Introduction
To enhance the diversity of species used in aquaculture, it is essential to source species from natural environments. This approach not only ensures the preservation of endangered species but also facilitates the production of endemic species by collecting gametes in their natural habitats. In the capture of these species, especially in freshwater environments, electric currents are employed, with techniques such as electrofishing being the most common in these practices. In electrofishing, since freshwater conducts electric current at a low level, a relatively high voltage is applied. This method allows for the capture of a large number of fish during breeding periods, potentially affecting the gametes of these fish.
The application of electroshock is influenced by several critical factors, including the chemical composition of the water (e.g., temperature and conductivity), as well as parameters such as the duration of the shock and the intensity of the applied voltage. Furthermore, even when using identical equipment in studies, variations in the effects of electric current on fish occur due to differing properties of each water source, such as conductivity.1 In particular, species belonging to the Salmonidae family are more affected by electrofishing compared to other species, and this is also extended to their gametes as well. Nowadays, there are no consistent results regarding the effect of electric currents on fish gametes. In some studies, it has been determined that the survival rates of gametes obtained from fish belonging to the Salmonidae family caught by electrofishing decreased compared to the control groups.2,3 On the other hand, similar results were obtained from the broodstock Chinook Salmon Oncorhynchus tshawytscha caught by electrofishing method in terms of fecundity, fertilized eggs and survival rate of offspring comparing with the control group.4 Eggs were successfully obtained from coho salmon, which had spinal damage due to electroshock application.5 A decrease of sperm motility is suspected in male broodstock fish caught by electrofishing. Only one study examined the effect of electric current on sperm motility under laboratory conditions. In this study spermatozoa motility was determined by exposing it to current at different voltage levels for applications in gene transfer studies.6
There are many evaluation criteria for assessing the quality of fish sperm, but the most important among them is motility performance, especially considering fertilization occurs outside their bodies in the aquatic medium. The chemical and physical properties of water such as temperature, pH, electrical conductivity, osmotic pressure, quality, and concentration of dissolved ions, are among the most important influencing factors for general and aquatic organisms, and their vital activities.7 Also, in freshwater fish, hypo-osmotic shock initiates sperm motility.8 The movement of sperm released by male fish during the spawning season is influenced by the characteristics and nature of their aquatic habitat, directly and indirectly affects the percentage of egg fertility. Additionally, the osmotic pressure of the seminal plasma decreases as a result of reduced ion concentrations due to diffusion in the aqueous medium.9 Therefore, the concentration and composition of ions in this medium affect the rate of the diffusion of seminal plasma, ions and consequently, the activation and motility of sperm.10
The electrolysis of water is the preferred method for conducting studies carried out in sustainable processes across various fields of science.11 Side reactions are not observed during the electrolysis of water, and the pH gradually changes due to the production of hydroxyl and hydroxide ions in electrolysis. At the cathode, H+ ions are reduced by gaining electrons, and H2 gas is formed. In the anode, O2 gas and H+ are formed as a result of the oxidation of H2O molecules. In an electric field, ions are in motion, where the speed of this movement depends on the type of ion, its charge, and the intensity of the field.12
To date, the quality of gametes obtained from fish exposed to electric current has been studied. It has been suggested that sensitivity of broodstock fish to electrofishing can impact gamete quality, potentially leading to significant implications for the continuity of fish populations. Rainbow trout, a member of the Salmonidae family, was selected for this study due to the sensitivity of Salmonidae species and their gametes to electric current applications compared to other species.13 Fish that were not exposed to electric current were used in the study. Sperm from these fish were activated using water exposed to electric current, with electrolyzed water also employed as a fertilization solution. The effect of electrolyzed water on spermatozoa motility, hatching rate, embryo and larval survival rates have been determined. The chemical parameters of water, an aspect often underemphasized in electrofishing but is recommended to be investigated,1 were also examined within the scope of this study.
Materials and Methods
ELECTRIC CURRENT EXPOSURE
Electric current was applied to the well water used for fertilization procedure and the activation solution of spermatozoa in the experimental groups. Well water was transferred to a 600 ml capacity electrophoresis tank (platinum electrodes included) (Thermo Scientific, EASYCASTTM B1) and exposed to 165 V and 3 mA electric current for 15 and 30 minutes. As a result, from the application of electric current at different duration times (15 and 30 minutes), six experimental groups were formed with water samples collected from positive (+), middle of the tank (N), and negative (-) poles [30(+), 30(-), 30N, 15(+), 15(-), 15N]. One experimental group was the control group, and no electric current was applied to this water. Immediately after 15 and 30 minutes of electrical application, water samples were transferred to 1.5mL microtubes to be used in spermatozoa activation and samples to be used in fertilization were transferred to 50 mL falcon tubes. After applying electric currentall water samples were kept at 12 °C for two hours before being utilized for fertilization and sperm activation. Water samples were measured for pH, temperature, dissolved oxygen, and oxidation-reduction potential using a multiparameter device (HANNA, HI98194), and osmolality using a micro-osmometer (Fiske Micro-osmometer 210). All trials were carried out in 3 repetitions.
