Introduction
The Chinese mitten crab, also known as the river crab, belongs to the class Crustacea and order Decapoda. It is a crucial aquaculture species in China, cherished by consumers for its rich nutrition and delicate flavor.1 Currently, the primary method of chinese mitten crab farming, is pond cultivation. However, as the scale of farming rapidly expands, the occurrence of diseases such as saprolegniasis, black gill disease, and limb rot disease has become increasingly prevalent, significantly impacting the profitability of mitten crab farming.2 Although antibiotics and chemical drugs can prevent and treat these diseases, their frequent use leads to decreased immunity in aquatic animals and the accumulation of heavy metals, posing risks to human and animal health. Thus, there is an urgent need to find eco-friendly additives that can enhance the disease resistance of aquatic organisms.
Probiotics are beneficial active microorganisms that colonize the host’s body, altering the composition of microbiota in specific areas to benefit the host. By modulating mucosal and systemic immune functions or balancing gut microbiota, they promote nutrient absorption and maintain intestinal health, leading to positive health effects.3 The gut microbiota refers to the community of microorganisms residing in the intestines, which, together with the intestinal system, form the gut micro-ecosystem, essential for maintaining normal intestinal function.4 The human gastrointestinal tract hosts a diverse and abundant array of microorganisms that impact health through mechanisms such as biological antagonism, immune response, and metabolic regulation.5,6 Research has shown that gut microbiota not only regulates the dynamic balance of the intestine but also breaks down complex polysaccharides in food,7 participates in the digestion and absorption of nutrients, and maintains the integrity of the intestinal epithelial barrier. The structure, function, and bioactive metabolites produced by gut microbiota are of vital importance in preserving intestinal health.8
L. rhamnosus is commonly found in the intestines of humans and animals. Taxonomically, it belongs to the genus Lactobacillus and the rhamnosus subspecies. This Gram-positive probiotic is anaerobic, acid-tolerant, and non-spore-forming, with characteristics such as resistance to acid, bile salts, and various antibiotics. As a member of the human symbiotic microbiome,9 L. rhamnosus has been proven to play a crucial role in human health, such as balancing the intestinal micro-ecosystem and providing both local and systemic immune modulation.10 It can withstand the digestive environment of animals, colonize the intestines of both humans and animals and enhance the host’s systemic immune response.11
Recent studies have demonstrated that L. rhamnosus enhances immunity, thereby improving eggshell quality by modifying the ultrastructure in hens.12 Additionally, L. rhamnosus increases the diversity of intestinal flora and enhances sperm quality in dogs.13 However, their effects on intestinal microbiota and the regulatory immune mechanism in Chinese mitten crab have not been reported. Therefore, this work aimed to evaluate the relationships between intestinal microbiota diversity and antioxidant properties after dietary L. rhamnosus supplementation in Chinese mitten crab.
Materials and Methods
Experimental material
L. rhamnosus was preserved by the Microbiology Research Laboratory of Jiangsu Green Biotech Co., Ltd., with an actual measured value of 1×10⁹ CFU/g. The Chinese mitten crab larvae were sourced from Yangzhou Dacheng Seed Industry Technology Co., Ltd., and the basal feed was procured from Taizhou Alpha Biological Co., Ltd., specifically designed for crab larvae. The primary nutritional levels (mass fraction) of the basal feed’s dry matter are as follows: crude protein ≥ 40.0%, crude fat ≥ 5.0%, crude fiber ≤ 4.0%, crude ash ≤ 14.0%, lysine ≥ 2.2%, calcium ≥ 2.0%, total phosphorus ≥ 1.2%, moisture ≤ 10.0%.
