Introduction
Megalobrama pellegrini (Tchang, 1930), the Black bream, is a type of fish found in the middle and upper reaches of the Yangtze River in China. It belongs to Cypriniformes, Cyprinidae, Culterinae, and Meglobrama, and it is economically important in the upper reaches of the Yangtze River. There is a lack of studies on the digestive physiology of juvenile Black bream in recirculating aquaculture systems (RAS), which hindered the development of high-quality, low-cost breeding processes. Therefore, it is essential to develop intensive rearing methods to meet the increasing demand.1
The cholecystokinin (CCK) gene was first discovered in the gastrointestinal tract of dogs2 and has since been found in humans,3 Oncorhynchus mykiss, Rainbow trout,4 and Salmo salar, Atlantic salmon.5 Chaudhri6 confirmed that CCK is a typical postprandial satiety signaling factor. Murashita5 proposed that the expression pattern of CCK in different species is dynamic and changes with environmental factors. In Atlantic salmon, the expression of CCK in the brain significantly increased three hours after a meal. Liddle7 demonstrated that the concentration of CCK in human plasma significantly increased within 15 minutes after a meal. Tartaglia8 reported that CCK can affect digestion and feeding in aquatic animals. The expression of CCK in the foregut decreased significantly after 72 hours of fasting in Pelteobagrus fulvidraco, Yellow catfish9 and Megalobrama amblycephala, Wuchang bream.10
According to Rojas-García, Morais, and Rønnestad,11 CCK is involved in an individual’s daily digestion, and its fluctuation rhythm is affected by the frequency of eating. The main biological effects of cholecystokinin (CCK) are to stimulate gallbladder contraction and bile secretion, promote the secretion and synthesis of trypsin, and regulate intestinal peristalsis. Additionally, it acts as a satiety factor to regulate food intake. CCK can release satiety signals, which are related to the secretion of trypsin. Then, CCK is released from the enteroendocrine cells of the enterocytes into body fluids and acts on target cells in the pancreas to induce the secretion of digestive enzyme precursors into the intestinal lumen. High intestinal trypsin activity acts as a negative feedback control on CCK release, suggesting a regulatory loop between these two factors.12 This regulatory loop has also been reported in Gadus morhua, Atlantic cod larvae,13 Atlantic salmon.14 A negative feedback loop between CCK and trypsin was observed in the daily rhythm test of juvenile Senegalese sole, Sole fish.15 However, little is known about the changes of CCK and trypsin in juvenile Black bream. Few reports have focused on the relationship between CCK and trypsin in this species.
Increasing fasting duration can harm the digestive system and negatively impact CCK levels. Feeding frequency is a crucial aspect of aquaculture management that affects fish growth, feed utilization, and management costs. It also influences the metabolic state and body composition of the fish. In intensive aquaculture, feeding frequency can reduce size differences between individuals in the same batch and improve water quality. The effects of fasting and feeding frequency on fish digestion have been extensively studied. Our research group has also conducted significant research on fasting and re-feeding of Black bream. However, there are few studies on monitoring CCK content or feedback regulation through fasting and changes in feeding frequency, with most of them focusing on marine fishes. The short-term daily rhythms of Atlantic cod,13 Sole fish juvenile,15 and Clupea harengus L, Atlantic herring11 had been reported after changing the feeding frequency and other influencing factors during individual development. The impact of fasting and refeeding on the growth and digestive enzyme activities of juvenile Black bream has been reported extensively. Additionally, research has reported the effects of refeeding on biochemical and non-specific immune parameters.
It has been documented that about 80 % of CCK in fish is produced by the central nervous system of the head, while only 6-10 % of CCK is secreted in the digestive tract.11 To accurately observe the regulation mechanism of CCK and trypsin in the intestine and remove the influence of head hormones, we took special treatment dissection steps (removing the head, back tissue, and tail peduncle of the individuals) during sampling.
This experiment selected Black bream, which has just entered a relatively stable development stage, for fasting and short-term circadian rhythm experiments. The aim was to explore the effects of CCK and trypsin on feeding in this critical period and supplement the theoretical basis for culture in a recirculating aquaculture system (RAS).