For all water samples used in experimental groups; Nitrate SM 4500 NO3ˉ - B; chloride SM 4500 Clˉ - B; Carbonate/Bicarbonate SM 2320 – B; Chemical Oxygen Demand (COD) was determined according to SM 5220 – C; K, Mg, Na and Ca 200.7 methods (Telliar and Martin 2001). Analyzes were performed with ICP-OES (Perkin Elmer, Optima 7000DV) system, Multimeter (Mettler Toledo, S213) and Thermo (Orion star, T910).
ANESTHESIA AND GAMETE COLLECTION
The trials were carried out between December 2022-February 2023 in Istanbul University Aquatic Vertebrate Experiment Unit (Sakarya, Turkey). In this study, 3+ years old three male and two female rainbow trout were used (1.6 ± 0.3 kg and length of 54 ± 0.4 cm). Commercial trout feed was used for rainbow trout feeding (44% protein, 18% fat, 1.9% sellulose. Özpekler, Denizli, Turkey). Fish were anesthetized in water containing 0.3 mL/L of 2-phenoxyethanol until gill movements stopped. For the experiment, a quality assessment based on morphological observation was made in the stripping of gamete from the broodstock fish. Since the gamete quality of two female and three male fish separated for the experiment was within the required criteria, they were used in the experiment. Since the first gamete taken from the broodstock fish during stripping were likely to be of poor quality, they were not used in the experiment. Eggs with a regular, circular shape and homogeneous protein content and color were used. Sperma obtained from male were not used in the study with an aqueous, a color other than milky white, a sperm kinematic speed value of less than 50µs and amount of sperm less than 10mL. Gamete collection and the procedures performed in this process were carried out according to the procedure applied by Ahmed et al.14 The obtained semen was collected in glass containers and transferred to Styrofoam boxes with lids containing ice cassettes. Direct contact of ice cassettes with sperm samples was prevented by using filter paper. The osmotic pressure of rainbow trout seminal plasma was determined using a micro-osmometer (Fiske Micro-osmometer 210). For this purpose, sperm samples were centrifuged at 10,000 x g for 10 min (4 °C) (Hettich Universal 32 R). Sperm osmolality was measured at 253.6 ± 4.03 mOsm/kg according to Ozdemir and Ekici15 protocol. Ciereszko and Dabrowski16’s method of counting (Neubauer counting chamber) was used to determine spermatozoa density. The collected sperm was diluted 1,000 times with 7‰ NaCl. The calculation was made using the following formula: semen concentration (ml) = 1000 ×number of counted semen/[area (mm2) × chamber depth (mm) × dilution ratio All trials were replicated 3 times.
EVALUATION OF SPERM KINEMATIC PARAMETERS
Sperm samples were activated with water samples belonging to 30(+), 30(-), 30N, 15(+), 15(-), 15N and control group. Sperm motility parameters were analyzed and recorded in the CEROS II (Hamilton-Thorne) Computer-Assisted Sperm Analysis (CASA) system by a digital camera (U-TV1X-2 Tokyo) attached to the CX41 microscope (Olympus). In the activation of sperm samples, a dilution ratio of 1:20 (sperm: water) was used and sperm kinematic parameters (Average path velocity, VAP; curvilinear velocity, VCL and straight-line velocity, VSL, µm/s) were determined using the standard count chamber slide (4- Chamber slide, 20 micron, Leja Standard Count, Nieuw Vennep The Netherlands).14 The kinematic parameters of the sperm samples of each fish were statistically analyzed ten seconds after the sperm samples were activated with the water (12 °C) of the experimental groups. In the CASA system, 50 μm/s was used as the lower limit of the progressive VAP value. A timekeeper was used to evaluate the duration of spermatozoa motility. Due to the brief motility period of rainbow trout spermatozoa identical tasks in each experiment were conducted for the same researchers. Trials were replicated 3 times.
FERTILIZATION AND HATCHING RATE
Fertilization was performed according to Billard and Jensen.17 Eggs (100 each) from two female rainbow trout were collected. A total of 200 eggs (22 g) obtained from two separate females were pooled in each group. Pooled sperm samples (175 μl) obtained from three male were added to the pooled eggs. For the fertilization, it was used spermatozoa:egg ratio of 3x106:1. About 20 minutes after fertilization, eggs were washed with well water and transferred to the cassettes (600 cm3) in the race-way systems in a single row. The fertilization procedure was carried out in 3 repetitions for each group. After the incubation of the fertilized eggs at 13 ± 0.5 °C, larvae hatched in 25 days. Eggs were found to be in the eyed stage on the 18th day (over 90%). During the process from the fertilization of eggs to the observation stage, unfertilized were removed. The fertilization rate was calculated according to the number of eggs with eyes using the following formula.
Fertilizationrate(%)=Numberofeyed−eggsNumberofestimatedeggsx100
The hatching rate was determined using the number of hatched larvae and the total number of fertilized eggs.
