Introduction
Litopenaeus vannamei holds significant global commercial value, anchoring a multibillion-dollar aquaculture industry that relies predominantly on this species.1 However, frequent disease outbreaks in recent years have led to a remarkable reduction in L. vannamei production and massive economic losses.2,3
Environmental stress is a crucial element in the genesis of numerous diseases, besides being directly infected by viruses and bacterial pathogens.4
Since nitrite levels in the intensive culture systems may approach 20 mg/L, environmental nitrite management has become a key concern in aquaculture.5 Studies have documented that increased nitrite-N content is associated with increased susceptibility to pathogens in crustaceans by depressing immunologic function and/or increasing oxidative damage.6–11 Wang et al.9 postulated that nitrite toxicity in Macrobrachium rosenbergii after subjection to increased nitrite-N results in antioxidant protection imbalances with the production of free radicals. Chand and Sahoo10 also reported that the activity of phenoloxidase (PO) along with the total hemocyte count (THC) contents were remarkably reduced in Macrobrachium malcolmsonii inoculated with nitrite-N levels of 0.063 mg/L and 0.314 mg/L, respectively. Furthermore, the mortality rate in prawns challenged with Aeromonas hydrophila was remarkably increased by subjection to higher nitrite-N (0.314 mg/L).10
Although the role of this immunomodulation is unknown, nitrite-N seems to have immunosuppressive properties. A comparison method might give some insight. Despite having simpler endocrine/neuroendocrine along with immunological systems relative to vertebrates, invertebrates have strong stress reactions that include the production of stress hormones/neurohormones. Crustaceans have evolved a unique neuroendocrine system that allows them to adapt to environmental stimuli by altering their critical systems to meet current requirements.12,13 Eyestalk is also crucial for the neuroendocrine system since it provides a location for the X-organ sinus gland. In the farming of the Penaeus species, eyestalk ablation is standard practice for ovarian maturation14,15 and has been found to increase the toxicity of some heavy metals.16 There is currently little information on crustaceans’ immunological and physiological responses to the ablation of eyestalks in nitrite-stressed crustaceans.
The purpose of the current research work was to evaluate the effects of increasing nitrite on some immunological (PO, antibacterial along with bacteriolytic activities) and physiological responses (lactate, hemolymph oxyhemocyanin, and glucose) of L. vannamei over time. The effect of nitrite subjection to eyestalk-ablated L. vannamei was also investigated.
Materials and Methods
Test shrimp
The white shrimp (L. vannamei) used in the research were obtained from farm in Haiyang (Yantai, China) and kept at the Qingdao Agricultural University (Qingdao, China). Shrimp were placed in 150 L polyvinyl chloride polymer (PVC) tanks for 1 week acclimation at 28 ± 0.5 °C and 30 % salinity before the studies. In the acclimatization phase, shrimp were given a formulation of shrimp meal twice every day (Aohua Feed Co., Ltd, Fujian province, PR China). In this investigation, only shrimp in the intermolt phase were utilized.
Test solutions
In nitrite-N exposure tests, five test solutions were employed, with nominal levels of 0, 2.5, 5, 7.5, and 10 mg/L derived from a stock solution of 49.3 g of NaNO2 dissolved in one liter of distilled water. The nitrite-N concentrations used as control and test solutions (0.04, 2.77, 6.01, 8.30, and 11.36 mg/L) were assessed via the spectrophotometric approach documented by Bendschneider and Robinson.17
Nitrite exposure conditions
A total of 450 shrimp, each weighing 2.65 ± 0.42 g, were randomly assigned into 18 tanks (150 L, 25 shrimp in each tank) and stratified into six groups, each with three tanks. Five tanks were filled with test solutions for nitrite-N exposure treatment solutions at 0, 2.5, 5, 7.5, and 10 mg/L, while unilateral eyestalk-ablated shrimp were added to the sixth group containing nitrite-N at 10 mg/L. Eyestalk ablation of shrimp was done 48 h prior to the start of the experiment. The shrimp underwent unilateral (left eyestalk only) ablation by excising the eyestalks underwater at the base of the peduncle, followed by applying pressure to the wound for 15 s to minimize fluid loss and facilitate coagulation. Removal of eyestalks was done, and then shrimps were returned to the tank for recovery immediately. Every morning, nitrite concentrations were assessed before being replaced with new solutions. Every day, half of the water was changed. At 3, 6, 12, 24, and 48 hours post nitrite-N exposure hemolymph (200 μL) was extracted using a 1 mL sterile syringe and transferred into plastic Eppendorf tubes on ice. The hemolymph of three shrimp from each tank was pooled for the following measurements. All samples were frozen and kept at -80°C immediately.
