Introduction
As the economy develops, the demand for electricity is increasing, leading to the construction of numerous thermal power plants in coastal areas of China. Currently, most thermal power plants rely on seawater as a cooling source. The favorable conditions of the seawater cooling system, such as temperature, provide a good environment for marine organisms to thrive. These organisms attach to and grow within the cooling system, causing significant operational issues such as pipeline blockages and equipment corrosion, which affect the normal operation of the system. Marine biofouling has become the biggest problem affecting the cooling systems of coastal power plants.1–3
Traditional biofouling control methods in domestic power plants, such as adding liquid chlorine,4 electrolyzing seawater, or directly adding sodium hypochlorite,5 are not very effective in killing biofouling organisms, are not easily degradable, and may cause some pollution to the marine environment.6 They also face issues related to safety, drug resistance, environmental protection, and cost. Mexel®432, a biocide, has been successfully used in military ships, condenser pipelines, air conditioning refrigeration systems, and other industrial and civil fields to control the growth of biofouling organisms.7 The main component of Mexel®432 is alkylamine-3-aminopropane (1.7%). As a surfactant or “amine film”, alkylamine can adhere to wet surfaces such as metal, plastic, concrete, or glass, forming a thin film that prevents microorganisms and biofouling organisms from attaching. Additionally, Mexel®432 is easily biodegradable in water, making it an environmentally friendly compound.7,8 However, the discharge of residues from Mexel®432 during use may have some potential negative impacts on marine organisms.
Studies have shown that the 96-hour LC50 of Mexel®432 for fish typically ranges from 0.26 to 3.70 mg/L.9–11 When treated with different concentrations of Mexel®432 (0.50, 1.00, 2.00 mg/L), the gill tissue Na+/K±ATPase activity and acetylcholinesterase activity in flatfish (Solea senegalensis) were significantly reduced, indicating that Mexel®432 could affect fish osmoregulation. Other studies have found that Mexel®432 treatment can lead to increased glutathione S-transferase (GST) activity and decreased catalase (CAT) activity in the gills of Mediterranean mussels (Mytilus galloprovincialis).12 The effects on heart cell lines of Pacific oysters (Crassostrea gigas) and gill cell lines of Manila clams (Ruditapes philippinarum) increased in a dose-dependent manner.10 While foreign scholars have conducted research on the acute toxic effects of Mexel®432 on marine organisms9,10,12 and its impact on metabolic enzyme activity,12–14 studies on its effects on DNA damage are rare. Moreover, research on the toxic effects of Mexel®432 on Chinese marine organisms is lacking, and there is a shortage of toxicity data on marine organisms.
Haliotis discus hannai Ino, belonging to the phylum Mollusca, is an important economic bivalve mollusk, mainly distributed in the Yellow Sea and Bohai Sea areas of the Liaodong Peninsula and Shandong Peninsula in China.15,16 Mesocentrotus nudus, belonging to the phylum Echinodermata, is an important economic sea urchin in southern China.17 Due to the limited range of activity and poor mobility of Haliotis discus hannai and Mesocentrotus nudus, they are considered ideal bioindicators for monitoring marine pollutants.18
This study used Haliotis discus hannai and Mesocentrotus nudus as experimental organisms and employed the single-cell gel electrophoresis (SCGE) (comet assay) method to detect the genetic damage in different tissues of Haliotis discus hannai and Mesocentrotus nudus after 20 days of exposure to Mexel®432. The study aimed to explore the feasibility of using DNA damage as a biomarker for Mexel®432 exposure and provide scientific evidence for the promotion and application of Mexel®432 in domestic thermal power plants in China.
Materials and Methods
Reagents and Instruments
Mexel®432 was provided by Shanghai Nuclear Environmental Protection Technology Co, Ltd. Low-melting agarose (LMA), normal-melting agarose (NMA), 4,6-diamidino-2-phenylindole (DAPI), Tris-HCl (pH 7.5) buffer, and PBS were purchased from Shanghai Bioengineering Co, Ltd.
