Introduction
Heat stress due to global warming poses a serious threat to fish, potentially altering growth, development and reproduction, especially cold-water fish such as sturgeon, which are closely linked to ambient temperature (Yang et al., 2022; Zhang et al.1). The effects of heat stress on fish are multilayered. It has been found that heat stress can lead to severe damage to tissue structures such as gills, intestine, liver, and kidney in fish (Yang et al.2; Yang et al., 2021). Heat stress also leads to a significant increase in apoptosis. Studies on Prochilodus lineatus embryos showed that heat stress-induced caspase-3-mediated apoptosis.3 Studies on Sander lucioperca livers revealed that heat stress significantly induced mRNA expression of apoptosis-related genes (p53, bcl-2, bax, apaf-1, caspase-3 and caspase-9) after exposure to 30 ℃, 32 ℃, and 34 ℃ for 2 h, respectively. Meanwhile, the apoptosis level in the liver increased significantly with increasing temperature (Liu et al., 2022). Erythrocytes are carriers of oxygen transport, and hemoglobin plays an important role in the body as a functional unit of red blood cells. The number of blood cells and the amount of hemoglobin in fish can reflect the health status of the fish body to a certain extent.4 It was also suggested that the number of erythrocytes in the blood of fish is mainly influenced by the temperature of the water, and that as the water temperature increases, the number of erythrocytes increases.5 Meanwhile, heat stress induces changes in mRNA expression levels of immune-related genes in fish. On the 7th day after treatment at 25 ℃ and 28 ℃, il-1β mRNA expression levels were significantly increased in gills and liver of Acipenser baerii, as well as tgf-β mRNA expression levels in spleen (Yang et al., 2021).
As a living fossil, the A. baerii is one of the oldest species in the class Actinopterygii. Due to its high nutritional value, it is an economically important fish for caviar production.6 In recent years, the morbidity and mortality rates of A. baerii have risen due to ambient temperatures surpassing their optimal growing conditions (Yang et al., 2022; Chen et al.7). As one of the immune organs in fish, the spleen plays an important role in regulating the body’s immune function against the unfavorable external environment. Currently, relatively little is known about the effects of heat stress on the spleen in fish, especially in the cold-water fish sturgeon.
In this study, we exposed A. baerii to heat stress and used H&E staining, flow cytometry, and qPCR to detect the effects of heat stress on A. baerii spleen. This study revealed the effects of heat stress on the sturgeon spleen and provides guidance for heat stress management in wild and farmed sturgeon.
Materials and Methods
Fish maintenance and treatment protocol
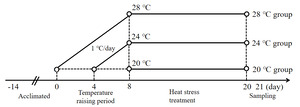
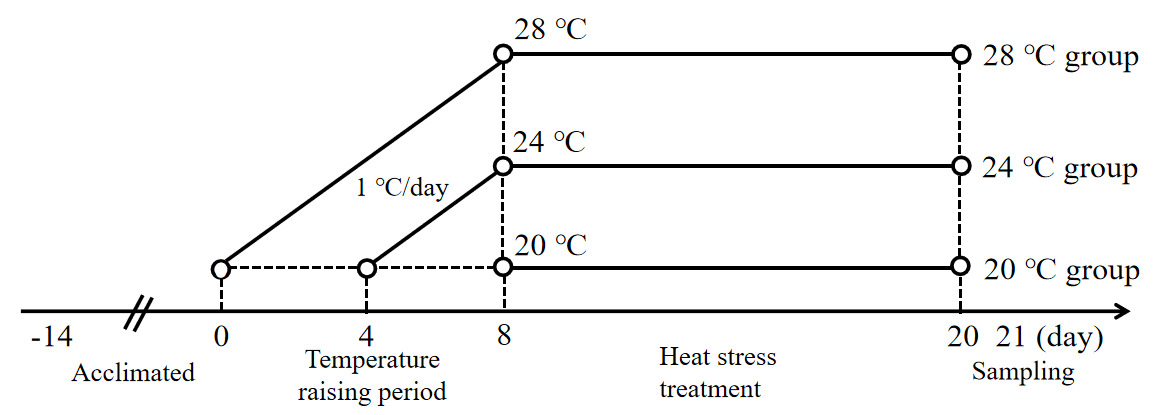