Materials and methods
Larval rearing in RAS
Black bream larvae were obtained from Dongping Aquaculture Co., Ltd, Chongqing, China. The recirculating aquaculture system (RAS) was established in a fish rearing room with a constant temperature of 28°C with air conditioning, and the water temperature was maintained at 28°C ± 0.5°C. On the fifth day after hatching (DAH5), fish larvae were randomly placed in the glass tanks of the RAS after being treated with 5000 ppm salt water for 15 minutes. Each tank had a volume of 40 L and a flow rate of 0.2 L/s. The RAS utilized LED tubes and was illuminated at a daily rate of 1200 lx from 7:00 to 19:00. The individuals were cultivated in tanks until DAH59 before the experiment, and the culture density was gradually reduced to 1.25 ind L-1 (40 tails per glass tank) as the individuals grew. The fish were fed with Floating Fish Species Matching Feed (Shuangliu Zhengda Co., Ltd., Type I-F1). The main nutrient values were: crude protein ≥ 36%, crude fiber ≤ 8.0%, crude ash ≤ 16.0%, crude fat ≥ 3.0%, and moisture ≤ 12.5%. Individuals were fed three times a day until apparent satiation (at 9:00, 13:00, and 17:00). After each satiation, any remaining bait in the cylinder was removed. The dissolved oxygen and ammonia nitrogen in RAS were monitored (DO ≥ 4 mg/L, NH4+ ≤ 0.1 mg/L, NO2-N ≤ 0.02 mg/L). Dead fish were cleaned daily, and data were recorded.
Short-term fasting trial
For the short-term fasting trial on DAH59, six glass tanks with a water volume of 32 liters were selected. A total of 240 juvenile individuals with an initial body weight of 183.75 ± 61.16 mg and length of 20.74 ± 4.08 mm were randomly divided into the 15-day fasting test group (FTG) and the three times-a-day feeding control group (FCG), with three replicates for each group. The stocking density was 40 individuals in each tank. During the test period, growth data such as weight gain rate (WGR) and specific growth rate (SGR) were measured using the method described by He et al.16 The tanks were emptied after each feeding. In the fasting trial, three individuals were randomly sampled from every three tanks of the two experimental groups. Three individuals were collected from each group randomly in the morning on days 1, 3, 5, 7, 9, 11, 13, and 15 from DAH60 to DAH75. Three individuals were randomly sampled at each sampling point, frozen, and analyzed in triplicate.
Daily rhythm experiment
Four glass tanks with a water volume of 32 liters of recirculating aquaculture system (RAS) were selected to prepare for different feeding regimes within 24 hours. One hundred and sixty juvenile individuals (DAH62) were randomly divided into four groups (40 individuals in each tank) with an initial body weight of 238.71 ± 68.84 mg and a length of 22.81 ± 2.42 mm (mean and SD). After a one-day fast, four tanks were assigned to each of the following feeding regimes on DAH64: (A) feeding once at 9:00, (B) feeding twice at 9:00 and 17:00, (C) feeding three times at 9:00, 13:00, and 17:00, and (D) fasting. The fish were fed to apparent satiation at each feeding time, with the proportion of feed being approximately 5.6% of individual body weight. Sampling was conducted at seven different times throughout the day: 9:00, 13:00, 17:00, 21:00, 1:00^+1; 9(p00)+1^, and 9:00+1, to study the daily feeding rhythm within 24 hours. Three individuals were randomly sampled from each group at each sampling point, frozen, and analyzed in triplicate.
Sampling preparation
The tests and procedures of Black bream in this study were approved by the Laboratory Animal Centre of Southwest University, and the qualification was obtained as a Laboratory Animal Practitioner (SWU _ LAC-20210794).
Body weight and total length were measured at each sampling. Body weight was calculated by weighing a sub-sample of individuals using a millimeter analytical balance. The total length, measured from the tip of the maxilla to the end of the notochord in millimeters, was determined using a caliper. The subjects were anesthetized with tricaine MS-222 (120 mg/L) and subsequently dissected on ice, with only the abdominal viscera being retained. The head, dorsal muscle, and tail were frozen using liquid nitrogen and stored in 2 ml enzyme-free test tubes at -80 °C until analysis. All samples were taken before feeding.
The study analyzed individual samples for CCK and trypsin enzyme activity using highly sensitive methods, as reported by Neda et al,15 which allowed for the resolution of both factors at the individual level. Previous research has indicated that a significant amount of CCK can be found in the head, primarily in the central nervous system, which may obscure changes in CCK in the gastrointestinal tract.11 Thus, all analyses in the current study were conducted on juvenile dissections, excluding the head, back muscles, and tail.
