Introduction
The Darkfin hind (Cephalopholis urodeta), also known as the Darkfin Grouper, is a marine fish species belonging to the family Serranidae. This species is widely distributed throughout the Indo-Pacific region, including the coral reefs of the Spratly Islands in Vietnam.1 As a commercially important fish and a key component of coral reef ecosystems, understanding the genetic diversity of C. urodeta is crucial for its conservation and sustainable management.
Genetic diversity is fundamental to species survival and adaptability, particularly in the face of environmental changes and anthropogenic pressures.2 In marine ecosystems, genetic diversity plays a vital role in maintaining population resilience, evolutionary potential, and ecosystem stability.3 For species like C. urodeta, which inhabit fragmented reef environments, genetic diversity can provide insights into population structure, connectivity, and local adaptation.4
Mitochondrial DNA (mtDNA) markers, particularly the Cytochrome c Oxidase subunit I (COI) and Cytochrome b (Cyt b) genes, have been widely used in genetic diversity studies of fish species.5 These markers are valuable due to their high mutation rates, maternal inheritance, and lack of recombination, making them suitable for assessing genetic variation within and between populations.6
Previous studies on the genetic diversity of groupers in the Indo-Pacific region have revealed complex population structure and connectivity patterns. For instance, Chen et al.7 found significant genetic differentiation among populations of the redspotted grouper (Epinephelus akaara) in the East Sea; Liu et al.8 reported genetic structuring and the presence of cryptic species in the neon damselfish (Pomacentrus coelestis) in the West Pacific Ocean; Jackson et al.9 reported high genetic diversity and population structure in the camouflage grouper (Epinephelus polyphekadion) across the Indo-Pacific. Similarly, Ma et al.10 found significant genetic differentiation in the leopard coralgrouper (Plectropomus leopardus) across the East Sea. However, specific studies on the genetic diversity of C. urodeta in the Spratly Islands are limited, highlighting a significant gap in our understanding of this species in this region.
The Spratly Islands in the East Sea represent a unique and ecologically important area. The complex oceanographic conditions and isolated nature of these islands may influence the genetic structure of marine populations.11 Understanding the genetic diversity of C. urodeta in this region is important for local conservation efforts and contributes to our broader understanding of reef fish population dynamics in the East Sea. Despite their ecological importance, the Spratly Islands face mounting threats from overfishing, pollution, and climate change.12 Therefore, assessing the genetic diversity and population structure of key species like C. urodeta is crucial for conservation efforts in this region.
This study aimed to investigate the genetic diversity and population structure of C. urodeta in the Spratly Islands using COI and Cyt b gene markers. Specifically, this study aimed to: (1) determine the haplotype and nucleotide diversity of C. urodeta populations in the study area; (2) analyze the distribution and patterns of genetic variation within these populations (3) investigate the phylogenetic relationships among the identified haplotypes and compare them with previously reported sequences; (4) evaluate the implications of our findings for the conservation and management of C. urodeta in the Spratly Islands.
By addressing these objectives, this study will provide valuable insights into the genetic structure of C. urodeta in a key part of its range, contributing to our understanding of reef fish population genetics in the East Sea and informing future conservation strategies for this important species.