Hatchability(%)=TotalnumberofhatchedeggsTotalnumberoffertilizedeggsx100
STATISTICAL ANALYSIS
Mean and standard deviation (±Sd) values (P<0.05) of all parameters were calculated using Microsoft Office Excel program. Water parameters were calculated using One-way analysis of variance (ANOVA) and Duncan post hoc test using SPSS 21 statistical program, all groups were analyzed. Sperm parameters, fertilization rates, and hatching rates were analyzed using Kruskal-Wallis Test, and Dunn’s post hoc multiple comparisons test for all groups using Minitab 18® software.
Results
WATER PARAMETERS
Chemical analyzes of water were conducted to determine the changes in water parameters resulting after the application of electric current. Differences in water parameter values were found among the anode (+), cathode (-), and the middle of the tank (N) area after the application of electric current (Table 1). Potassium, which has a significant effect on rainbow trout spermatozoa motility, was found to be highest in the 30N group and lowest in the 30(+) group. Also, in the 30(+) group, in addition to sodium and magnesium, the chemical oxygen demand (COD) was measured at the lowest level.
Conductivity, dissolved oxygen, and pH values of water samples to be used in the activation of rainbow trout sperm and fertilization are shown in Table (1). The 15(+) and 30(+) groups detected the highest dissolved oxygen values. Statistically, no difference was observed between 15(+), 30(+) and control groups (P>0.05). The pH value was determined in the highest 30(-) and the lowest 30(+) groups.