Experimental design and feeding trial
The crab larvae were temporarily reared in canvas tanks measuring 300 cm × 100 cm × 40 cm, and were fed commercial feed during this period. After 10 days of temporary rearing, 120 healthy and similarly vigorous crabs with an average body weight of (0.80±0.12) g were randomly selected from 200 Chinese mitten crabs and divided into 4 groups, including the control group (C) and the L. rhamnosus group, with 4 replicates per group and 8 crabs per replicate. The crabs were individually housed in 180 boxes (28 cm × 21 cm × 17.5 cm, water depth 12 cm) with small holes on all sides and the bottom, placed in polyvinyl chloride (PVC) water tanks (3 m × 0.3 m × 0.8 m) under an indoor recirculating water system. Tiles were placed at the bottom of the boxes as hiding places. During the rearing period, experimental feed was provided daily at 16:00, with a feeding rate of approximately 2% to 3% of the total body mass. Feces and leftover feed were removed every other day using siphon methods. The conditions were maintained with 24-hour aeration, 40W fluorescent lighting (12 hours light/12 hours dark), water temperature > 20°C, pH 7.0–9.0, average dissolved oxygen concentration > 4 mg·L–1, ammonia nitrogen concentration < 0.5 mg·L–1, and nitrite concentration < 0.15 mg·L–1. The body mass of each crab was measured and recorded 3–5 days after molting, and the experiment was concluded once over 80% of the crabs completed their second molt.
The control group (C) was fed the basal diet, while the experimental groups were supplemented with L. rhamnosus at concentrations of 2% (S-1 group), 5% (S-2 group), and 8% (S-3 group) added to the basal diet. The specific method for adding L. rhamnosus involved calculating the required grams of basal feed, the volume of L. rhamnosus solution, and the volume of purified water according to the desired concentration gradient. The prepared L. rhamnosus solution was evenly sprayed onto the feed surface using a spray bottle, ensuring an even coating. The feed was then air-dried naturally before being weighed and administered. The experiment lasted for 40 days.
Sample collection
During the experiment, juvenile crabs in the intermolt stage, approximately 3 to 7 days after completing their second molt, were selected. After wiping off surface moisture with absorbent paper, the crabs were weighed using an electronic balance with an accuracy of 0.01 g. The crabs were then anesthetized on ice, and 0.5 mL of hemolymph was drawn from the base of the third walking leg using a 1.0 mL sterile syringe and placed in a 1.5 mL centrifuge tube, then stored at –20°C. The carapace was separated from the body along the side, and the hepatopancreas was dissected and accurately weighed, and used for subsequent detection and analysis. Intestinal tissues were collected, with three entire intestines from river crabs taken per parallel sample, and placed into sterile EP tubes, then stored at –80°C for future use.
The survival rate (SR), weight gain rate (WGR), specific growth rate (SGR), intermolt duration (ID) [the interval between the second and first molting (days)], and hepatosomatic index (HSI) of each group of juvenile crabs were calculated using the following formulas:
SR = (N2 / N1) × 100%
WGR = [(M2 - M1) / M1] × 100%
SGR (%·d⁻¹) = 100 × (ln M2 - ln M1) / T
Where:
N2 is the number of surviving juvenile crabs at the end of the experiment.
N1 is the initial number of juvenile crabs in each group.
M2 is the body mass of juvenile crabs at the end of the experiment.
M1 is the initial body mass of juvenile crabs.
T is the duration of the rearing period.
MH is the mass of the hepatopancreas.
MC is the body mass of the juvenile crabs.
Antioxidant-related parameters assay
Hepatopancreas homogenate and serum were prepared following the method described by Zhao14 et al. and stored at -40°C for later use. Alkaline phosphatase (APK), aspartate aminotransferase (AST), alanine aminotransferase (ALT), superoxide dismutase (SOD), malondialdehyde (MDA), and catalase (CAT) were measured using assay kits from Nanjing Jiancheng Bioengineering Institute.
Intestinal microflora analysis
Intestinal microbiota analysis was conducted by Shanghai Majorbio Bio-Pharm Technology Co., Ltd. Bacterial genomic DNA was amplified using specific primers 338F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT) targeting the hypervariable V3 and V4 regions of the 16S rDNA gene. High-throughput sequencing of the gut microbiota was performed on the Illumina MiSeq platform. The raw data were processed to obtain optimized sequences, which were then clustered into OTUs at a 97% similarity level. Alpha diversity, including microbial richness (Chao index) and diversity (Shannon index, Simpson index), was analyzed based on OTUs using UPARSE (V7.1) on the Majorbio Cloud Platform (www.majorbio.com). Taxonomic identification results were classified based on OTU annotation, and statistical analysis was performed accordingly.