Materials and Methods
Sample collection
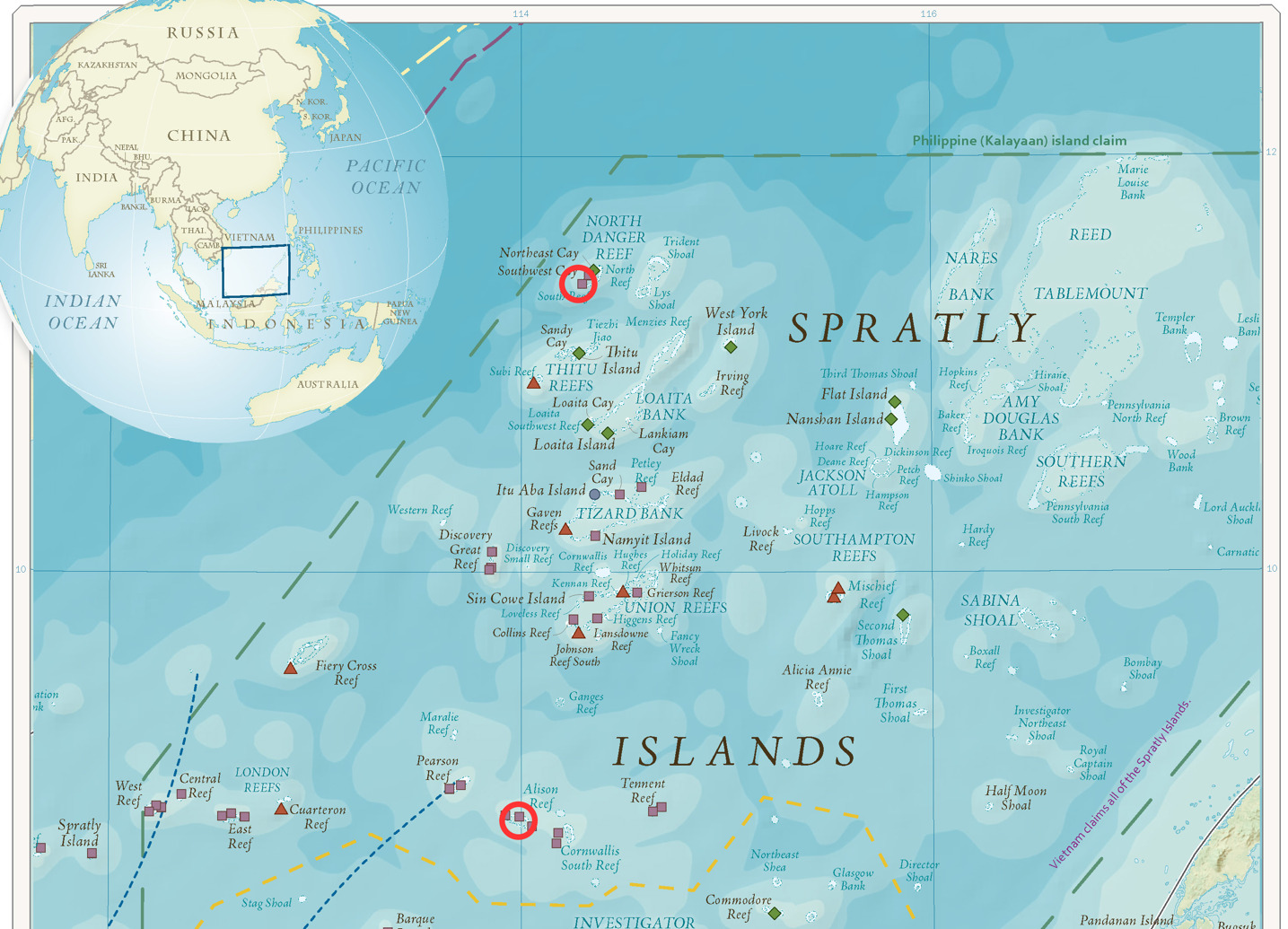
A total of 30 specimens of C. urodeta were collected from various locations within the Spratly Islands of Vietnam in August 2022. This study focused on samples collected from Song Tu Tay Island (Southwest Cay) and Toc Tan Reef (Alision Reef) (Figure 1). Sampling sites were selected to represent different reef habitats across the archipelago. Fish were caught using hook and line or hand spears. A small piece of muscle tissue (approximately 1 cm³) was excised from each specimen and stored at -20°C until DNA extraction. Voucher specimens were photographed, labeled, and deposited in the Ho Chi Minh City University of Education for future reference.
DNA extraction and amplification
Genomic DNA was extracted from muscle tissue samples using the the TopPURE® Genomic DNA Extraction Kit (spin-column method) following the manufacturer’s instructions. The quality and quantity of extracted DNA were assessed using a NanoDrop spectrophotometer and gel electrophoresis.