A total of 90 healthy experimental juvenile A. baerii (76.37 ± 9.45 g) were purchased from Sichuan Runzhao Fishery Co., Ltd., Sichuan, China, and cultured in 3 circular PE material tanks (1.5 m diameter and 1 m height) in the laboratory. During the temporary domestication of the fish, water quality parameters, including temperature, dissolved oxygen, and pH, were maintained at 20 ± 0.5 ℃, 7.1 ± 0.8 mg/L, and 7.8 ± 0.3. These fish were fed three times a day (at 8:00,14:00 and 20:00) on a full stomach, and the remaining feed was siphoned out after 30 minutes. After 2 weeks of domestication, 90 fish were randomly divided into three groups (n = 30): a control group (20 ℃) and a heat stress group (24 ℃ and 28 ℃). Each group had 3 parallel replicates (3 tanks) with 10 fish in each tank. Fish in the two heat stress groups were warmed from 20 ± 0.5 ℃ to 24 ± 0.5 ℃ and 28 ± 0.5 ℃, respectively, with a heating rod at a rate of 1 ℃/d for 8 d, and then maintained at the heat stress temperatures for 12 d (Figure 1).
Sample collection
After the heat stress experiment, aseptic sampling was performed on the experimental fish in each group. 15 fish from each group were randomly selected and anesthetized with MS-222. Blood was collected through the tail vein for hematological testing and the fish were dissected for spleen sample collection. Of these spleen tissue samples, 5 of them were stored at -80 ℃ for gene quantification, five were used for flow cytometry to detect apoptosis, and the remaining five were fixed in 10% neutral buffered formalin for at least 24 h for histological observation. All animal handling procedures were approved by the Animal Care and Use Committee of Sichuan Agricultural University, following the guidelines of animal experiments of Sichuan Agricultural University under permit number TCL-2021302188.
Histological observation
The fixed spleen tissues were dehydrated by conventional alcohol gradient, then cleared with xylene and embedded in paraffin. Paraffin sections of 5 μm were prepared and affixed to slides for hematoxylin-eosin (H&E) staining. After sealing with neutral glue, these sections were observed under an optical microscope (Nikon, Tokyo, Japan).
Hematological indicators
The number of RBC were counted with a blood count board (FK-KN253-267, Corning, USA). The hemoglobin (Hb) content of the blood was determined with Hb diluent kit (C021, Nanjing Jiancheng, China) following the instruction manual.
Flow cytometry (FCM) detection of apoptosis
Spleen tissues from five fish in each group were collected and fixed separately in pre-cooled 1× PBS solution for subsequent detection of apoptosis. The kidney was incubated with Annexin V-FITC/PI (Thermo Fisher, Waltham, Massachusetts, USA) for 25 min at room temperature, and the percentage of apoptotic cells was measured using a flow cytometer (Backman, Pasadena, CA, USA). FITC was excited at a wavelength of 488 nm and emitted at 525 nm, while PI was excited at a wavelength of 535 nm and emitted at 615 nm.
For the flow cytometry detection of apoptosis, a single-cell suspension of 100 μL at a concentration of 1 × 106 cells/mL was taken, centrifuged at 300× g for 5 min, and the supernatant was discarded. The cells were then resuspended in 200 μL of freshly prepared 1× binding buffer. Annexin V-FITC staining fluorescent dye (5 μL) was added, and the cells were stained for 10 min at room temperature while being protected from light. Next, PI staining (10 μL) was added and incubated for 5 min at room temperature, avoiding light. Finally, 400 μL of PBS was added, and the cells were resuspended and immediately assayed on the machine. The data were analyzed using Kaluza 2.1 software after being detected by a CytoFLEX flow cytometer (Backman, Pasadena, CA, USA).