Analysis of CCK and trypsin enzyme activity
The samples were homogenized in 0.05M PBS (pH 7.4) at a ratio of 1:9 using a tissue grinder. After centrifugation at 2500 rpm for 20 minutes at 4°C, the samples were immediately analyzed. All assays were performed in triplicate.
Trypsin activity was measured to detect total protein content using assay kits from Nanjing Jiancheng Bioengineering Institute. Prior to conducting the experiment, the diluted supernatant was further diluted tenfold with 0.05 M PBS (pH 7.4). Enzyme activity was determined by measuring the change in absorbance.
The detection method used was based on the protocol developed by Neda et al.15 All groups were analyzed using the Fish Cholecystokinin (CCK) Elisa Assay Kit from Nanjing Jiancheng Bioengineering Institute, which employs a competition method to measure the CCK content.
Histology
In the short-term fasting trial, Black bream underwent routine histology and hematoxylin-eosin (H-E) staining. The samples were fixed in 4% paraformaldehyde for 24 hours, washed, dehydrated, cleared, and embedded in paraffin. Sagittal sections (5 μm) were cut using a conventional microtome (RM2016, Shanghai Leica Instrument Co., Ltd.), placed on gelatin-coated slides, rehydrated, and stained. The tissue sections were analyzed using a Leica four-camera digital camera and ImageJ, after being observed under a light biomicroscope (B302, Chongqing Aote Optical Instrument Co., Ltd.).
Statistical analysis
The mean ± SD expression of CCK content and trypsin enzyme activities in the abdominal extracts of Black bream were evaluated. Homogeneity of variance and normality tests were conducted. The specific activity of CCK was assessed using one-way ANOVA. Multiple comparisons of enzymatic activity over time were performed using the Duncan test. The analysis of variance between groups was conducted using an independent samples t-test. Histological sections were analyzed with Image J (National Institutes of Health). The statistical analysis was conducted using IBM® SPSS® Statistics26 (IBM, International Business Machines Corporation), and the graphs were created using Origin 2021 (OriginLab Corporation). A significance level of P < 0.05 was utilized.
Results
Short-term fasting trial
Trypsin enzyme activity and CCK Content
All groups exhibited an increasing trend in body weight of Black bream from DAH60 to DAH75 (Fig. 1), with a significant difference between groups (P < 0.05). The weight gain of both groups did not significantly increase during the first three days. The weight gain of the FCG was significantly higher than that of the FTG (P < 0.01). There was no significant difference in the weight gain of FTG (P > 0.05), and the SGR of FTG was 0.49%. The SGR of FCG reached 8.68 %.
There were no extreme values of trypsin activity in FCG, and the final trypsin activity level was higher than the initial value (Figs. 2,5). FCG exhibited a higher level of trypsin activity compared to FTG (Fig. 4). The trypsin activity of FTG showed a decreasing trend on the first day of fasting and reached its lowest value of 279.43 U/mgprot on the ninth day of fasting (DAH72), which was significantly different from other time points (P < 0.05). After a slight increase, the trypsin activity fell to a level lower than the initial activity on the fifteenth day of fasting. There was a significant difference in trypsin enzyme activity between FTG and FCG (P < 0.05). The values of each point in FCG were greater than those in FTG (Fig. 2).
The trypsin enzyme activity test revealed significantly higher levels in FCG on days 11 and 13, with peaks exceeding adjacent points (Fig. 5). The enzyme activity levels of all sampling points in FCG were higher than the baseline value. FTG exhibited two downward trends, reaching a low of 279.43 U/mgprot on the ninth day of fasting (Fig. 6). The enzyme activity level of FTG was lower than the baseline value on the fifteenth day of fasting (Fig. 6). The enzyme activity level of FCG was consistently higher than that of FTG, with a significant difference between the two groups (Fig. 4).