Two mitochondrial genes were targeted for amplification: Cytochrome c Oxidase subunit I (COI) and Cytochrome b (Cyt b). The COI gene was amplified using the universal fish primers FishF1 (5’-TCGACTAATCATAAAGATATCGGCAC-3’) and FishR1 (5’-ACTTCAGGGTGACCGAAGAATCAGAA-3’).13 For the Cyt b gene, the primers Cyt01F (5’-ACCATCGTTGTTATTCAACTACAAAAACCC-3’) and Cyt34R (5’-AAACTGCAGCCCCTCAGAATGATATTTGTCCTCA-3’) were used Linacre and Lee.14 PCR amplifications were performed in 25 μL reactions containing 12.5 μL of MyTaq™ Mix (Meridian/Bioline), 1 μL of each primer (10 μM), 2 μL of template DNA (approximately 50 ng), and 8.5 μL of nuclease-free water. The thermal cycling conditions for COI were as follows: initial denaturation at 95°C for 5 minutes, followed by 35 cycles of 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 30 seconds, with a final extension at 72°C for 7 minutes. For Cytb, the PCR conditions were as follows: initial denaturation at 95oC for 4 minutes, followed by 35 cycles of 95°C for 15 seconds, 55°C for 15 seconds, and 72°C for 10 seconds, with a final extension at 72°C for 5 minutes.
Sequencing
PCR products were purified using a PCR purification kit (Qiagen) and sequenced bidirectionally using the same primers used for PCR amplification. Sequencing was performed on a Thermo Fisher at DNA Sequencing Company Limited.
Sequence Alignment and Haplotype Identification
Raw sequences were edited and assembled using FinchTV 1.4. The sequences were aligned using the ClustalW algorithm implemented in MEGA X.15 To eliminate areas with missing data, aligned sequences were trimmed to a uniform length of 650 base pairs for COI and 467 base pairs for Cyt b. Haplotypes were identified using DnaSP v6.16
Genetic diversity indices
Genetic diversity indices, including haplotype diversity (Hd), nucleotide diversity (π), and the average number of nucleotide differences (k), were calculated using DnaSP v6. The number of polymorphic sites and synonymous and non-synonymous substitutions were also determined.
Phylogenetic analysis
The best-fit nucleotide substitution model for each gene was determined using jModelTest 217 based on the Akaike Information Criterion (AIC). Phylogenetic trees were constructed using the Maximum Likelihood (ML) method in MEGA X with 1000 bootstrap replicates. Additional sequences of C. urodeta and related species were retrieved from GenBank for comparative analysis. Cyprinus carpio (GenBank accession: NC001606) was used as an outgroup.
Population Structure Analysis
An Analysis of Molecular Variance (AMOVA) was performed to assess population structure using Arlequin v3.5.18 Pairwise FST values were calculated to estimate genetic differentiation between sampling locations. A haplotype network was constructed using the median-joining method in NETWORK v1019to visualize the relationships among haplotypes.
Results
Genetic diversity and haplotype analysis
A total of 30 C. urodeta individuals were successfully sequenced for the COI and Cyt b genes, respectively. A high level of genetic diversity was observed for the COI gene, with 20 unique haplotypes identified (Table 1). Haplotype diversity (Hd) was 0.9563±0.0226, indicating a high level of haplotype richness within the sampled population. The most common haplotype, Hap_2, was found in 16.67% of the samples, followed by Hap_1, present in 13.33% of the individuals. The remaining haplotypes were relatively rare, with frequencies ranging from 3.33% to 6.67%.
The COI gene’s nucleotide diversity (π) was calculated to be 0.010199±0.005561, indicating a moderate level of nucleotide variation within the population. The average number of nucleotide differences between haplotypes (k) was 6.048276±2.963196, further supporting the presence of substantial genetic variation within the COI gene.