DNA damage kits were purchased from Nanjing Jiancheng Bioengineering Research Institute. The analysis was conducted using a TG16-WS benchtop high-speed centrifuge, DYCP-31BN agarose gel horizontal electrophoresis apparatus, Leica DMi8 inverted fluorescence microscope, and LAS X Synapse fluorescence imaging system.
Experimental Organisms
Juvenile Haliotis discus hannai were obtained from an abalone farm in Rongcheng, with an average shell length of 20.70±0.50 mm, shell width of 11.00±0.90 mm, and body mass of 1.76±0.26 g, approximately 12 months old.
Juvenile Mesocentrotus nudus were obtained from a sea urchin hatchery in Rongcheng, with an average shell diameter of 22.74±0.16 mm and body mass of 1.03±0.50 g.
Mexel®432 Exposure Experiment
Haliotis discus hannai were exposed to three concentrations of Mexel®432: high (0.70 mg/L), medium (0.35 mg/L), and low (0.175 mg/L).
Mesocentrotus nudus were exposed to three concentrations: high (1.10 mg/L), medium (0.55 mg/L), and low (0.275 mg/L).
Natural seawater served as the control group, with 50 individuals of each species used per concentration group. The experiment lasted for 20 days, with continuous aeration and daily 100% water changes.
Preparation of Cell Suspensions
Tissue samples were ground into a homogenate and filtered into sterilized centrifuge tubes. The cell suspensions were prepared using pre-cooled PBS buffer and adjusted to a concentration of 1×10⁸ cells/mL.
Single-Cell Gel Electrophoresis
The comet assay was performed with modifications based on Singh et al.'s method. Cell viability was assessed using trypan blue staining.
Data Analysis
CASP software was used to analyze comet images, measuring DNA damage in cells. Data were analyzed using SPSS 20.0, with significance levels set at p=0.05.
Results and Analysis
Impact of Mexel®432 on DNA Damage in Digestive Gland Cells of Haliotis discus hannai
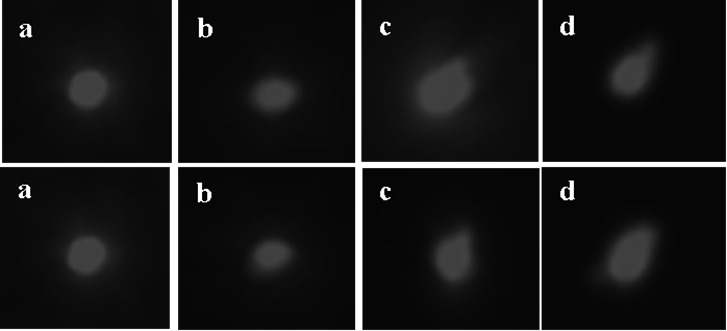
Figure 1 shows the comet assay images illustrating the effects of different concentrations of Mexel®432 on the DNA of the digestive glands of Haliotis discus hannai. As seen in the images, most DNA structures in the digestive glands of the control group were intact, with the nuclei appearing as round fluorescent bodies with uniform intensity and no noticeable tailing (Figure 1a). The degree of DNA damage in Haliotis discus hannai varied with different concentrations of Mexel®432. At a concentration of 0.175 mg/L, the DNA in the digestive glands began to disintegrate, with some tailing observed (Figure 1b), indicating that the DNA had been damaged. As the concentration of Mexel®432 increased, the DNA damage in the digestive glands became more severe, with more prominent tailing and longer comet tails. In the high-concentration group (0.70 mg/L), the tails were diffuse and dim, indicating significant damage (Figures 1c and 1d), demonstrating a clear concentration-effect relationship of Mexel®432 on DNA damage in the digestive glands.