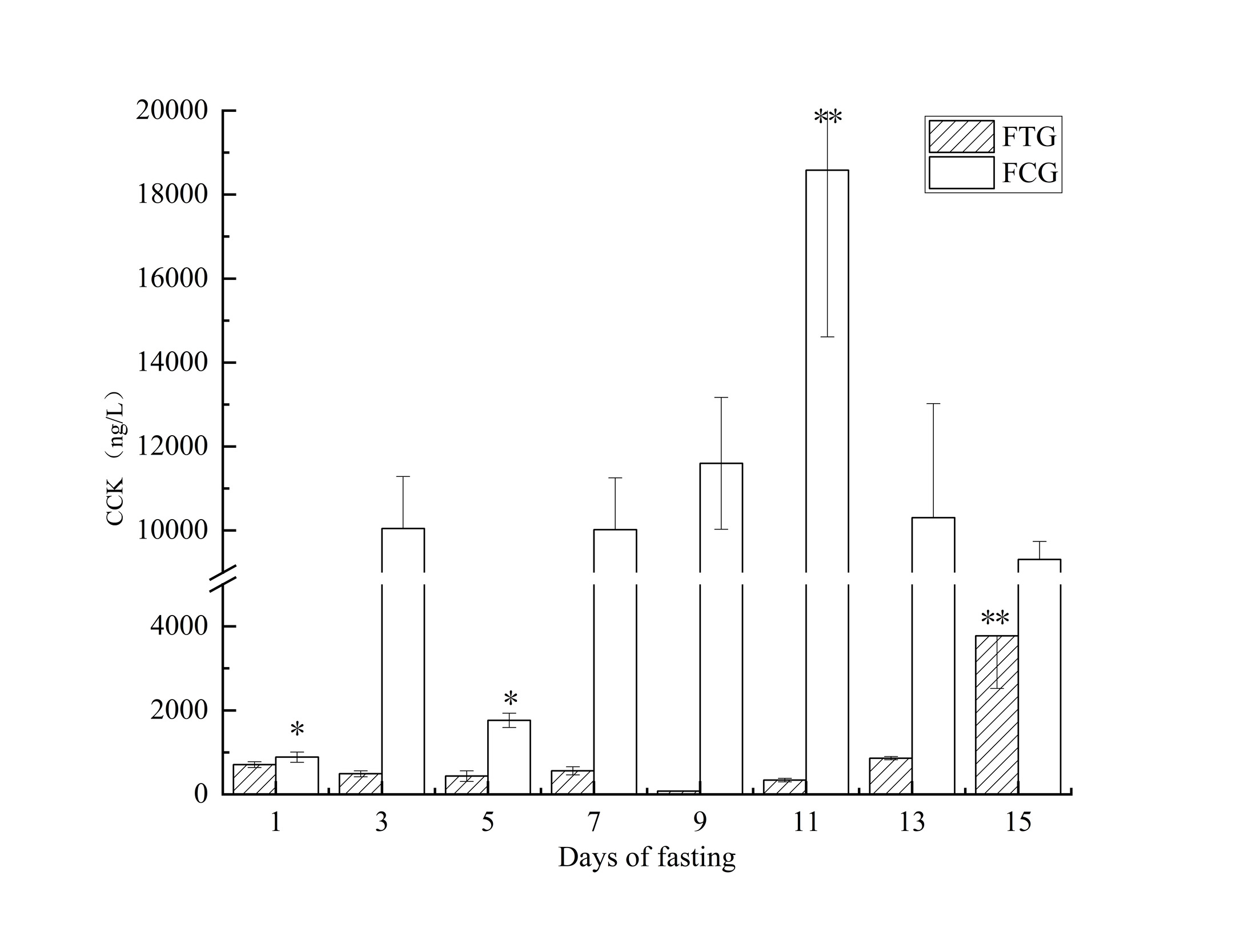
The CCK content in FCG exhibited two upward fluctuations, with the second peak occurring at DAH70, reaching the highest value of 18583.85 ng/L (Figs. 3,5). In FTG, a continuous upward trend was observed after the lowest value of 77.41 ng/L on day 9. During the first 14 days, there was no significant fluctuation of CCK in FTG (Fig. 6). However, on the 15th day, the CCK content reached its highest value of 3777.26 ng/L, which was still significantly lower than that in FCG. When compared with the mean values (Fig. 4), the CCK content in FCG remained significantly higher than that in FTG. The final values of both groups were significantly higher than the initial values.
During the test, the control group experienced multiple negative feedback fluctuations, although these were not stable due to factors such as developmental stage. The fasting group, on the other hand, only showed a reverse trend on the 15th day. Additionally, the CCK content did not change with the variation of trypsin in the early stage of the experiment.