The Cyt b gene analysis revealed 16 unique haplotypes from the 30 sequenced individuals, indicating slightly lower haplotype diversity than the COI gene (Table 2). However, haplotype diversity (Hd) remained high at 0.9402±0.0229, suggesting considerable genetic variation within the Cyt b gene. Like the COI dataset, the Cyt b haplotypes showed a skewed frequency distribution, with Hap_5 being the most frequent, occurring in 16.67% of the samples.
Nucleotide diversity (π) for the Cyt b gene was estimated to be 0.007358±0.004530, indicating a lower nucleotide variation level than the COI gene. However, the average number of nucleotide differences between haplotypes (k) was 2.531034±1.400415, suggesting the presence of moderate genetic diversity within the Cyt b gene.
Nucleotide composition and polymorphic Sites
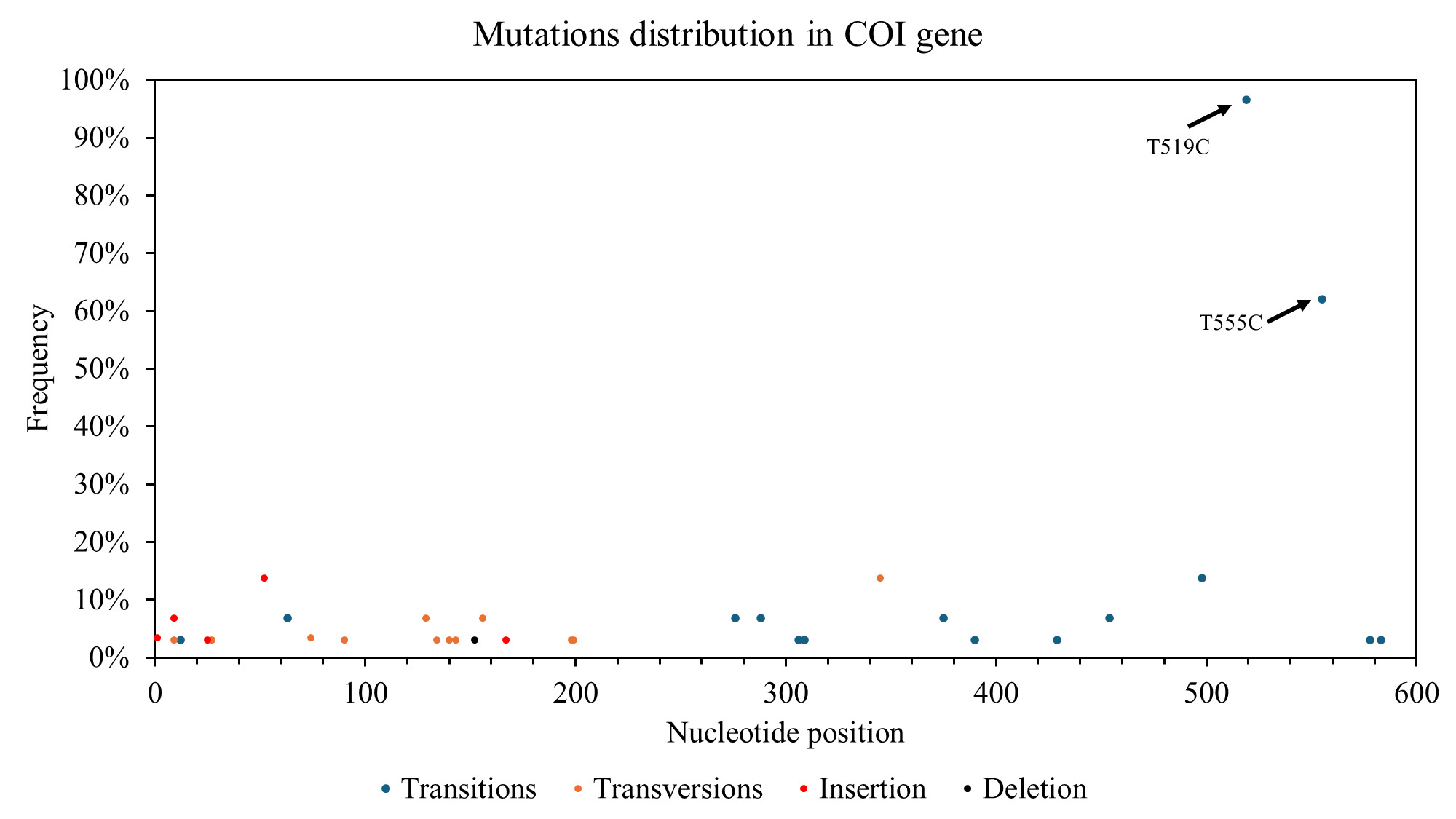
The nucleotide composition analysis for the COI gene revealed a higher proportion of thymine (T) at 31.86%, followed by cytosine (C) at 27.39%, adenine (A) at 23.85%, and guanine (G) at 16.91%. 68 polymorphic sites were identified within the COI gene, with 26 synonymous substitutions, 21 non-synonymous substitutions, and the remaining sites representing insertions/deletions (indels). The distribution of polymorphic sites along the COI gene was not uniform, with a higher concentration observed within the first 203 base pairs (Figure 2).
