Introduction
Cysteamine (2-mercaptoethylamine) is an aminothiol compound used as a drug for the treatment of cystinosis,1,2 a disease caused by cystine accumulation because of deficiency of a cystine carrier in the lysosomal membrane. According to several reports,1,3 cysteamine is a decarboxylation product of cysteine, an intermediate metabolite that exists in the metabolism of sulfur amino acid and an ingredient of acetyl-CoA that participates in protein, carbohydrate and lipid metabolism. Hence, cysteamine plays important roles in neuroprotection,4 hepatosomatic and glomerular rehabilitation,5,6 immune and antioxidant promotion,7 reproductivity and breastfeeding function8,9 as well as improvement of growth caused by regulating the growth hormone.10–12 Although cysteamine naturally exists in most animals and plants,13 the exogenous addition of cysteamine in diets was validated to be effective by many previous reports.3,5,7,10
The positive effects of cysteamine on growth performance have been reported by numerous types of research for freshwater fish, including common carp,14,15 rainbow trout,16 and grass carp.17 Compared with most freshwater fish (less than 0.5%), the optimum dietary cysteamine level for marine fish and special aquatic livestock was relatively high (more than 0.5%).12,18–21 Therefore, it is generally believed that different fish species do not affect growth improvement caused by dietary cysteamine.22 However, to some degree, the optimum addition of cysteamine varies with fish species, size, diet, and experimental conditions.22
Turbot (Scophthalmus maximus L.), a popular commercial carnivorous fish, has been widely farmed in East Asia and Europe because of its delicious meat and rapid growth. A dependency on taurine in diets was suggested by the positive correlation between growth promotion in turbot of varying body weights and increasing taurine levels in their diet,23,24 which was validated to be the metabolic product of cysteamine in vivo by growth performance, hepatosomatic histological structure and metabolism of taurine synthesis for most livestock and poultry as well as a few aquatic animals.1,3,17,25 However, no information is available on the effects of cysteamine supplemented in plant-based diets for juvenile turbot, the relationships between the effects, and whether cysteamine can convert to taurine to take effects in turbot. Therefore, this study aimed to investigate the effects of cysteamine in high-plant diets on growth performance, body composition, hepatosomatic histological structure, and metabolism of taurine synthesis.
Materials and Methods
Feed Preparation
The formulation of a high plant diet consists of 53% crude protein (23% soybean meal, 30% gluten meal, and 14% white fish meal) and 13% crude lipid (Table 1), which can meet the nutritional requirements for growth of turbot (Kaushik SJ, 1998). 0.00%, 0.05%, 0.10%, and 0.15% cysteamine hydrochloride (purity, 99%; Qingdao Masite Feedstuff Co., Ltd, China) was supplemented separately to the plant-based diet to obtain 0.00%, 0.05%, 0.10%, and 0.15% (of dry weight) cysteamine, respectively. Five diets were isonitrogenous and isolipidic, including the fishmeal-based (72.5% white fish meal) control diet. Ingredients were ground into fine powder through 246-um mesh. All ingredients were thoroughly mixed with fish oil and soybean oil, and water was supplemented to produce stiff dough. The dough was then pelleted and dried for about 12 h in a ventilated oven at 60°C. After drying, the diets were broken up, sieved into proper pellet size, and stored at −20°C until used. Actual nutrients compositions, including crude protein, crude lipid, and cysteamine content in pellet diets, were analyzed, and gross energy was calculated by the formula: (Crude Protein×4) + (Ether Extract×9) + (Total Carbohydrate×4) (Goddard et al., 2003) in Table 1.
Fish and Feeding trial
Turbot juveniles (2.0±0.04 g) were purchased from Yellow Sea Fisheries Co., Ltd (Haiyang, Shandong, China) and were fed the control diet for 2 weeks to acclimate to the diet and conditions in a 500L cylindrical fiberglass tank. Before starting the trial, the fish were in abrosia 24h to prevent any inconclusive data. At the initiation of the trial, Fish of similar sizes were stochastically distributed into 15 tanks (500L cylindrical fiberglass tank), and per tank was stocked with 42 fish.