Using CASP software, we analyzed comet tail DNA content, tail length, tail moment, and Olive tail moment to assess the extent of DNA damage in the digestive glands. The data in Table 1 show that at each experimental time point, the indicators of digestive gland cells in the control group of Haliotis discus hannai remained at the same level, indicating no DNA damage (Table 1). After 10 days of the experiment, as the concentration of Mexel®432 increased, the comet tail DNA content, tail length, tailing rate, and tail moment in the digestive gland cells of the experimental groups of Haliotis discus hannai gradually increased, showing a dose-response relationship. Compared with the control group, the tailing rate in the 0.35 mg/L experimental group was significantly higher (p<0.05). After 20 days of the experiment, compared to the control group, there were no significant differences in the indicators of the 0.175 mg/L experimental group (p>0.05); however, the tailing rate in the 0.35 mg/L experimental group was significantly higher than that of the control group (p<0.05), with no significant differences in other indicators. The tail DNA content and Olive tail moment in the 0.70 mg/L experimental group were significantly higher than in the control group (p<0.05).
The experimental results indicate that a medium concentration of 0.35 mg/L of Mexel®432 can cause DNA damage in the digestive glands within a short time, and higher concentrations of 0.70 mg/L of Mexel®432 can exert certain genotoxic effects on Haliotis discus hannai, leading to DNA damage in the cells over prolonged exposure.
Impact of Mexel®432 on DNA Damage in Intestinal Cells of Mesocentrotus nudus
Figure 2 shows the comet assay images that illustrate the impacts of various concentrations of Mexel®432 on the DNA of intestinal cells in Mesocentrotus nudus. In the control group, most of the cells in the intestinal tissue had intact DNA structures, with the nuclei appearing and round fluorescent bodies of uniform intensity and no noticeable tailing (Figure 2a). In the 0.275 mg/L concentration group, the nuclei appeared elliptical with some tailing, indicating that DNA in the nuclei had begun to disintegrate, with some DNA spilling out (Figure 2b). In the 0.55 mg/L concentration group, the number of cells with tailing increased, and the comet tails became longer (Figure 2c). As the exposure concentration increased, the 1.1 mg/L group exhibited more significant tailing and an increase in the number of affected cells, with noticeably elongated comet tails, indicating an increase in DNA fragment breakage (Figure 2d).
After processing the comet images using CASP software, the parameters such as tail DNA content, tail length, tail moment, and Olive tail moment were analyzed using SPSS software, with the results shown in Table 2. At various time points after Mexel®432 treatment (0 days, 10 days, and 20 days), the control group’s intestinal cells exhibited minimal DNA damage, with tail DNA content, tail length, and tail moment values lower than those in the treatment groups. After 10 days of Mexel®432 treatment, as the exposure concentration increased, the tail DNA content, tail length, and tail moment gradually increased, showing a dose-response relationship, although the differences were not significant compared with the control group (p>0.05). After 20 days of treatment with Mexel®432, there were no significant differences in the indicators between the control group and the 0.275 mg/L and 0.55 mg/L concentration groups (p>0.05). However, in the 1.10 mg/L concentration group, the tailing rate and Olive tail moment were significantly higher than those in the control group (p<0.05), with no significant differences in the other indicators (Table 2). The experimental results indicate that prolonged exposure to a high concentration (1.10 mg/L) of Mexel®432 exerts a certain level of genotoxicity on Mesocentrotus nudus.