For the Cyt b gene, the nucleotide composition was C (29.20%), T (30.76%), A (23.99%), and G (16.04%). A total of 18 polymorphic sites were identified within the Cyt b gene, including 9 synonymous substitutions, 8 non-synonymous substitutions, and 2 indels. In contrast to the COI gene, the distribution of polymorphic sites in the Cyt b gene was more evenly spread across the gene, with some clustering observed between nucleotide positions 14-89 and 144-201 (Figure 3).
Haplotype network and phylogenetic analysis
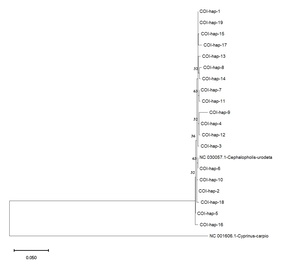
The haplotype network analysis for the COI gene revealed a complex interconnectedness among the identified haplotypes (Figure 4). Haplotypes Hap_1 and Hap_2 emerged as central hubs in the network, suggesting their ancestral nature and potential contribution to diversifying other haplotypes within the population.
The haplotype network constructed for the Cyt b gene showed a distinct pattern compared to the COI gene (Figure 5). Haplotype Hap_5 was directly connected to the reference sequence of C. urodeta obtained from GenBank, indicating a close genetic relationship. The remaining Cyt b haplotypes formed separate clusters, suggesting a degree of genetic divergence among them.
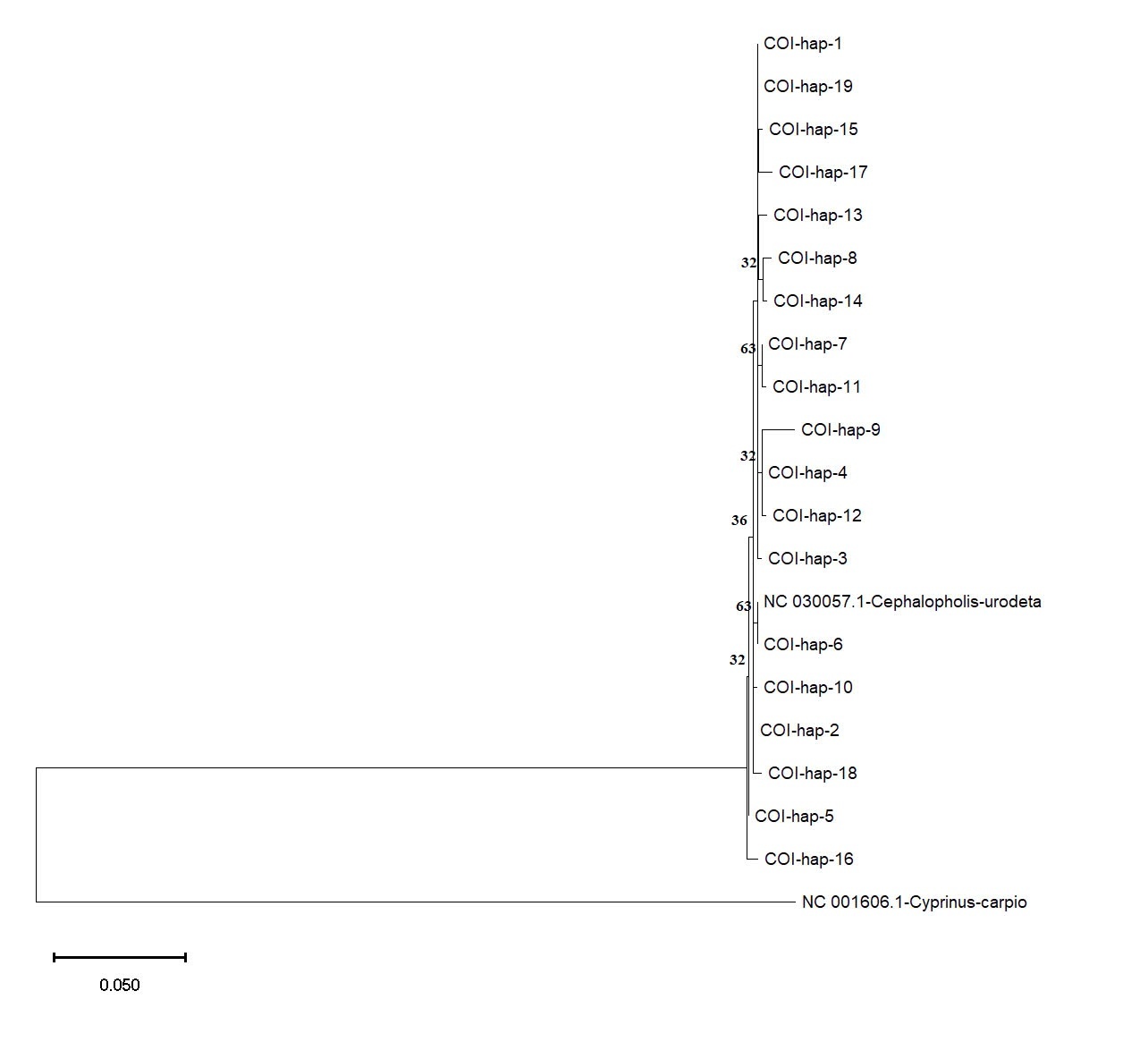
Based on the COI and Cyt b datasets, phylogenetic analysis using the Maximum Likelihood method provided further insights into the genetic relationships among the C. urodeta haplotypes (Figure 6). The phylogenetic tree constructed using the COI gene revealed a distinct cluster comprising most of the haplotypes from Vietnam. Interestingly, Hap_6 from Vietnam grouped separately with the C. urodeta reference sequence, suggesting a closer genetic affinity to the reference sequence than to other Vietnamese haplotypes (Figure 7).