The experiment was carried out in the recirculating farming systems of Yellow Sea Fisheries Co., Ltd, Haiyang, China. Triplicate groups of fish were fed to apparent satiation twice daily (06:30 and 18:30) for 9 weeks. Any uneaten feed was collected and dried to constant weight at 70°C and reweighed as residual bait. Leaching loss in the residual diets was measured by leaving five samples of each diet in tanks without fish for 40 min, recovering, drying, and reweighing. Water was altered every 2 days in the initial 4 weeks and then 50% every day in the last 5 weeks. All tanks were provided with continuous aeration through air stones to ensure the dissolved oxygen was near saturation. During the actual trial, the mortalities were noted daily, and specific indicators of rearing water were assayed every 7 days. Besides, the environmental factors were controlled as follows: water temperature 15-20oC, pH 7.5-8.0, and salinity 30-33‰. Ammonia nitrogen was lower than 0.25 mg/L; nitrite was lower than 0.1 mg/L, and the dissolved oxygen was higher than 6.0 mg/L. At the termination of the trial, the fish were fasted for 24 h before harvest. The total number and body weight of fish per tank were measured.
Sample Collection
This research protocol was approved by the ethics review board of Hebei Normal University of Science and Technology (ethics approval code: 2024035). The procedures conformed to the Declaration of Helsinki and related policies in China. Ahead of the start of the trial, sufficient pellet diets in every treatment were stochastically selected for assay of nutrient compositions, including crude protein, crude lipid, carbohydrate, and cysteamine, separately. At the termination of the trial, all experimental fish were anesthetized with pentobarbital sodium salt and eugenol (Shanghai Reagent Corporation, Shanghai, China), which were proved to be effective and reduced anatomical pain.26,27 Pentobarbital sodium salt was administrated with a dosage of 0.2 mg/kg fish body weight by intramuscular injection, while eugenol was administered with a concentration ratio of 1:10000 in physiological saline to relieve pain. After all the terminal fish per tank were weighted for evaluating the growth performance, 7 fish per tank were sampled and stored at -20 oC for whole body composition analysis, and the other 7 fish per tank were sampled to anatomize for calculation of morphometric parameters and investigation of hepatosomatic histological structure. Individual body weight, body length, liver, and visceral weight were noted to calculate condition factor, hepatosomatic index, and viserosomatic index. After calculating morphometric parameters, the whole liver samples were frozen in liquid nitrogen and stored at -80 oC for subsequent enzymatic activity and taurine content assay.
Growth Performance
All fish were deprived of food for 24 h before weighing and sampling; the following parameters were measured at the end of 9 weeks using the following equations:
Specific growth rate (SGR) (%/d) = 100 × (Ln (final body weight) − Ln (initial body weight))/days
Feed intake (FI) (%/d) = 100 × total consumed amount of the diet / [(initial body weight + final body weight) / 2] / days
Feed efficiency rate (FER) = wet weight gain (g) / total consumed amount of the diet (g)
Protein efficiency rate (PER) = wet weight gain (g) / total consumed amount of the diet (g) / protein percent in diets
Survival rate (SR) (%) = 100 × (final fish number / initial fish number)
Condition factor (CF) = 100 × fish weight (g) / [body length (cm)]3
Hepatosomatic index (HSI) (%) = 100 × (liver weight / body weight)
Viscerosomatic index (VSI) (%) = 100 × (visceral weight / body weight)
Activity of CSD / CDO (nmol•mg-1 prot•min-1) = 1000×[A (absorbance at 400nm) -0.0561] /0.0076/125.5×dilution multiple/reaction time (12min)/protein concentration in homogenate (mg prot•ml-1)
Body composition assay
Body compositions, including moisture, crude protein, crude lipid, and ash in samples and feed, were analyzed. Moisture was assayed by drying the samples to constant weight at 105 oC. Crude protein was analyzed using the Kjeldahl method (1995), which was estimated by multiplying nitrogen by 6.25. Crude lipid was determined by ether extraction using the Soxhlet method. Ash was assayed by combustion in a muffle furnace at 550 oC. Duplicate analyses were carried out per sample.