RNA extraction and qPCR
The mRNA expression of β-actin, tgf-β, il-1β and il-8 in spleen tissue was detected by qPCR. The Primer 6.0 software was used to design primers (Table 1). Total RNA was isolated from the spleen with an animal tissue total RNA extraction kit (Fuji, Chengdu, China). cDNA was synthesized from 2 μg of RNA using a RT Easy™II kit (Fuji). qPCR was performed using a SYBR green real-time PCR kit (Takara, Kyoto, Japan) and a Thermo Cycler (BioRad, Hercules, CA, USA). The relative expression ratio of target genes was calculated using the 2−△△Ct method and normalized with the expression of the reference gene β-actin.
Statistical Analysis
All data were expressed as mean ± SD. Statistical analysis was conducted using SPSS version 22.0 software, employing one-way ANOVA. To assess the differences between the groups, Least Significant Difference (LSD) was utilized. *p < 0.05, **p < 0.01.
Results
Heat stress damages spleen structure and leads to reticular cell proliferation
It can be seen that the red pulp, white pulp and cell structure of spleen in A. baerii were relatively complete and clear at 20 ℃. However, at 24 ℃ and 28 ℃, the spleen of sturgeon showed vacuolization of the cells, proliferation of reticular cells and macrophage infiltration. Notably, fibrous connective tissue hyperplasia was seen at 28 ℃ (Figure 2).
Effect of heat stress on hematological indicators
With the increase in temperature, the levels of RBC in the blood increased and were significantly higher in group 28 ℃ than 20 ℃ (p < 0.05). While the hemoglobin content tended to decrease with the increase of temperature, there was no significant difference (p > 0.05) (Figure 3).
Heat stress induced apoptosis of spleen cells
To further investigate the effect of heat stress on apoptosis of A. baerii spleen cells, apoptosis was detected by flow cytometry. The apoptosis rate of spleen cells gradually increased with the increase of temperature. The apoptosis rate in the 28 ℃ group was significantly higher than that in 24 ℃ group (p < 0.05), and significantly higher than that in 20 ℃ group (p < 0.01) (Figure 4).
Heat stress leads to an immune response in the spleen
It can be seen that heat stress caused a significant increase in the expression of inflammation-related factor (tgf-β, il-1β and il-8) (p < 0.05), and the increase was linear with temperature. The expression of il-1β in 24 ℃ group was significantly higher than 20 ℃ group (p < 0.05), and the expression of tgf-β, il-1β and il-8 in 28 ℃ group were significantly higher than 20 ℃ group (p < 0.05). The expression of il-1β and il-8 in 28 ℃ group were significantly higher than 24 ℃ group (p < 0.05) (Figure 5).
Discussion
Global warming has increased average summer temperatures and the frequency of extreme weather, posing a threat to the survival of cold-water fish.8 The spleen is important to the organism as an important immune organ in fish. However, there is limited information on the effects of heat stress on the spleen in cold-water fish. Therefore, in this study, we analyzed the changes of heat stress on spleen architecture, apoptosis level and expression of immune factors in A. baerii.
In this study, we observed vacuolization, reticulocyte hyperplasia and macrophage infiltration in the spleen after heat stress at 24 ℃ and 28 ℃, with hyperplasia being more pronounced, especially at 28 ℃. These results suggest that splenic damage becomes more prominent with increasing heat stress temperature. This is consistent with studies on grass carp (Ctenopharyngodon idella) where heat stress caused spleen damage (Liu et al., 2022). It has been shown that spleen structural damage is closely related to apoptosis (Liu et al., 2022; Guo et al.9). In this study, we found that the apoptosis rate of spleen cells in the 28 ℃ group was significantly higher than that in the 20 ℃ and 24 ℃ groups. It indicated that heat stress-induced the up-regulation of apoptosis levels in spleen cells. This is consistent with the study on Sander lucioperca, where heat stress led to a significant increase in the level of apoptosis in its cells with increasing temperature (Liu et al., 2022). This is consistent with the histological observations wherein heat stress treatment at 28 ℃ caused severe damage to the spleen. Erythrocytes are key cells for gas transport and exchange in sturgeon, and changes in their numbers play an important role in maintaining organismal stability (Yang et al., 2021). In this study, it was found that the erythrocyte content of blood increased significantly with an increase in temperature. This is consistent with the study of Oncorhynchus mykiss.10
The mRNA expression levels of immune-related factors such as tgf-β, il-1β and il-8 have been used as an indicator to measure the immune response in fish.11,12 Tgf-β plays an important role in down-regulating the immune response, and il-1β and il-8 are potent pro-inflammatory cytokines.13 In this study, we observed that heat stress resulted in significant up-regulation of tgf-β, il-1β and il-8 mRNA in the spleen. This is similar to the findings in Micropterus salmoides, where tgf-β, il-1β and il-8 mRNA levels were also significantly elevated after heat stress.14 These results suggest that heat stress activates immune responses in fish to defend against unfavorable external environments. The combined analysis suggests that inflammatory cell activation in the spleen may lead to the release of immune-related factors (tgf-β, il-1β and il-8), which in turn induces apoptosis, which may be one of the mechanisms for the increased level of apoptosis induced by chronic heat stress in the spleen.