EFFECT OF WATER EXPOSED TO ELECTRIC CURRENT ON KINEMATIC PARAMETERS OF SPERM MOTILITY
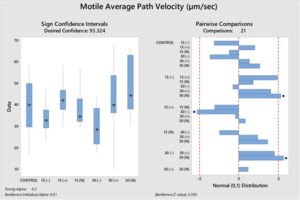
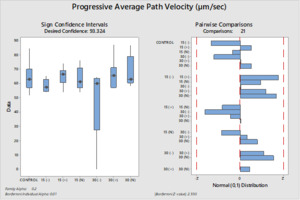
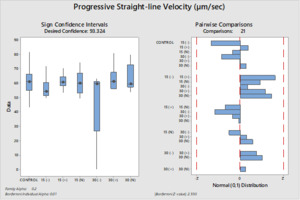
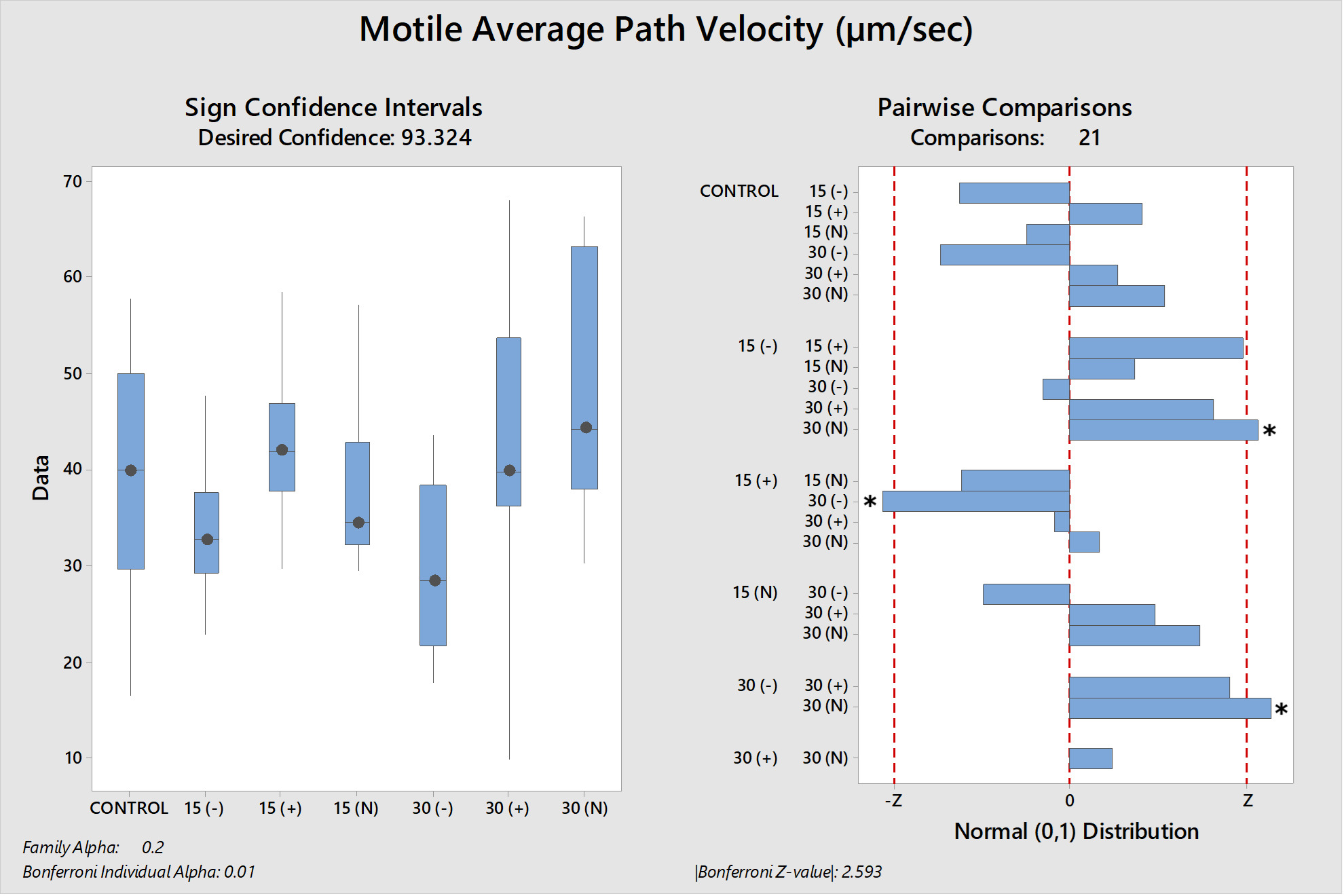
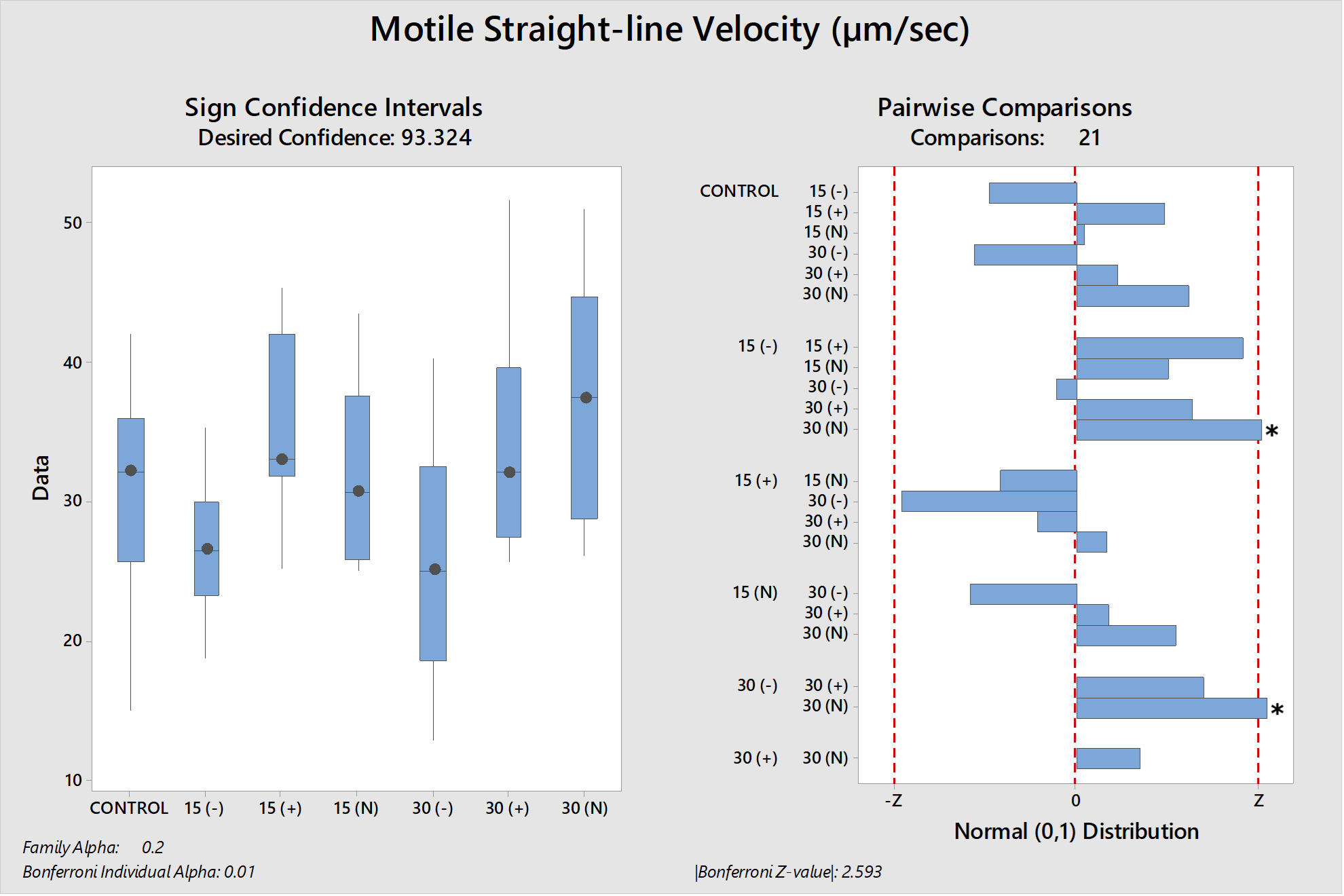
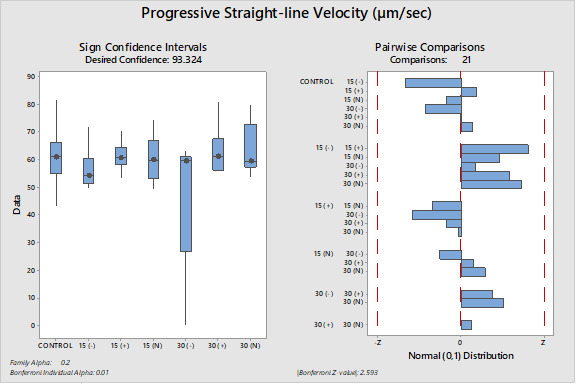
The differences between the groups were determined as a result of the use of well water samples without electric current and water samples exposed to electric current in sperm activation. The use of water samples in which electric current was applied at different duration times (15 and 30 min) in sperm activation, sperm kinematic parameters differed depending on the different regions of the tank where the water samples were taken and the duration time of electric current application. As a result of the use of water samples exposed to electric current for 30 minutes in sperm activation, the best sperm motility parameters were determined statistically [except for the 30(-) group]. In addition, in the control group, motility parameters were determined at the lowest values in sperm activated by water samples obtained from (-) poles, regardless of the duration of electric current application. Almost similar results were obtained in all groups in terms of progressive and motile motility kinematic parameters (Table 2). Progressive, and motile sperm motility values were observed to be highest in the 30N group. Significant differences between groups are shown in figures 1, 2, 3, 4, 5 and 6.
