% and reduce existing biofilms by 25-31% on a 96-well microplate.54 In another study, 48-hour biofilms of P. aeruginosa, S. Typhimurium, and S. aureus were treated with a combination of two bacteriophages (PRD1 and P22). The bacterial loads in both planktonic and biofilm forms were monitored for 72 hours. It was observed that the S. Typhimurium biofilm decreased significantly below the control after 8 hours of treatment, but increased above the control with longer incubation time (although not statistically significant). In all tested bacterial species, the biofilm levels were significantly reduced below the control for only 12-24 hours.55 It has been suggested in the literature that a slight increase in biofilm levels after phage treatment may be due to extracellular DNA accumulation from cell lysis caused by the phages.55,56 In our study, phage VT223 was able to reduce biofilm levels by up to 57.7% below the control level after 4 hours. This highlights the potential of phages as a promising approach for combating biofilm-related infections.
The lytic ability of phage VT223 was found to effectively degrade the bacterial strain S. enteritidis in the study. Other studies have also demonstrated similar results, with bacteriophage vB_SalP_TR2 showing the ability to inhibit the growth of Salmonella spp. effectively. In untreated samples, the OD600nm value reached the logarithmic phase after one hour and increased rapidly. However, when treated with bacteriophage vB_SalP_TR2, the OD600nm values remained consistently lower than those of the control treatment (P<0.05).28 Additionally, bacteriophage vB_SalP_LDW16 significantly reduced the bacterial load of S. Enteritidis in the blood, improved pathological changes in the intestines, liver, and heart, and enhanced the growth and development of chickens.42
The genome of VT223 is circular dsDNA with a length of 43,062 bp, which is consistent with most phages of the Jerseyvirus. Although the similarity between the VT223 genome and known Jerseyvirus viruses ranged from 76% to 87%, our collinearity analysis suggests VT223 as a novel Salmonella phage.
Acknowledgments
The authors thank the Department of Research Affairs, the Institute of Food and Biotechnology, and Can Tho University for their valuable support. This study is fully funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106.05-2019.335.
Authors’ Contribution
Conceptualization: Truong T. Bich Van (Lead). Methodology: Truong T. Bich Van (Equal), Nguyen T. Loan Anh (Equal). Writing – review & editing: Truong T. Bich Van (Equal), Nguyen T. Loan Anh (Equal), Vo V. Thanh (Equal). Funding acquisition: Truong T. Bich Van (Lead). Resources: Truong T. Bich Van (Lead). Supervision: Truong T. Bich Van (Lead). Formal Analysis: Nguyen T. Loan Anh (Supporting), Nguyen P. Anh Thi (Equal), Le Viet Dung (Supporting). Writing – original draft: Nguyen P. Anh Thi (Equal).
Competing of Interest – COPE
No competing interests were disclosed.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.

_and_transmission_electron_microscopy_images_of_phage_vt2.png)




_and_transmission_electron_microscopy_images_of_phage_vt2.png)