Histology
On the first day of fasting, intact intestinal chorion and a single layer of enterocytes were observed (Fig. 7a). The intestinal tissue structure appeared healthy, with goblet cells distributed among the intestinal epithelial cells. The muscular mucosae and basement membrane were closely arranged, and the connective tissue in the lamina propria was dense. On the ninth day of fasting (Fig. 7b), there was evacuation between the monolayer of enterocytes of Black bream. The number of goblet cells significantly decreased compared to the first day of fasting, and the muscular mucosa was loosely arranged within the chorion. On the 13th day of fasting, partial detachment of the striated border was noted (Fig. 7c). The degree of villus shrinkage increased, and the villus height decreased. The intestinal mucosa exhibited necrotic enterocytes that sloughed off, edematous lamina propria, and a looser shape than on the 9th day of fasting (Fig. 7b). On the 15th day of fasting, complete goblet cells were difficult to observe (Fig. 7d), although the area of a single goblet cell did not significantly change (Table 1). On day 15, the intestinal chorion had partially detached, the monolayer of columnar epithelial cells showed signs of vacuolation and degeneration, and the loss of the striated border was more pronounced than on the 13th day of fasting (Fig. 7c). The lamina propria’s connective tissue was loose, and the mucous membrane and basement membrane were somewhat damaged. The mucosal muscle was loose and deformed, and the gap between the mucosal muscle and the lamina propria increased.
The Black bream lacks an independent liver and pancreas, instead having a combined hepatopancreas in its digestive system. After one day of fasting, the hepatocytes contained large lipid vacuoles, causing the nuclei to be displaced and the cells to become compact (Fig. 8a). By the third day, the liver cells displayed noticeable cord-like connections, with the dense pancreas embedded within them (Fig. 8b). On day 9, the volume of liver cells significantly decreased, intracellular fat decreased, intercellular spaces became loose, cells became irregularly arranged, cell borders blurred, and the alveolar cells of the pancreas shrunk (Fig. 8c). The degree of vacuolar degeneration of liver cells increased, pancreatic cells gradually shrank and became loose, and the nucleoli of pancreatic cells deformed and displaced on day 11 (Fig. 8d). After 13 days of fasting, the liver cell vacuoles were observed to have degenerated, the cell borders were blurred (Fig. 8e), the nucleoli were severely displaced, and the zymogen particles were reduced (Fig. 8f). On the last day of fasting, the hepatocytes had almost no cytoplasm, the intercellular space of liver cells increased, and the vacuoles were almost damaged and ruptured (Fig. 8h). Additionally, the pancreatic acinar cells gradually shrank (Fig. 8g).
Daily rhythm experiment
There were no significant differences in body length or weight among the four groups in the daily rhythm experiment, and this trend continued throughout the experiment.
The CCK content of the first group (Fig. 9A) peaked at 17:00, which was the only peak at this time among the four groups. It then showed a downward trend until an upward trend began after the lowest level appeared at 1:00+1 the next day. The CCK content at 1:00+1 and 5:00+1 the next day was significantly different from other nodes. The trypsin activity of group A tended to increase after the first feeding and to decrease after 17:00, but there was no significant difference between the trypsin activities in this group.
The CCK content of the second group exhibited a single peak at 21:00 (Fig. 9B), which was significantly different from the other three groups. After 21:00, a significant decreasing trend was observed, which was also significantly different from the trypsin activity at 5:00+1.
In group C, two peaks of CCK were observed at 13:00 and 1:00+1 (Fig. 9C), with the CCK content at 1:00+1 being significantly increased. Trypsin activity peaked at 13:00 and showed a continuous downward trend until 1:00+1. Group C exhibited higher trypsin activity levels in the first 12 hours compared to the other groups.
The CCK content of the fasting group (Fig. 9D) reached its maximum at 1:00+1 and continued to decrease to a minimum at 9:00. At 13:00, the CCK content of this group was significantly lower than that of the third group. The CCK content at 9:00 AM was 236.01 ng/L, which was significantly lower than that of the second and third groups. In the fasting group, trypsin activity exhibited two peaks at 17:00 and 1:00+1. The peak at 1:00+1 was significantly higher than the levels before and after, while a significantly low level was observed at 5:00+1. The change in CCK content and baseline at 5:00+1 was less than that at 9:00+1.
Discussion
The objective of this study was to examine the feedback-regulatory effects of short-term fasting and different feeding frequencies in a single day on the secretion of CCK and trypsin in juvenile Black bream. The juveniles had an initial body weight of 183.75 ± 61.16 mg. Although this was not strictly a growth test, the FCG still exhibited a better growth trend. This may suggest that the experimental individuals were still in the early stages of ontogeny.