The phylogenetic tree for the Cyt b gene showed a different pattern of relationships. Consistent with the haplotype network analysis, Hap_5 (Cyt b) clustered closely with the reference sequence of C. urodeta, confirming their close genetic relationship (Figure 8). The remaining Cyt b haplotypes were distributed across the phylogenetic tree, forming several distinct clades. This pattern suggests a higher level of genetic structuring and potential for population differentiation within the Cyt b dataset than the COI gene.
Discussion
This study delved into the genetic diversity and population structure of the Darkfin hind, Cephalopholis urodeta, within the ecologically significant Spratly Islands, utilizing mitochondrial COI and Cyt b gene markers. Our findings revealed compelling evidence of high genetic diversity within the studied populations, as reflected in both genes’ high haplotype diversity (Hd) and nucleotide diversity (π) values. These findings align with previous studies on C. urodeta and other reef fishes in the Indo-Pacific region, reinforcing the notion of this region as a reservoir of marine biodiversity.20–22
Several factors likely contribute to this remarkable genetic diversity. The Spratly Islands, characterized by their vast and intricate coral reef systems, offer a mosaic of habitats and ecological niches, reducing competition and fostering diversification within C. urodeta populations.23 This habitat heterogeneity promotes local adaptation, leading to genetic divergence among populations inhabiting different reef environments.