Taurine and enzyme activity assay
The determination of taurine was conducted with High-Performance Liquid Chromatography (HP1100, America) according to the method of Sakai and Nagasawa,28 which used a standard curve to input absorbance measured at 400 nm into the curve equation to obtain concentration. L-cysteinesulfinate decarboxylase (CSD) activity in the liver was assayed by using the method described by Goto et al,29 based on the assay of taurine from the substrate cysteine sulfonic acid catalyzed by the enzyme. The reaction mixture consists of phosphate buffer (10mM, ph7.4), cysteine sulfonic acid (1mM), Pyridoxal 5-phosphate (0.2mM), 2-hydroxy-1-ethanethiol (4mM) and the crude enzyme solution, which was obtained by homogenate, centrifugation and dialysis. Cysteamine dioxygenase (CDO) was measured by estimating the increasing taurine translated from the substrate cysteamine hydrochloride due to the catalytic reaction of the enzyme according to the method of Goto et al.29 The reaction mixture consists of phosphate buffer (10mM, ph7.4), cysteamine hydrochloride (1mM), sodium sulfide (2mM), and the crude enzyme solution, which was also obtained by homogenate, centrifugation, and dialysis. Determining protein content in homogenate referred to the method described by Lowry et al.30 The reaction mixture was incubated for 60 minutes at 35°C, and the reaction ended by heating at 70°C for 3 minutes. The reaction products were both hypotaurine, which was converted to taurine by adding a 200ul mixture composed of hydrogen peroxide (31%) and ammonia solution (29%) in proportion of equal volume. Then, the sample solution was stored overnight for subsequent determination of taurine content.
Investigation of hepotosomatic histological structure
The hepatosomatic histological structure was observed by paraffin section, which was manufactured by dehydration (Hydroextractor, Leicatp1020, China), transparency (xylene), embedding (Thermo Scientific HistoStar, Leicaeg1160, America), sectioning (Microscope Slicer, Leicarm2145, China), staining (Automatic Rotary Dyeing Machine, Varistain24-4, Japan) and capping (Thermo Scientific ClearVue, HP1100, America) according to the description of Deepika et al.31 Seven fish of each tank were sampled for morphometric parameters and histological observation. After measuring morphometric parameters, the dissected livers were preserved in Bouin’s fixative in advance for no more than 24 hours. Before the official preparation of the paraffin section, the livers were removed from the fixative and washed with 70% ethanol to eliminate the fixative and ensure that it did not affect terminal observation and photographing.
Statistical analysis
The SPSS 11.0 microcomputer software package (SPSS, Chicago, IL, USA) was applied to statistical evaluations. A homogeneity test for the variance was carried out. All data were subjected to a one-way analysis of variance (ANOVA) followed by Tukey’s test. Differences and no differences were respectively regarded as significant and insignificant when p<0.05 and p>0.05. Data are expressed as means with pooled S.E.M, F-value, and P-value.
Results
Growth Performance and Survival
FI of the DietCS-0.05% group was significantly higher than that of the DietPP group (p<0.05), but no notable differences were observed compared to the DietCS-0.1%, DietCS-0.15%, and DietFM group (p>0.05)(Table 2). As dietary cysteamine in plant-based diets increased from 0.00% to 0.15%, an increasing trend in FBW, SGR, and WGR appeared, although no notable differences were discovered (p>0.05), and those in fish-fed plant-based diets were conspicuously lower than those in fish-fedDietFM (p<0.05). FER and PER declined firstly and thereafter increased (p>0.05) with dietary increasing cysteamine, although there were no notable differences among dietary treatments (p>0.05). SR also showed no notable differences (p>0.05) among dietary treatments (Table 2).
Morphological index
CF of fish-fedDietCS-0.1% was conspicuously lower than that of DietFM group (p<0.05), but no notable differences compared to DietCS-0.05%, DietCS-0.15% and DietPP group (p>0.05) (Table 3). VSI of fish-fed plant-based diets was conspicuously higher than that of fish-fedDietFM (p<0.05), but no notable differences were found among plant dietary treatments (p>0.05). HSI in fish-fed plant-based diets showed a decreasing trend compared to that with a lower level in fish-fed diet (p>0.05), and no notable differences were discovered among dietary treatments (p>0.05) (Table 3).
Body composition
The moisture content (%, wet matter) of the DietPP group was conspicuously higher than that of the DietFM group (p<0.05), but no notable differences compared to the other plant dietary groups (p>0.05) (Table 4). As dietary cysteamine in plant-based diets increased from 0.00% to 0.15%, crude protein content (%, dry matter) of plant dietary groups increased first, and then declined (p>0.05) compared to the higher level of DietFM group, while a reverse changing trend appeared in crude lipid content (%, dry matter). However, no notable differences were discovered among dietary treatments in crude protein and lipid content (p>0.05). The ash content (%, dry matter) in fish-fedDietCS-0.05% was conspicuously higher than that of DietPP group (p<0.05), but no notable differences were discovered between DietCS-0.05% group and DietCS-0.1%, DietCS-0.15% and DietFM group (p>0.05) (Table 4).