Statistical analysis
The alpha diversity was calculated using QIIME software, including Chao1, Observed species, and the Shannon index. The PCoA diagram was drawn by R package. SPSS version 20 was used for the data analyses. The microbial community and relative abundance values were tested for significance using the non-parametric Manne Whitney U oneway ANOVA test for independent samples. Mean differences were considered significant at P ≤ 0.05. Tables were generated using Excel 2021, while figures were made using Excel 2021 and GraphPad Prism 9.
Results
Growth performance
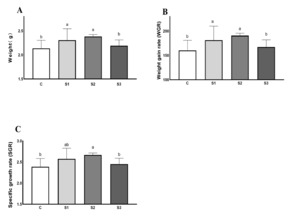
The growth performance of crabs is presented in Figure 1. The FBW, WGR, and SGR of crabs in S1 and S2 were higher than that of crabs in the control group (P < 0.05). There was no significant difference in S3 than that of crabs in the control group (P > 0.05).
Activities of immune- and antioxidant-related enzymes.
The activities of immune- and antioxidant-related enzymes of Eriocheir sinensis changed after dietary L. rhamnosus supplementation. The results showed that the enzymatic activity of immune-related increased after dietary L. rhamnosus supplementation (Figure 2A to F). The enzymatic activity of alkaline phosphatase (AKP) significantly increased between the S1, S2, and S3 compared to the C group (P < 0.05, Figure 2A). while the content of AST in all groups was not significantly different from the C group (P > 0.05, Figure 2B). The content of ALT was significantly decreased in the S1, S2 and S3 group compared with the C group (P > 0.05, Figure 2C). The superoxide dismutase (SOD) activity was significantly increased in the S2 group compared with the C group. The similarity regularity was present in the enzymatic activity of catalase (CAT) (Figure 2E). However, the enzymatic activity of malondialdehyde (MDA) was not significantly different in S1 and S3 comparison with the C group (Figure 2F).
OTU clusters and alpha diversity of microbiota in intestinal contents
To explore the diversity of intestinal microbiota of Chinese mitten crab after dietary L. rhamnosus supplementation, the intestinal microbial composition and species distribution of Chinese mitten crab was investigated by 16s rRNA sequencing. The samples from the intestine of the Chinese mitten crab were analyzed by assigning OTUs at the 97% identity level. The rarefaction curves indicated that there were sufficient samples of the bacterial communities (Figure 3H). Our results showed that the ace, chao1, shannon and sobs indices of alpha diversity were increased after dietary L. rhamnosus supplementation (Figure 3A-3C, 3E). However, the simpson index shows no significant differences between them (Figure 3D). The results of difference analysis showed that there were many significantly different genera after dietary L. rhamnosus supplementation, wherein 244 genera were same between the S2 group and control group (Figure 3G).
Analysis of Beta diversity of bacteria and microbial composition differences after dietary L. rhamnosus supplementation
The separated principal co-ordinates analysis (PCoA) plot of overall diversity based on weighted normalized unifrac revealed that the two groups had different microbiota composition (Figure 4A-B). The taxonomic classification displayed that Proteobacteria, Bacteroidota, Firmicutes, Actinobacteriota and Chloroflexi are the dominant phyla in intestine of this species (Figure 4C-D). The statistical analysis exhibited that dietary L. rhamnosus supplementation enhanced the abundance of Proteobacteria and Firmicutes, reduced the abundance of Actinobacteriota in intestine compared with the control (Figure 4E,P < 0.05). At the genus level, the taxa whose abundance values are in the Top 10 were Dysgonomonas, Candidatus Bacilloplasma, Roseimarinus, Paracoccus, Thiothrix, Alphaproteobacteria, Rhodobacter, Shewanella, Acinetobacter, Pragia in the intestine of this species. The statistical analysis displayed that dietary L. rhamnosus supplementation increased the abundance of Paracoccus and Thiothrix in intestine of crabs (Figure 4F, P < 0.05).