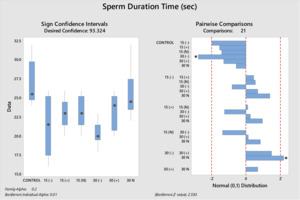
EFFECT OF WATER EXPOSED TO ELECTRIC CURRENT ON SPERM MOTILITY AND DURATION TIME
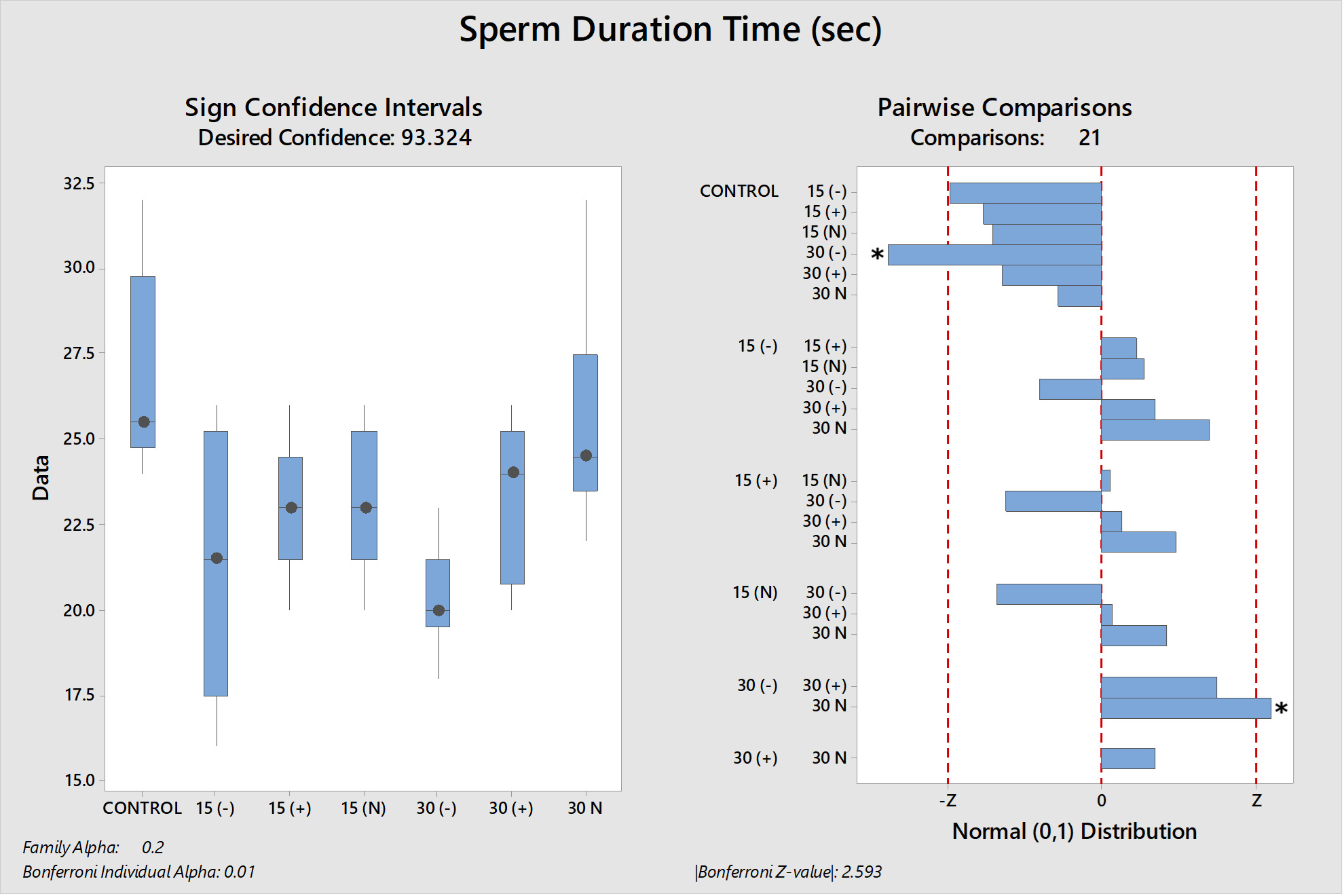
The results of the study suggest that the electric current applied to the water does not positively affect the sperm motility time. The control group statistically had the longest motility period (P<0.05). It was determined that the 30N group, which had the best kinematic parameter values, was the group with the longest sperm motility time among the experimental groups other than the control group. As in other analysis results, the groups with the lowest motility time are the (-) groups, regardless of the electric current application time (Table 3). Significant differences between groups are shown in figures 7 and 8.