Taurine content
As dietary cysteamine increased from 0.00% to 0.15%, the body taurine content increased (447.4±37.6 mg/100g-784.8±73.2 mg/100g) (p<0.05) and thereafter reached a plateau (p>0.05), but no notable differences were discovered between 0.05%-0.15% cysteamine addition groups and DietFM group (p>0.05) (Table 5). The liver taurine content in fish-fedDietCS-0.1% was conspicuously lower than that of DietFM group (p<0.05), but no notable differences were found between DietCS-0.1% group and DietCS-0.05%, DietCS-0.15% and DietPP group (p>0.05). The serum taurine content of fish-fed plant-based diets increased first and then declined (p>0.05) compared to the higher level of fish-fed the fishmeal based diet, but no notable differences were found among dietary treatments (p>0.05) (Table 5).
Enzymes activities
As dietary cysteamine in plant-based diets increased from 0.00% to 0.15%, the hepatosomatic CSD activity decreased firstly, then increased, and declined finally (p>0.05). However, no notable differences were recorded among dietary treatments (p>0.05) (Table 6). The hepatosomatic CDO activity of the DietCS-0.15% group was higher than that of the DietFM group (p<0.05), but no notable differences were discovered between the DietCS-0.15% group, and DietCS-0.05%, DietCS-0.1% and DietPP group (p>0.05). Besides, the protein concentration of hepatosomatic crude enzyme solution used for CSD and CDO assay increased firstly and thereafter decreased (p>0.05) compared to the higher level of DietFM group, but no notable differences were discovered among dietary treatments (p>0.05) (Table 6).
Hepatosomatic histological investigation
As the red circles in Figure 1 showed, the cellular stripes of liver tissues in the DietFM group were more obvious than those in plant dietary groups, and the condition of cellular stripes showed improvements to a certain degree with dietary increasing cysteamine in plant-based diets. According to the black arrows in Figure 1, as dietary cysteamine in plant-based diets increased from 0.00% to 0.15%, the hepatosomatic cellular morphology changed from rectangle to circle compared to the approximate polygon cell of the DietFM group. In cellular structure, the plant-based dietary groups showed more cells lacking a nucleus compared to the DietFM group, but this situation was improved with dietary increasing cysteamine. Besides, similar homogeneity was observed in cellular size between the DietFM and DietCS-0.15% group compared to the longer cell of the DietPP group, according to Figure 1.
Discussion
Cysteamine, an aminothiol compound, is typically used as a drug for the treatment of cystinosis,1 asthma,32 melanosis33 and cancer34 for humans. Besides, for livestock and poultry, cysteamine participates in massive metabolic processes, including a precursor for synthesizing taurine, and plays important roles in lactation,8 hormone secretion,10 antioxidative defense (Kessler et al35; Huang et al, 2022), immunoregulation,7 defense against infection36 as well as protection of neural,37 reproductive9 systems and hepatic injury.38 It is well-recognized that exogenous supplementation of cysteamine can facilitate improving growth performance of animals, including chicks,39 sheep,40 pigs,27,41 canines42 and cows (Chowdhury et al43; Meenongyai,2024). However, for aquatic animals, extremely limited information is available on the effects of cysteamine addition on marine aquatic animals44 except for a small number of reports on grass carp, red tilapia, and Blood-red Parrot Cichlid,17,45–47 which demonstrated that exogenous cysteamine addition was beneficial for growth improvements through hormone regulation, antioxidant enhancement, and immunologic defense. In mammals, cysteamine, as an intermediate metabolite in sulfur amino acids metabolic pathways, can be converted from 4’-phosphopantetheine to hypotaurine, which is the precursor of taurine synthesis.48 