Discussion
Effects of Temperature Rise on Thermal Shock in Four Fish Species
The primary component of Mexel®432, alkylamine, functions as a surfactant and is known to degrade easily in water. Studies have demonstrated that surfactants have toxic effects on aquatic organisms, especially benthic organisms that lack avoidance responses to pollution.19–21 Once inside an organism, surfactants can trigger the denaturation of metabolic enzymes and proteins, cause significant damage to antioxidant enzyme systems, and induce DNA mutations.22 Research by some scholars on the effects of the surfactant nonylphenol (NP) on the hemolymph cells of the bay scallop (Argopecten irradians) and the Pacific oyster (Crassostrea gigas) showed that NP can cause DNA breakage and damage in hemolymph cells. There is a clear dose-response relationship between NP concentration and DNA damage.23,24
The results of this experiment reveal that as the concentration of Mexel®432 solution increases from 0.175 mg/L to 0.70 mg/L, the DNA integrity of the digestive gland cells in Haliotis diversicolor (small abalone) is significantly lower than that in the control group (p<0.05). The digestive gland cells in the abalone experience varying degrees of DNA fragmentation and damage. This damage is evidenced by an increase in DNA break points, higher comet tail DNA content, and more severe damage as the concentration of Mexel®432 rises (Figure 1). A similar DNA damage effect is observed in the intestinal tissues of Mesocentrotus nudus (northern sea urchin) (Figure 2). However, only when the concentration of Mexel®432 reaches 1.10 mg/L does it cause significant DNA damage to the intestinal cells of the sea urchin (Table 2), indicating a concentration-dependent relationship between Mexel®432 and DNA damage in these two marine organisms.
As the duration of Mexel®432 exposure increases, the compound gradually accumulates in the bodies of both Haliotis diversicolor and Mesocentrotus nudus. Under the action of biotransformation enzymes, reactive oxygen species (ROS) and other metabolites are produced. Intermediate metabolites can bind to DNA, and the resulting free radicals may directly or indirectly lead to DNA strand breaks and damage. If these breaks are not promptly repaired, they can impair DNA function, leading to biotoxicity.25 As shown in Figures 1 and 2 and Tables 1 and 2, high concentrations (0.70 mg/L in Haliotis diversicolor and 1.10 mg/L in Mesocentrotus nudus) result in severe and irreversible genotoxicity. When Haliotis diversicolor is exposed to 0.175 mg/L of Mexel®432 for 10 days, DNA damage in its digestive gland cells is already apparent. In contrast, Mesocentrotus nudus only shows significant damage effects when exposed to a relatively high concentration (0.55 mg/L).
These differences may be attributed to the two species’ distinct ecological habits and physiological structures, leading to variations in their sites and mechanisms of action when exposed to the same xenobiotic, Mexel®432. Haliotis diversicolor appears more sensitive to pollutants, possibly due to its feeding behavior, which involves scraping food with its radula and ingesting large amounts of seawater, and the direct exposure of its foot to the environment, resulting in higher toxicity after prolonged exposure. On the other hand, echinoderms like sea urchins have coelomic cells that can coagulate, initiating immune responses when stimulated externally. Through both cellular and humoral immunity, they can break down exogenous pollutants into harmless substances or expel them directly from the body.26 Additionally, pigment cells release pigment granules that may have protective effects,27 potentially preventing or mitigating bodily damage. Thus, physiological and ecological differences are the main reasons for the varying toxic responses to Mexel®432 among marine organisms.
Authors’ Contribution
Conceptualization: Zhenglou Zhang (Lead). Project administration: Jiaying Cai (Lead). Writing – original draft: Jiaying Cai (Lead), Ke Sun (Supporting). Writing – review & editing: Zhengqiang Miao (Lead), Mei Jiang (Supporting). Methodology: Zheng Tao (Lead), Xianling Liu (Supporting). Supervision: Mei Jiang (Supporting), Lei Li (Lead). Formal Analysis: Ye Wang (Lead), Shouxia Zhao (Supporting). Investigation: Ye Wang (Lead), Shouxia Zhao (Supporting). Resources: Baojun Tang (Lead), Qiyi Chen (Supporting).
Ethical Conduct Approval – IACUC
Our experiment was conducted within the ethical guidelines of the author’s institution and country. We adhered to the Convention on Biological Diversity and the Convention on International Trade in Endangered Species of Wild Fauna and Flora and confirmed that every effort had been made to alleviate any suffering of the animals.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.