FERTILIZATION AND HATCHING RATES
Data on fertilization and hatching rates are shown in Table (3). Although there was no statistically significant difference between the groups.
The fertilization rate has been found to be high especially in groups where electrical current (regardless of the pole from which the water is taken) is applied to the water for 30 minutes. Hatching rate; water samples taken from the middle of the tank (regardless of the duration of electric current application) were found to be high in the groups used in fertilization (15N ve 30N).
A high correlation coefficient was found between in motile sperm motility average path velocity (MVAP), motile sperm motility straight -line velocity (MVSL), with the duration time of spermatozoa motility (correlations of 0.843 and 0.824 respectively). The P-values associated with these correlations were 0.017 and 0.022 respectively, indicating a high confidence in the observed relationship, supporting the statistical significance of these associations at the 5% significance level. (Figure 9).
Discussion
Electrofishing is one of the anthropogenic activities that impacts aquatic life. To date, studies on this subject have primarily focused on electrofishing effects on broodstock fish, as well as the quality of eggs and larvae obtained from these broodstocks. In these studies, the chemical parameters of water were not adequately evaluated. For this reason, the effect of electrofishing on the chemical parameters of water is determined in this study. In addition, After the application of electric current to water, the effect of changes in the chemical structure of the water on fish gametes was examined. Water was electrolysed with the use of an electrophoresis device in the laboratory., Numerous studies have demonstrated the effectiveness of electric currents at various voltages for electrofishing. For consistency with previous research, this study utilized 165 volts, a value commonly employed in similar studies. Additionaly, the use of electrofishing during fishing also affects invertebrates. For this reason, there are studies examining the use of electrofishing in invertebrate catching and its effects on living organisms.18,19 Although studies on marine fish are limited, there are studies on direct exposure of eggs and larvae to electric current20 However, the effect of electrolyzed water on spermatozoa motility is unknown. In this study, a single electric current intensity, but two different application times were used. As a water source, well water was used for rainbow trout spermatozoa activation and fertilization. Spermatozoa were activated by well-water samples from (+) pole, (-) pole, and the middle area (N) between two poles of electrophoresis device after exposure to 165 V at 15 and 30 minutes. The electric current passed easily through water due to the presence of dissolved ions, which facilitate electron transfer.
The results of the effects of electrofishing on fish reproduction, as well as gamete quality, are contradictory. However, it has been stated that electrofishing in spawning areas can damage embryos. Huysman et al.3 reported that the survival rates of eggs obtained from O. tschawytsca were lower compared to the control group, while Tipping and Gilhuly5 successfully obtained eggs from injured broodstock coho salmon through electrofishing. In the study conducted by Tesch et al.,21 an increase in the survival rates of eggs was found in the use below 200 volts. Dwyer and Erdahl22 subjected the eggs of Oncorhynchus clarki to 150 and 225 volts, a similar survival rate at 150V was found compared to the control group. Keefe and Whitesel23 stated that exposing brook trout embryos to 600 V has negative consequences for the survival and development of both embryos and larvae. Cho et al.2 reported that an electric current of 200 V adversely affected the survival rates of chinook salmon embryos. For this reason, 165 V, a value lower than 200 V, was used in our study.