Analysis of microbial composition differences groups under L. rhamnosus supplement
LEfSe analysis was performed to explore the key microbiota of the changes among the groups. As presented in Figure 5A-C, two genera were markedly enriched in the S2 group, including Rhizobiaceae and deonella.
Discussion
An increasing body of research has shown that probiotics can enhance fish growth performance, improve feed efficiency, boost immune responses, and alter gut microbiota, typically following treatment with a single probiotic species.15 Earlier studies have demonstrated that the inclusion of L. rhamnosus in the diet can significantly enhance body weight gain and augment the immune response in chickens.16 For the first time, the present study was designed to examine the role of 2%, 5%, 8%, 109 CFU/g L. rhamnosus inclusion in the diet of crabs. Our results showed that L. rhamnosus supplementation in the diet enhanced the FBW and SGR in Chinese mitten crabs, indicating that L. rhamnosus could improve the growth of this species. This observation is in agreement with earlier studies that probiotics (Lactobacillus sp.) supplementation significantly increased the growth performance of gilthead sea bream (Sparus aurata),17 red sea bream (P. major),18 and olive flounder (P. olivaceus).19 In addition, the enhanced growth of crabs can be ascribed to improvements in immune function, antioxidative ability, and intestinal microbiota.
Like other crustaceans, Chinese mitten crabs mainly rely on their non-specific immune system, including various endogenous expression or inducer factors, as well as the activities of various immune-related enzymes against pathogens. Serum AST, ALT, AKP, SOD, CAT, and MDA, which are considered vital indicators for assessing the immune status in crustaceans, AST and ALT are the two most important transaminases in fish. In this experiment, there was no significant difference in AST and ALT levels between the experimental groups and control group, indicating that the crab liver’s metabolic function was normal. During normal metabolic processes in the body, there is a dynamic balance between the production and metabolism of reactive oxygen species (ROS), which include superoxide anion (O2-), hydrogen peroxide (H2O2), ion (•OH), and others. Excessive free radicals can lead to lipid peroxidation, and O2- can be produced during immune activities such as phagocytosis and secretion of cytokines. The antioxidant enzyme system composed of superoxide dismutase (SOD), and catalase (CAT) can clear excess free radicals and play an important role in clearing oxidative stress, enhancing the defense capacity of phagocytic cells, and improving the body’s immune function. The results of this experiment demonstrate that the S2 group exhibited a significant increase in the CAT and SOD levels, indicating that L. rhamnosus addition to the diet can enhance the antioxidant capacity of the crab. Dawood and colleagues discovered that supplementing red sea bream feed with L. rhamnosus significantly enhances serum superoxide anion radical scavenging activity and lysozyme activity, thereby boosting the fish’s immune and antioxidant capabilities.20 Consequently, this study corroborates the finding that L. rhamnosus can enhance the organism’s antioxidant capacity. MDA, the end product of lipid peroxidation, often indicates the extent of lipid peroxidation in the body and indirectly reflects the degree of cellular damage. The results of this experiment show that as the levels of L. rhamnosus in the feed increased, MDA levels did not change significantly, indicating that the addition of L. rhamnosus did not cause any damage to the organism.
The intestinal microbiota, often described as the “hidden organ” or “organ within an organ,” hosts tens of trillions of microorganisms residing in the host’s intestine, playing a role in metabolic health that rivals the importance of the organs themselves.21 However, the composition of intestinal microbiota varies between individuals, influenced by both genetic and environmental factors, with dietary habits being a key environmental determinant.22 Therefore, 16S rRNA gene sequencing was conducted to detect variations in intestinal microbiota composition across different diets. In present study, the increase in the shannon and chao1 indexes after L. rhamnosus treatment indicated an increase in microbial diversity and richness; the increase in intestinal microbial diversity can enhance nutrient absorption, facilitate the digestion of food, and bolster immunity.23 This is consistent with the findings of the separated PCoA in the present study.