Furthermore, the Spratly Islands are strategically located within the Coral Triangle, a globally recognized marine biodiversity hotspot.24 This region’s high species richness and intricate connectivity patterns contribute significantly to the genetic diversity of its marine inhabitants, including C. urodeta.
The species’ life history characteristics further contribute to its genetic diversity. As a relatively long-lived species with a pelagic larval duration, C. urodeta possesses considerable dispersal potential, facilitating gene flow and counteracting genetic drift among populations, even those geographically separated.25 This dispersal capacity is crucial for maintaining genetic connectivity and resilience across the archipelago.
However, despite the high overall genetic diversity, our results highlight a nuanced scenario with indications of genetic structuring within C. urodeta populations in the Spratly Islands. The haplotype networks, particularly for the Cyt b gene, reveal distinct clusters, suggesting limited gene flow and potential for genetic differentiation among some populations.
The phylogenetic analysis further corroborates this observation of genetic structuring, particularly for the COI gene. This finding suggests that factors beyond the species’ dispersal potential shape the genetic structure of C. urodeta populations in the Spratly Islands.
To provide a broader context for our findings, we have expanded our discussion to include comparisons with genetic studies on other reef fish species in the Spratly Islands and the wider Coral Triangle region.
For example, a study by Ma et al.26 on the leopard coral grouper (Plectropomus leopardus) in the East Sea revealed significant genetic structuring, which aligns with our findings for C. urodeta. Similarly, research by Timm et al.27 on the blue-spotted grouper (Cephalopholis argus) across the Indo-Pacific showed high genetic connectivity in some regions but distinct genetic breaks in others.
These comparisons highlight that while some patterns of genetic structuring may be species-specific, others might reflect broader regional trends in marine connectivity. Understanding how C. urodeta fits into these regional patterns of marine biodiversity can inform more comprehensive conservation strategies for reef ecosystems in the Spratly Islands and beyond.
One potential driver of this structuring is the complex interplay of oceanographic currents within the East Sea. The region’s hydrodynamics, influenced by monsoonal winds and the Kuroshio Current, can create barriers to larval dispersal, isolating populations and leading to genetic divergence.28,29 Geographic distance, acting in concert with ocean currents, can further restrict gene flow, exacerbating genetic differentiation among geographically distant populations.
Furthermore, habitat discontinuities, such as deep channels or areas of unsuitable substrate, can disrupt larval dispersal and contribute to genetic structuring. These discontinuities can effectively isolate populations, limiting gene flow and leading to genetic divergence.30,31 Future research incorporating oceanographic modeling, habitat mapping, and larval dispersal data would be invaluable in clarifying the relative contribution of these factors to the observed genetic patterns.
The observed genetic structuring within C. urodeta populations holds profound implications for their conservation and management. Recognizing the existence of genetically distinct populations is crucial for developing effective conservation strategies that consider the unique needs of each population.32
Implementing spatially tailored management measures, such as strategically placed marine protected areas or targeted fishing regulations, can help preserve the genetic diversity and adaptive potential of C. urodeta within the Spratly Islands. Ignoring this genetic structuring could lead to management strategies that erode genetic diversity, making populations more vulnerable to environmental change and compromising their long-term resilience.33,34
It’s crucial to acknowledge that while our study provides valuable insights, it is not without limitations. Mitochondrial DNA provides a single locus perspective of the evolutionary history and may not fully capture contemporary gene flow or hybridization events. Future research incorporating nuclear markers, such as microsatellites or single nucleotide polymorphisms (SNPs), are crucial to obtain a more nuanced understanding of population structure, particularly at finer spatial scales.35,36 These markers are inherited from both parents and offer higher resolution for detecting recent gene flow and population bottlenecks and identifying potential hybrid zones.