The effective factors in the motility of spermatozoa differ according to living organisms. This circumstance is more diverse among fish species. The final evaluation of the sperm motility rate is determined by the quality of the spermatozoa synthesized inside the testicles of males and by the nature and characteristics of the aqueous medium (osmolality, pH, ion composition and quantity) in which they motile after release from the males.24 Mature sperm are found inside the testicular ducts in the inactive form, surrounded by seminal fluid, which works to adjust the osmotic pressure between the intracellular and extracellular sperm cells to remain the sperm in an immobility state. The cations (sodium, potassium, calcium, and magnesium) considered are the main ionic components of the sperma of rainbow trout, and the potassium ion concentrations have chief role in immobility state of spermatozoa.8,25 When the sperma of freshwater fish mixes with the aqueous medium, the osmotic pressure of the seminal plasma changes and tends to decrease by reducing the concentrations of its ions due to their diffusion in the aqueous medium.8,26 This leads to a change in the osmotic pressure difference between intracellular and extracellular the sperm cells, which is the point of transition of sperm cell from the quiescent phase to the motile phase. Therefore, the concentration and composition of ions in the aqueous medium affect the degree of the diffusion rate of seminal plasma ions and thus the osmotic pressure difference around the sperm and its rate of movement.24 It has been determined that in various species of freshwater fish, sperm motility decreases at values between 0-100 or 200-300 mOsmol/kg.27 In our study; The use of semen with an osmotic pressure of 250 mOsmol/kg with all groups of activation solution with an osmotic pressure of 2.8 ± 1.2 mOsmol/kg has given motility to spermatozoa. Although the osmotic pressure values of the water samples used in the study were quite low, it was observed that it did not adversely affect sperm activation (due to being outside the limit values) as stated by Cosson.27 Although, all water samples groups had similar osmotic pressure values, differences in motility time and velocity parameters were observed between the groups (P<0.05). It was thought that the source of these differences between the groups (P<0.05) may not be related to osmotic pressure because the water samples groups have similar osmotic pressure values. As Morisawa and Suzuki28 similarly stated, our study shows that osmotic pressure only initiates spermatozoa motility, but different factors may have an effect on velocity parameters and motility duration. Seminal plasma osmolality is not a factor that inhibits the motility of trout in the sperm duct, and sodium chloride can slightly stimulate motility.25 Potassium, which is an important component of seminal plasma, is the factor that prevents sperm motility in the sperm duct.25 In addition to the fact that the increase in sodium concentration in rainbow trout decreases potassium sensitivity,25 there is a possibility that the presence of Ca2+ prevents the passage of K+ through the cell membrane. The presence of Na+, K+ and Cl– ions in S.t. macrostigma reduces motility ratios, while the presence of Ca2+ and Mg2+ ions have the opposite effect.24 In rainbow trout sperm was immobile in KCl solution containing 1-300 mmol/kg,25 the K value in all groups was found to be below 1 mmol/kg in our study. Although K+ was the highest in the 30N group (6.73 mg/l) and the lowest value was (0.9 mg/l) in the 30(+) group. The fertilization and hatching rates and kinematic parameters of sperm motility were found to be the highest in the 30N group. K+ and osmotic pressure together are an important ion that controls spermatozoa motility in the Salmonidae family.8 Since the K+ and osmotic pressure values of the experimental groups were outside the limit values specified in the literature,25,27 it shows that the sperm kinematic velocity parameters, fertilization and hatching rate in our study were not determinants. A sperm activation solution in which the ionic concentration is less than the ionic concentration of the seminal plasma, but the concentration of cations (which are similar to the positive ions of the seminal plasma) is slightly higher than the anions, thus reducing the rate of diffusion of seminal plasma ions (mostly cations) in the aqueous medium due to the similar repulsion force between the plasma ions and the medium, which reduces the rate of activation and movement of sperm. Yamaner et al.29 found that; there is an inverse relationship when increasing the concentrations of ionic solutions (NaCl and CaCl2) (high osmosis) as activation mediums with sperm motility (%) of Black Sea trout (Salmo trutta labrax). From Figures (1 and 2); sperm that were activated by dilution with 30N had the highest values of motile and progressive sperm motility (VAP, VCL, VSL) parameters than other treatments. The ions were run to the poles in the electrophoresis tank depending on their charge and application time (15-30 min). Depending on the ion charges they have, the separation of ions other than calcium ions into (+) and (-) poles was relatively achieved in 30 minutes. When the electric current passes for thirty minutes (+ and N groups) and used to activate the sperm, the semen plasma ions spread well and quickly, which reflected positively on the speed of activation and the movement of the sperm (P<0.05).
The 30N group was the group with the highest spermatozoa motility kinematic parameters, fertilization and hatching rates. 30(+) group was in the second rank of experimental groups for fertilization rate. In the 30(+) and 30N groups, chemical oxygen demand (COD) values, which is one of the water pollution parameter indicators, were quite low. The fact that the rate parameters detected in these groups were at the highest level indicates that the Chemical Oxygen Demand (COD) value was likely the determinant of the sperm kinematic velocity parameters and fertilization rate in our study.
Under hypoxic conditions, the spermatozoa of some fish species consume more oxygen and swim faster. This can increase the swimming rate of spermatozoa by increasing the rate of oxidative phosphorylation.30 However, in many fish species, there is also a decrease in the percentage of motile spermatozoa and the velocity of sperm swimming in hypoxic conditions.31 The differences detected in the sperm kinematic velocity parameter values of the water samples groups that were observed to be hypoxic compared to the other groups (P<0.05) showed that hypoxic conditions (at least alone) were not determinants of the velocity values.