Determination of immune function
The generation of dopachrome from L-3,4- dihydroxyphenylalanine (L-DOPA, D-9628, Sigma-Aldrich, St Louis, MO, USA) was assessed spectrophotometrically at 490 nm, as documented by Mason18 and Hernández-López et al.19 The antibacterial and bacteriolytic activities in plasma were determined spectrophotometrically at 570 nm using Micrococcus lysodeikticus and Vibrio harveyi (Sigma), respectively, as documented by Jiang et al.20 V. harveyi was grown for 18-20 hours at 28°C on 2116E-beveled solid substrates, then rinsed with 0.1M phosphate buffer (pH 6.4) and dispersed in the buffer to O.D.570nm=0.3. In 96-well plates in cold water (0°C), each suspension (250μL) was combined with 50μl plasma, and the absorbance (A0) was assessed. The absorbance (A) was measured again after 30 minutes of incubation at 37 °C.
Antibacterial activity (Ua) along with bacteriolytic activity (UL) were calculated as follows:
Ua=√[(A0 −A)/A]
UL=(A0 −A)/A
Determination of metabolic indices
Lactate and glucose levels were assessed using assay kits (Nanjing Jiancheng Institute, China) as described by the manufacturer, and measurement of absorbance was done at 490 nm and 540 nm with a microplate reader (MD Model SpeciraMaxiD5, USA); the levels were computed using a standard solution of substrate. The levels of oxyhemocyanin in hemolymph samples diluted 1:20 with isotonic saline were assessed via measuring absorbance at 335 nm. An extinction coefficient (EmM) of 17.26 was adopted to compute the oxyhemocyanin content.21
Statistical analysis
All data is provided as means with standard errors (SE) and analyzed using SPSS (v16.0; SPSS Inc., Chicago, IL, USA). One-way ANOVA, Duncan’s multiple range tests, along with independent-samples t-tests were adopted to analyze the data. Statistical significance was defined as a P value of less than 0.05.
Results
The effects of nitrite-N on metabolic function parameters in unilaterally ablated and non-ablated shrimp eyestalks
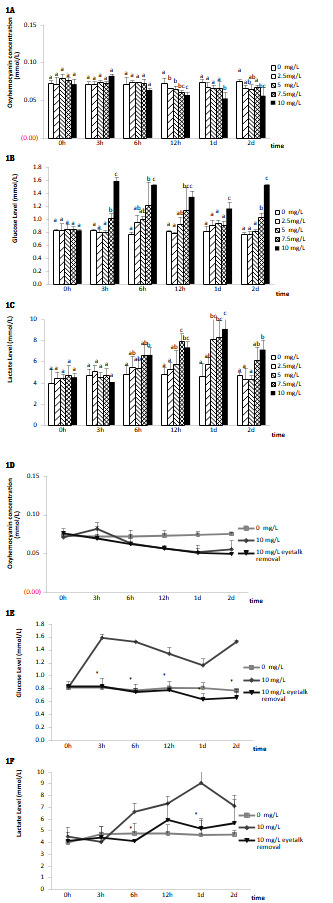
Figure 1 illustrates the influence of nitrite-N on the metabolic function parameters of L. vannamei. The contents of oxyhemocyanin in the hemolymph of shrimp subjected to ambient nitrite-N at 2.5, 5, and 7.5 mg/L for 12 hours and to nitrite-N at 10 mg/L for 12, 24, and 48 hours were remarkably lower in contrast with those in control (Fig. 1A, P < 0.05). The hemolymph oxyhemocyanin contents in the ablated, as well as non-ablated eyestalk groups subjected to ambient nitrite-N at a dose of 10 mg/L did not vary remarkably (Fig. 1D).