Additionally, a number of reports indicate that taurine plays important roles in many important physiological processes, including lipid absorption and utilization,49 cellular regulation,50 antioxidative defense,51,52 neural maintenance53 and muscle improvement.54 It was reported that the growth of different sizes of juvenile turbot was increased by 1% taurine added in plant-based and casein-based diets.24,26 However, the impact of dietary cysteamine on growth performance and the relationship between cysteamine and taurine conversion in turbot remains unclear. Therefore, this research focuses on investigating these aspects. The FBW, SGR, WGR, FER, and PER in the present study showed an increasing trend (p>0.05) with dietary increasing cysteamine in plant-based diets while FI in fish-fedDietCS-0.05% was conspicuously higher than that in fish-fedDietPP (p<0.05). According to related reports,10,17 cysteamine can promote the growth of animals in two ways: firstly, changes the configuration of somatostatin (SS) to reduce the inhibition of gastrin, which plays a role in increasing food intake; secondly, induce growth hormone (GH) secretion by inhibiting dopamine hydroxylase activity to increase dopamine content, which can promote synthesis and secretion of GH. The lack of notable growth improvement in juvenile turbot may be attributed to the nutritional deficiencies in plant-based diets, which appear to hinder the effective functioning of cysteamine. Cysteamine is known to exert its physiological effects through the involvement of enzymes and hormones.10,17,45,55
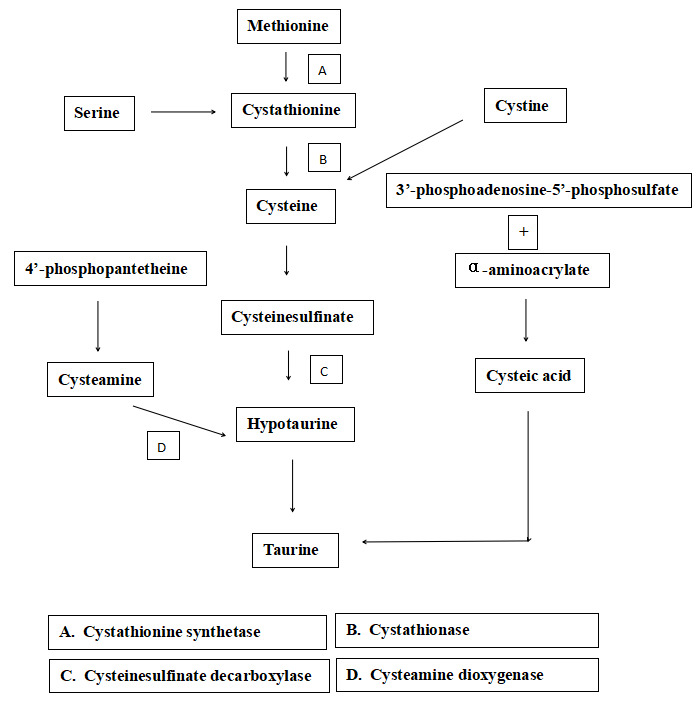
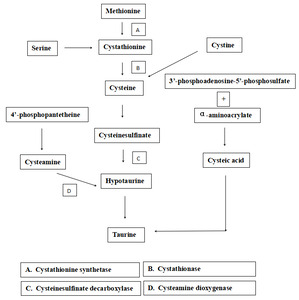
It has been well documented that taurine is synthesized through several pathways in mammals (Jacobsen and Smith. 1968; Rodehutscord et al.56; Tabatabaie et al.48), which involves the 4’-phosphopantetheine route. 4’-phosphopantetheine, as a component part of Coenzyme A (CoA) and Acyl carrier protein (ACP), widely takes part in glucose, lipid, and protein metabolism, can be dephosphorylated to generate pantothenic acid and cysteamine, which is further carboxylated to cysteine. Cysteine is oxidated to cysteine sulfinate by cysteine dioxygenase, and cysteine sulfinate is then decarboxylated to hypotaurine. Hypotaurine is oxidated to taurine at last (Figure 2). However, the transformation mechanism from cysteamine to taurine in marine fish is unclear.57 Therefore, in the present study, to verify whether the transformation exists in turbot, cysteamine is supplemented in plant-based diets, which lack taurine.
Numerous types of research indicated that non-essential or semi-essential amino acids play crucial roles in fish-fed with plant-based diets.24,58,59 In the present study, with increasing dietary cysteamine in plant-based diets, body taurine content of turbot considerably increased from 447.4±37.6 mg/100g to 784.8±73.2 mg/100g (P<0.05) compared to 828.1±68.2 mg/100g of fishmeal-based diet, which indicated cysteamine addition in plant-based diets can cause an increase of taurine. Similar results were obtained in ricefield eel60 and mice.61 However, no significant increment was discovered in taurine content and CDO activity in the liver, which indicated that the transformation pathway from cysteamine to taurine possibly does not exist in the liver of the turbot in this study. It was also reported that different pathways of taurine synthesis were detected in chick brain and liver.62,63 Besides, no significant differences were found in serum taurine content and CSD activity in the liver in the present study. Factually, serum taurine assay is considered unstable,64 which varies with fish species, diet, and assay time.57 Taurine and CDO mainly exist in muscle, not in other tissues of fish.26,27 Therefore, to some degree, the metabolic pathway of cysteamine to taurine depends on animal species, dietary ingredients, and tested parts.