Above pH 8.5 in rainbow trout, while an increase in spermatozoa motility was observed at pH 8.18 in rainbow trout26; in our study, a decrease in kinematic velocity parameters and motility duration was detected in rainbow trout spermatozoa under alkaline conditions. Control, 30(-) and 15(-) groups are the groups with the lowest sperm kinematic velocity values and hatching rates. Among these groups, especially in the 30(-) group; It is noteworthy that pH is the highest and conductivity is the lowest (Table 1). Oberlercher and Wanzenböck32 in their study of whitefish, a species belonging to the Salmonidae family, found a decline in survival rates of these eggs when conductivity was measured at 330 μS, but the survival rates of the offspring was similar with the control group. In our study, the 30N group with the highest fertilization and hatching ratio has a conductivity value similar to the Oberlercher and Wanzenböck32’s results. In the 30(-) group with the value of conductivitiy has 178 μS, sperm motility was at its lowest level and fertilization rate was higher than the control group.
It is stated that the application of electrofishing can increase the mortality of trout eggs (Dwyer et al., 1993) These results led us to conclude that the duration of the application of electric current was significant and the ions were dispersed to the poles after 30 minutes. Since the increase of H+ inhibits sperm motility, the accumulation of H+ observed in the cathode with the effect of electric current in the 30(-) group adversely affected the kinematic parameters. These results show that the electric current time has an effect on pH and conductivity. The group of 30(-) with the lowest rate of spermatozoa kinematic parameters was found to have the highest pH, Chloride, Calsium, bicarbonate, nitrate values among the experimental groups. On the other hand, it is stated that pH does not have a significant effect on trout sperm motility.8 In Onchorynchus mykiss, sperm motility increased as a result of the use of a high-pH water samples in the activation of sperm stored at low pH. In addition, it was stated that there was no motility at pH≤7,33 motility values were very low at pH≤7.5. Contrary to these results, in our study, it was determined that the sperm motility parameters in the 30(+) group where the lowest pH value was determined had higher sperm motility kinematic parameters than all groups, except for 30N. O2- accumulation was expected to be observed at the anode, and in our study, these findings were supported by the highest dissolved oxygen levels in the groups (+). At the (-) pole, there is an accumulation of H+. At the cathode, the water was expected to be alkaline, and in our study, it was determined that as the electric current application time was prolonged, the (-) groups gained alkaline properties, the (+) groups gained acidic properties, and the acidic character of the water increased with the prolongation of the electric current application time.
As a result of the study; it was determined that the 30 (N) group has the value of the highest sperm motility kinematic parameters, fertilization and hatching rate. The most noteable result that can be evaluated as different from other groups in the chemical parameters of the water belonging to the 30N group is that the COD is at low levels and the K ion is at high level. Although K-ion has the highest value in groups, it is not at the limit of inhibiting spermatozoa motility. Despite the duration of the applied electric current, the use of water samples taken from different poles, and the changes in the amount of ions in the water, these results did not have a negative effect on the fertilization of eggs and the survival rates of embryos and larvae. Also; while the broodstocks and the offsprings obtained from these broodstock fish can be affected positively/negatively during the application of electric current to the water during electrofishing, the gametes left to this environment after the application of electric current are also positively affected due to changes in the chemical structure of the water.
After all, in aquaculture; it is thought that water exposed to electric current can be used during fertilization. It is thought that the results will be evaluated more clearly by increasing the number of samples and analyzing the waters of different characters in the future studies.
Acknowledgments
We would like to thank Prof. Sabriye Perçin Özkorucuklu for her valuable contributions and guidance during the study process, as well as the staff of Sapanca Inland Fisheries Production Research and Application Unit-IUSUCAN. We would like to thank José Antonio Azpilcueta Vásquez for his grammar and statistical revisions to the article and thanks to Assoc. Prof. Özcan Gaygusuz, we have gained insight into electrofishing.
Authors’ Contribution
Conceptualization: Aygül Ekici (Equal), Mohmmed Abdalftah Hassan Ahmed (Equal). Supervision: Aygül Ekici (Equal), Mohmmed Abdalftah Hassan Ahmed (Equal). Methodology: Aygül Ekici (Equal), Mohmmed Abdalftah Hassan Ahmed (Equal), İlker Keskin (Equal), Suat Özkorucuklu (Equal). Formal Analysis: Aygül Ekici (Equal), Mohmmed Abdalftah Hassan Ahmed (Equal), İlker Keskin (Equal), Suat Özkorucuklu (Equal). Investigation: Aygül Ekici (Equal), Mohmmed Abdalftah Hassan Ahmed (Equal), İlker Keskin (Equal), Suat Özkorucuklu (Equal). Writing – original draft: Aygül Ekici (Equal), Mohmmed Abdalftah Hassan Ahmed (Equal). Writing – review & editing: Aygül Ekici (Lead).
Competing of Interest – COPE
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Conduct Approval – IACUC
The experiments were carried out in accordance with the guidelines approved by the Istanbul University Animal Experiments Local Ethics Committee (IUHADYEK) for animal experimental procedures, and this study received Ethics Committee approval numbered 2020/25 from IUHADYEK. The trials were carried out at Istanbul University Aquatic Vertebrate Experiment Unit.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.