Throughout the test period, hemolymph glucose contents in shrimp subjected to ambient nitrite-N at 7.5 for 3, 6, 12, and 48 hours, as well as 10 mg/L for 3, 6, 12, 24, and 48 hours were remarkably greater in contrast with those in control (Fig. 1B, P <0.05). Shrimp challenged with nitrite-N at 2.5 and 5 mg/L had no remarkable variations in hemolymph lactate contents. Hemolymph glucose contents were remarkably lower in unilateral eyestalk-ablated shrimp challenged with 10 mg/L ambient ammonia-N during the test period in contrast with the non-ablated shrimp kept under the same parameters (Fig. 1E, *P *< 0.05).
Figure 1C illustrates hemolymph lactate contents in shrimp subjected to ambient nitrite-N. Hemolymph lactate contents rose remarkably after subjection to nitrite-N at 5 mg/L for 24 hours, 7.5 mg/L for 12 and 24 hours, and 10 mg/L nitrite-N for 6, 12, 24, and 48 hours (P < 0.05) in contrast with the controls. Following the challenge with ambient nitrite-N at 2.5 mg/L, no remarkable variations in hemolymph lactate contents were found (Fig. 1C). Hemolymph lactate contents were remarkably lower in unilaterally eyestalk-ablated shrimp challenged with ambient nitrite-N at 10 mg/L for 6 and 24 hours relative to shrimp with intact eyestalks kept under the same parameters (Fig. 1F, P < 0.05).
Effects of nitrite-N on immune response parameters of eyestalk-ablated and non-ablated shrimp
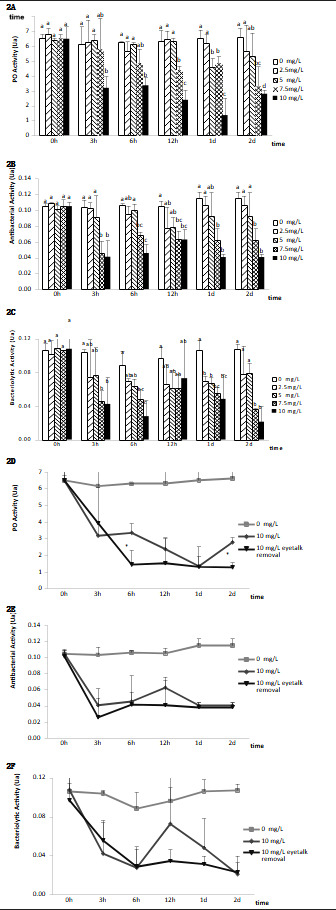
Figure 2 depicts the impact of nitrite-N on immunological parameters in L. vannamei. Hemolymph PO activity was substantially reduced after subjection to nitrite-N at 5 mg/L for 24 hours, 7.5 mg/L for 12, 24, and 48 hours, and 10 mg/L for 3, 6, 24, and 48 hours in contrast with the controls (P < 0.05). Following the challenge with nitrite-N at 2.5 mg/L, no remarkable variations in hemolymph PO activity were noted (Fig. 2A). Antibacterial activity was remarkably reduced after subjection to nitrite-N at 7.5 and 10 mg/L for 3, 6, 12, 24, and 48 hours (Fig. 2B, P < 0.05). Bacteriolytic activity was remarkably reduced after subjection to nitrite-N at 2.5 and 5 mg/L for 24 hours, as well as at 7.5 and 10 mg/L for 3, 6, 24, and 48 hours (Fig. 2C, P < 0.05). Hemolymph PO contents were remarkably lower in unilaterally eyestalk-ablated shrimp subjected to ambient nitrite-N at 10 mg/L for 6 and 48 hours relative to shrimp with intact eyestalks kept under the same parameters (Fig. 2D, P < 0.05). Following subjection to ambient nitrite-N at 10 mg/L, there were no remarkable variations in antibacterial along with bacteriolytic activity in the hemolymph of eyestalk-ablated along with non-ablated shrimp (Fig. 2E and 2F).