Firmicutes, Proteobacteria, Bacteroidota, and Actinobacteriota have been identified as the dominant microbiota in the intestines of crustaceans.24 Similarly, our study detected that Firmicutes, Proteobacteria, Bacteroidota, and Actinobacteriota are the predominant phyla in the intestinal tract of Chinese mitten crabs, further validating the reliability of our findings. Firmicutes, which include many probiotics such as Lactobacillus, Enterococcus, and Bacillus, play a crucial role in aiding digestion through the release of various digestive enzymes, preventing gut diseases by inhibiting pathogen adhesion in aquatic animals, modulating intestinal mucosal immunity, and maintaining intestinal barrier function.25 Previous studies have demonstrated that a higher ratio of Firmicutes to Bacteroidetes in the gut microbiota of animals is associated with improved nutrient digestibility and greater weight gain.26 In this study, we observed an increased abundance of Firmicutes and a decreased abundance of Bacteroidetes, which may enhance nutrient utilization and, consequently, promote the growth of crabs fed with L. rhamnosus.
Candidatus Bacilloplasma, a commensal microorganism in the gastrointestinal tract, plays a role in the digestive system of crustaceans and is considered a novel lineage of Mollicutes.27 Previous research has shown that Candidatus Bacilloplasma is closely linked to the digestive function of Chinese mitten crabs.28 In our study, Candidatus Bacilloplasma was identified as the dominant genus in the intestines of crabs in the L. rhamnosus treatment group, correlating with the growth observed in this research. Furthermore, prior studies indicated that Candidatus Bacilloplasma positively influences the maintenance of intestinal microbiota in shrimp suffering from acute hepatopancreatic necrosis disease.29 However, whether Candidatus Bacilloplasma could be utilized as a probiotic, as well as its specific role among intestinal bacteria, requires further investigation.
Conclusion
In conclusion, the incorporation of L rhamnosus into the diet of Chinese mitten crabs significantly enhanced growth performance, as evidenced by increased final body weight, weight gain rate, and specific growth rate. The study also demonstrated that L. rhamnosus positively influenced intestinal function by promoting beneficial microbial diversity and enhancing the activity of immune-related enzymes. Notably, dietary supplementation altered the intestinal microbiota composition, increasing the abundance of Proteobacteria and Firmicutes while decreasing Actinobacteriota. These findings underscore the potential of L. rhamnosus as a valuable probiotic to improve growth performance and bolster non-specific immunity and antioxidant functions in the Chinese mitten crab, highlighting its promising applications in aquaculture practices.
Acknowledgments
This work was supported by School-level scientific research project of Jiangsu Agri-animal Husbandry Vocational College (NSF2024ZR09); Development and application of aquatic functional bacteria and algae (NSF2023TC05); Jiangyan District Science and Technology Support Program (Agriculture) (TN202434); Horizontal Enterprise Cooperation Projects (S20240093).
Authors’ Contribution
Conceptualization: Zhenfei Yang (Equal), Haiyue Cao (Equal). Formal Analysis: Zhenfei Yang (Equal), Haiyue Cao (Equal). Investigation: Zhenfei Yang (Equal), Haiyue Cao (Equal). Writing – original draft: Zhenfei Yang (Equal), Haiyue Cao (Equal). Software: Zhenfei Yang (Lead). Methodology: Jianguo Wang (Equal), Apeng Lin (Equal). Writing – review & editing: Wei Li (Lead). Funding acquisition: Lin Song (Lead). Supervision: Xiaofeng Tang (Lead). Project administration: Fugang Qi (Lead). Data curation: Pingping Meng (Lead). Resources: Zhongyu Feng (Lead).
Competing of Interest – COPE
No competing interests were disclosed
Ethical Conduct Approval – IACUC
This study involving animals has consulted the ‘Animal Research: Reporting In Vivo Experiments’ (ARRIVE) guidelines 2.0, ensuring that the data from animal experiments can be thoroughly scrutinized and utilized.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.




_the_taxonomic_cladogram_from_lefse_analysis._(b)_(c)_the_histogram_of_lda_score_from_l.jpeg)




_the_taxonomic_cladogram_from_lefse_analysis._(b)_(c)_the_histogram_of_lda_score_from_l.jpeg)