Moreover, incorporating environmental data, particularly those related to oceanographic currents, habitat suitability, and the potential impacts of climate change, will be crucial for developing holistic and effective conservation strategies for C. urodeta and other reef fishes in the Spratly Islands. Understanding how these environmental factors interact with the species’ biology and influence genetic connectivity is essential for predicting future population trends and guiding conservation efforts.
It is important to acknowledge that this study provides valuable preliminary insights, but further investigation with a larger sample size and wider geographic scope is warranted. Our sampling was limited to 30 individuals collected from a subset of reefs within the Spratly Islands during a single expedition in August 2022. While this provided initial evidence of genetic structure, expanding the study to encompass a larger number of individuals and reefs across a wider geographic range (e.g., including populations from neighboring regions in the East Sea) would strengthen the inferences drawn about C. urodeta population connectivity and allow for more robust comparisons with other grouper species in the Indo-Pacific.3,10 Additionally, repeated sampling across multiple seasons or years would provide a more comprehensive understanding of temporal variation in genetic diversity and potential fluctuations related to environmental factors or fishing pressure.
Conclusion
Our study sheds light on the genetic diversity and population structure of C. urodeta within the Spratly Islands. The high genetic diversity observed underscores the ecological significance of this region and highlights the need for science-based conservation measures to safeguard this valuable resource. Recognizing and incorporating the observed genetic structuring into management plans will ensure the long-term sustainability and resilience of C. urodeta populations within this dynamic and ecologically valuable region.
Acknowledgments
The author sincerely thanks Dr. Hoang Thi Thuy Duong and Dr. Do Huu Quyet (Institute of Tropical Ecology, Vietnam-Russia Tropical Center, Vietnam) for facilitating the sampling trip to Truong Sa and the crew of naval ship 632 (in 2022) for their support throughout the sampling process. This research is funded by Ho Chi Minh City University of Education Foundation for Science and Technology under grant number CS.2023.19.47.
Authors’ Contribution
Conceptualization: Van-Thanh Vo (Equal), Quan Ke Thai (Equal). Methodology: Van-Thanh Vo (Equal), Quan Ke Thai (Equal), Nguyen-Thanh-Thao Le (Equal), Thi-To-Nhien Doan (Equal). Formal Analysis: Van-Thanh Vo (Equal), Quan Ke Thai (Equal), Nguyen-Thanh-Thao Le (Equal), Thi-To-Nhien Doan (Equal), Thi-Hieu Tran (Equal), Thanh Tri Do (Equal). Investigation: Van-Thanh Vo (Equal), Quan Ke Thai (Equal), Nguyen-Thanh-Thao Le (Equal), Thi-To-Nhien Doan (Equal), Thi-Hieu Tran (Equal), Thanh Tri Do (Equal). Writing – original draft: Van-Thanh Vo (Equal), Nguyen-Thanh-Thao Le (Equal). Writing – review & editing: Van-Thanh Vo (Equal), Quan Ke Thai (Equal), Nguyen-Thanh-Thao Le (Equal), Thi-To-Nhien Doan (Equal), Thi-Hieu Tran (Equal), Thanh Tri Do (Equal). Funding acquisition: Van-Thanh Vo (Lead). Supervision: Van-Thanh Vo (Lead).
Competing of Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
This study, focusing on the genetic diversity and structure of the Darkfin hind (Cephalopholis urodeta) in the Spratly Islands, Vietnam, involved the collection and handling of wild fish. While the study did not involve vertebrate animal experiments requiring ethical approval from an Institutional Animal Care and Use Committee (IACUC) or equivalent ethical body, we adhered to ethical guidelines to minimize stress and harm to the fish.
Furthermore, all research activities followed the Convention on Biological Diversity and the Convention on the Trade in Endangered Species of Wild Fauna and Flora. No permits were required for the collection of Darkfin hind for this study.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.