Discussion
Shrimp are often raised in an environment that is susceptible to physicochemical changes. Nitrite is a prevalent contaminant in aquaculture systems, and high levels have been linked to lower growth, survival, antioxidant enzyme activity, and immunity.22–24
The mechanisms of nitrite toxicity have been documented in various aquatic species, with the main effect being the loss of function of oxygen-carrying pigments causing hypoxia, then ultimately resulting in death.25 In fish, increased levels of NO2- in the blood plasma leads to oxidation of iron atoms (Fe2+→Fe3+). Consequently, functional hemoglobin (Hb) is converted to methemoglobin (metHb; Camargo et al.25) causing a reduction in efficiency of oxygen transfer.26,27 In our study, the observed decrease in oxyhemocyanin levels with increasing nitrite-N concentrations is consistent with previous reports on the effects of ambient nitrite in Astacus astacus,28 Penaeus monodon21 and Eriocheir sinensis.29 In shrimp, the mechanism underlying this effect involves nitrite accumulation in blood plasma, leading to oxidation of copper (Cu1+→Cu2+), which prevents the reversible binding of molecular oxygen by hemocyanin.25
Our results showed that exposure of L. vannamei to increasing levels of nitrite resulted in decreased hemolymph oxyhemocyanin while lactate content increased, which indicates that the oxygen capacity of the shrimp is reduced and conversion from aerobic to anaerobic respiratory metabolism is exacerbated by hypoxia-induced nitrite stress to ensure that readily metabolizable energy stores are available to meet an increased energy demand.30
Cheng and Chen23 reported that exposure of kuruma shrimp Penaeus japonicus to nitrite for 24 h led to increased oxygen consumption and ammonia excretion, indicating increased energy production and protein catabolism. As a stress response, nitrite exposure increased the quantity of glucose in the current investigation. According to Hong et al.,29 glycogen in the hepatopancreas is reduced following nitrite exposure. Therefore, the buildup of glucose in the hemolymph might be attributable to glucose transfer from the hepatopancreas to the hemolymph. Furthermore, nitrite exposure’s remarkable impact on hemolymph glucose and lactate contents suggests that an increase in metabolic substrates is necessary to fulfill the increased energy requirement under high nitrite-N stress conditions. Besides, the hyperglycemic response generated by nitrite exposure seems to be an adaptive mechanism that ensures metabolizable energy reserves are available throughout the organism.
In the current research, removing the eyestalk resulted in a remarkable drop in glucose along with lactate contents in the hemolymph, revealing that ablated shrimp utilized energy less efficiently than intact shrimp after being subjected to nitrite stress. Although the major hormones from XO-SG include crustacean hyperglycemic hormone (CHH), molt-inhibiting hormone (MIH), gonad-inhibiting hormone (GIH) or vitellogenesis-inhibiting hormone (VIH), and mandibular organ-inhibiting hormone (MOIH),31,32 we postulate that the primary factor among these is CHH.6,33 CHH is considered to have a predominant effect due to its high concentration in the eyestalk and its direct role in regulating glucose metabolism.34 This suggests that CHH is involved in modulating glucose metabolism in response to nitrite stress to meet the energy demands of organs and tissues. Consequently, eyestalk ablation was performed to evaluate its impact on CHH levels and the shrimp’s response to elevated nitrite levels. Moreover, the complex interaction between CHH and other hormonal pathways highlights the intricacy of endocrine regulation in crustaceans, which is crucial for their survival and adaptability in variable environments. However, the lack of a clear link between oxyhemocyanin levels and ablation outcomes complicates the interpretation of our findings, particularly regarding the influence of other hormones on anaerobic metabolism.