Cysteamine addition in plant-based diets failed to improve growth performance in the present study, which agrees well with the results of routine body composition. The ash content in fish-fed with plant-based diets significantly increased with increasing dietary cysteamine (p<0.05). A similar conclusion was described in related research.27,65 It is inferred that the participation of cysteamine in the regulation of physiological, metabolic processes by affecting the secretion of hormones including GH, INS (insulin), TH (thyroid hormone), GAS (gastrin), and CCK (cholecystokinin) is accompanied by the participation of enzymes and active ions, which results in elevation of mineral elements.10,65,66 The crude protein and crude lipid content in the body showed an increasing trend, as well as protein concentration in the liver with dietary increasing cysteamine in plant-based diets, although no notable differences (p>0.05) were detected (Table 4). On the contrary, a decreasing trend was discovered in moisture content (p>0.05), which is consistent with the results of growth performance in the present study and other related reports.67 Factually, limited by the difference in assay and sampling methods, it is widely believed that the insignificance of body compositions caused by dietary amino acid or analogs addition was common.26,49,57
According to Shunji et al.,68 taurine supplemented in high plant-based diets was supposed to act as a regulatory factor facilitating the amelioration of structure and function in the liver. Lack of taurine in low fishmeal diets resulted in green liver syndrome,29,69,70 which led to hepatomegaly accompanied by abnormal cellular morphology and disorderly intercellular substance.71,72 In the present study, VSI in plant-based treatments showed a significant increment (p<0.05) compared to fishmeal-based treatment, although no conspicuous difference was discovered among the plant-based treatments (p>0.05), and an opposite trend was observed in CF (p<0.05). Histologically, cellular stripes of liver tissues in fish-fed fishmeal-based diets were more obvious than that of the other groups, and some improvements have been shown in the conditions of cellular stripes with dietary increasing cysteamine in plant-based diets (Figure 1). As secretory ducts, intercellular substances are important in secreting digestive juices to facilitate digestion and absorption.72 It seems that taurine was transformed from cysteamine and replaced with cysteamine to play roles in the liver of turbot fed with plant-based diets. However, the evidence is insufficient because of insignificant taurine increment and HSI in the liver with dietary increasing cysteamine. It is believed that a faint half-conversion capacity may exist in the liver of juvenile turbots, as reported in previous related studies (Savolainen58; Al-Asmari, 2016; Hu60). The cellular morphology alternation from rectangle to circle in the liver indicated that cysteamine addition facilitated the morphological improvement of hepatosomatic cells, which is probably due to the alternation of cellular osmoregulation and is supposed to be the crucial proof of cysteamine conversion to taurine.50,60 It is widely reported that the semi-synthesis capacity of taurine exists in fish species based on low activities (Kim SK, 2003; 2010) and inactive gene expression of key rate-limiting enzymes.73,74
In conclusion, an exogenous 0.05% cysteamine addition in plant-based diets resulted in significant increases in feeding, mineral accumulation, body taurine concentration, and obvious improvement in histological structure in the liver. Cysteamine supplemented in plant-based diets causes increasing in body taurine concentration by feeding improvement of dietary added cysteamine, and increasing body taurine concentration causes mineral accumulation as well as improvement of hepatic histology. Therefore, under the premise of taurine deficiency, it is inferred that cysteamine can probably convert to taurine to some degree in juvenile turbot.
Acknowledgments
This study was financially supported by the Science and Research Funds of Hebei Normal University of Science and Technology (Grant no: 2020YB008), the Innovation team building project of the third stage of the modern agricultural industrial technology system in Hebei province (Grant no: HBCT2023220207), and the China Agriculture Research System (Grant no: CARS-50-G08).
Authors’ Contribution
Data curation: Guoshan Qi (Equal), Lu Wang (Equal). Methodology: Guoshan Qi (Equal), Lu Wang (Equal). Formal Analysis: Guoshan Qi (Equal), Lu Wang (Equal). Investigation: Guoshan Qi (Lead). Writing – original draft: Guoshan Qi (Equal), Lu Wang (Equal). Writing – review & editing: Lu Wang (Supporting), Yanying Zhang (Supporting), Qinghui Ai (Equal). Funding acquisition: Yanying Zhang (Lead). Supervision: Yanying Zhang (Lead). Conceptualization: Qinghui Ai (Equal), Kangsen Mai (Equal). Resources: Kangsen Mai (Lead).
Competing of Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
The present study was carried out strictly according to the recommendations in the Guide for the Use of Experimental Animals of Hebei Normal University of Science and Technology.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.

.png)
.png)