Our results showed that chronic heat stress caused splenic vacuolization, reticulocyte hyperplasia and macrophage infiltration in Siberian sturgeon. Meanwhile, blood erythrocyte count, apoptosis rate in the spleen and mRNA expression of immune-related factors (tgf-β, il-1β and il-8) were significantly elevated, suggesting that heat stress caused severe damage to the spleen. These findings contribute to the understanding of sturgeon spleen coping strategies to chronic heat stress.
Heat stress induced by global warming is a serious threat to fish growth, development and reproduction. In particular, the impact on cold-water fish is most severe. In this study, only the spleen was investigated, and future studies should combine multiple tissues and organs to better reflect the multilevel effects of heat stress on A. baeri. In addition, the present study was explored by the method of histopathology and biochemical index detection. In the future, it can be combined with multi-omics research, such as transcriptomics and proteomics, which can reveal the mechanism of the spleen of A. baeri to cope with heat stress at the gene and protein levels.
Acknowledgments
We thank all authors for stimulating discussions and support. All authors read and approved the final manuscript. This study was supported by the Sichuan Science and Technology Program (2021YFYZ0015), Project of Sichuan Innovation Team of National Modern Agricultural Industry Technology System (SCCXTD2024-15), 2023 Aquaculture Breeding Research Project, Integration and application demonstration of key technologies for sturgeon breeding (kczx2023-2025-19) and Youth Foundation of Natural Science Foundation of Sichuan Province (2022NSFSC1723, 2023NSFSC5806 &2023NSFSC1218).
Authors’ Contribution
Conceptualization: Chaolun Tan (Equal), Xiaojian Pang (Equal), Shiyong Yang (Equal). Writing – original draft: Chaolun Tan (Equal), Xiaojian Pang (Equal), Jiajin Zhang (Equal). Resources: Chaolun Tan (Equal), Chaozhan Yan (Equal), Zihan Xu (Equal), Wuyuntana Shao (Equal), Jiayun Wu (Equal), Yunkun Li (Equal), Shiyong Yang (Equal). Writing – review & editing: Chaolun Tan (Equal), Shiyong Yang (Equal). Formal Analysis: Xiaojian Pang (Lead). Data curation: Jiajin Zhang (Equal), Chaozhan Yan (Equal). Validation: Jiayun Wu (Lead). Software: Yunkun Li (Equal), Xiaogang Du (Equal).
Competing of Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
This study was conducted according to the guidelines of the Helsinki Declaration, and all animal handling procedures were approved by the Animal Care and Use Committee of Sichuan Agricultural University following the Animal Experiment Guidelines of Sichuan Agricultural University, license no. TCL-2021302188.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
Data available on request due to privacy/ethical restrictions (the data that support the findings of this study are available on request from the corresponding author).

.png)
.png)
.png)
*il-1*_(b)_and_*il-8*_(c)_in_the_spleen_of_.png)
.png)
.png)
.png)
*il-1*_(b)_and_*il-8*_(c)_in_the_spleen_of_.png)