Nitrite can depress immunological responses,11 causing an increased susceptibility to pathogens.6–10 However, reports on the neuroendocrine effects of nitrite stress on the crustacean immune system are relatively limited.
In the present study, while immunological parameters fluctuated extensively throughout the 48 h of exposure, there was a general trend of immunological compromise in L. vannamei following nitrite-N exposure. These findings are in accordance with those of Chand and Sahoo10 who also reported reduced PO activity along with THC and increased resistance to A. hydrophila in M. malcolmsonii exposed to nitrite-N. Conversely, Cheng and Chen6 demonstrated that subjection to increased nitrite-N for 7 days had no remarkable effect on THC or PO in M. rosenbergii but caused a remarkable reduction in phagocytic activity and clearance efficiency. Furthermore, a remarkable increase in susceptibility to Enterococcus was observed. Similarly, the mortality rate of L. vannamei challenged with Vibrio alginolyticus was increased following subjection to nitrite-N in the range from 4.94 mg/L to 19.99 mg/L.7 These findings illustrated that exposure of L. vannamei to nitrite-N caused impairment of physiological and immunological functions, which resulted in increased susceptibility to pathogens and toxins.
The current study demonstrated that eyestalk ablation in shrimp led to a decrease in blood parameters such as PO activity compared to non-eyestalk-ablated shrimp under the same conditions. Consistently, a reduction in total hemocyte count was observed in unilaterally ablated female Farfantepenaeus paulensis35 and in L. vannamei.36 Therefore, our results suggest that eyestalk hormones mediate a protective immune response against nitrite stress in L. vannamei. Further investigations are required to elucidate the mechanism by which CHH modulates immune functions in shrimp to protect against nitrite stress.
In summary, the results of the current study indicate that exposure of L. vannamei to nitrite-N at 10 mg/L results in impaired metabolic and immunological responses. Moreover, eyestalk hormone mediates a protective response against nitrite-N stress via a mechanism that may involve increased availability of metabolic substrates and enhanced immune function.
Acknowledgments
This work was supported by the earmarked fund for the Natural Science Foundation of Shandong Province (ZR2024MC077/ZR2024MC208), the Modern Agro-industry Technology Research System in Shandong Province (SDAIT-13), the High-level Talents Research Fund of Qingdao Agricultural University (663/1119054), and the “First Class Fishery Discipline” program in Shandong Province.
Authors’ Contribution
Conceptualization: Yuquan Li (Equal), Yanting Cui (Equal). Funding acquisition: Yuquan Li (Lead). Methodology: Pengyuan Hao (Lead). Formal Analysis: Pengyuan Hao (Equal), Long Zhang (Equal), Xiaofan Wang (Equal), Zhongkai Wang (Equal). Investigation: Pengyuan Hao (Equal), Long Zhang (Equal), Xiaofan Wang (Equal), Zhongkai Wang (Equal). Writing – original draft: Pengyuan Hao (Lead). Visualization: Xuan Song (Lead). Resources: Long Zhang (Equal), Zhongkai Wang (Equal). Validation: Fei Liu (Lead). Data curation: Renjie Wang (Lead). Writing – review & editing: Yanting Cui (Lead). Supervision: Yanting Cui (Lead).
Competing of Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
In the course of this study, we utilized the Pacific whiteleg shrimp (Litopenaeus vannamei), an invertebrate species, as our experimental model. Given the current scientific consensus and guidelines, ethical approval for the use of invertebrates is not mandatory as they are not considered to have the capacity for pain or suffering in the same way vertebrates do. Despite this, we remain committed to the principles of the 3Rs (Replacement, Reduction, and Refinement) and have taken extensive measures to ensure the welfare of the animals used in our research.
Our study was designed to minimize any potential stress to the shrimp. All experimental procedures were carried out swiftly and with minimal handling to reduce any disturbance. Furthermore, we use anesthetizing agents prior to sampling to ensure that the shrimp did not experience any discomfort. We confirm that all efforts have been made to alleviate any suffering of the animals used in this research, and we adhere to the highest standards of animal welfare in the conduct of our scientific investigations.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.