Short-term fasting trial
Extended fasting can cause damage to the epithelium of the digestive tract and the hepatopancreas. The degree of damage to the digestive system is directly proportional to the duration of fasting. While there are numerous reports on fasting experiments, there are relatively few studies on the effects of fasting during the early stages of individual development. Hunger is a significant obstacle to individual development and a major negative factor, assuming the same commodity feed. To obtain more reasonable experimental data, this study selected Black bream in its juvenile stage as the test subject. The results of WGR and SGR indicate that FTG was significantly lower than FCG, suggesting that during the juvenile stage of Black bream, the individual was in the rapid development stage of nutrient accumulation. In the absence of exogenous nutrient intake, individuals stagnate and consume their own stored energy to maintain life. Thus, the combination of detecting enzyme activity and analyzing tissue structure through histology can provide a more intuitive understanding of the relationship between fluctuations in CCK and trypsin during fasting trials.
The hepatopancreas, being a resilient and regenerative organ, typically remains structurally intact even during short-term fasting. In contrast, the intestine is more susceptible to feeding as it is directly in contact with food. The monolayer columnar epithelial cells in the intestinal villus membrane are metabolized relatively quickly, making damage more intuitive. This study found that fasting for 15 days caused more severe damage to the intestinal tissue. Damage to the lamina propria and muscularis can lead to decreased intestinal peristalsis, resulting in reduced nutrient absorption efficiency. Short-term fasting reportedly has no significant effect on the nutritional status of juvenile fish, but long-term fasting can cause the starvation effect to persist and affect the nutritional status of juvenile Black bream.17 Therefore, we hypothesized that short-term fasting of 15 days may not induce the desired hunger effect. Prolonging the fasting cycle could potentially worsen hepatopancreatic damage.
The trypsin activity in FTG exhibited a decreasing trend, followed by an increasing trend, and was lower than that in FCG. This study found that the CCK content reached its lowest level on the 9th day of FTG, which is consistent with the findings of Ye et al18 regarding the effects of fasting on Trachinotus blochii, Snubnose pompano. They suggest that fasting can cause overall degeneration of the fish digestive tract and its accessory glands, but the lowest value appeared later than in Black bream. In our experiment, we observed a downward trend in the content of CCK within one week of fasting, with the lowest value appearing on the 9th day. The values in FTG did not show any significant difference, which is different from the results obtained in studies on Atlantic herring,11 Yellow catfish,9 Wuchang bream,10 and Atlantic salmon.5 It is worth noting that the studies mentioned above usually use mature individuals as test subjects. The development of the intestines in mature individuals is more advanced, and the cells in the intestines are more responsive to CCK secretion. This study utilized juvenile Black bream, which may explain the discrepancies between our findings and those of others.
During the later stage of fasting, CCK and trypsin levels increased. The reason for this increase was that Black bream entered the juvenile stage, which leads to increased intestinal permeability. While there is still debate on whether the midgut and hindgut are involved in the secretion of CCK. Micale et al19 confirmed the presence of CCK in the hindgut of fish through immunohistochemistry and discussed its distribution. It was found that CCK is a polypeptide hormone with a complex and variable structure. Different isomers can replace the existing digestive and metabolic processes in different intestinal segments. According to Murashita et al,5 CCK could act as a mediator in the anti-inflammatory response and is present in the hindgut, detecting gene expression. Bermúdez et al.20 found an increase in the number of CCK-IR cells in the hindgut of parasitic fish, indicating their participation in the CCK immune response. Gilliam Vigh et al21 reported a decreasing trend in the expression of CCK mRNA and the density of CCK immunoreactive cells from the duodenum to the ileum, but still found a small number of CCK immunoreactive cells and low levels of CCK mRNA expression in the large intestine. Meng and Tang22 noted that the development of olfactory bulbs, intestines, gills, and other organs of Black bream matured gradually and intermittently during the observed period. This change in growth may explain the simultaneous recovery of CCK content and trypsin activity level after reaching their lowest values on the ninth day. According to Li et al,23 Black bream reached adulthood 68 days after hatching. This finding may explain the observed phenomenon in our experiment where the individuals were in the adult stage.
Short-term fasting caused more damage to the foregut epithelial cells of early juvenile Black bream. Trypsin activity and CCK content increased after 9 days of fasting, while CCK and trypsin in the digestive system showed a negative correlation fluctuation trend only after 13 days of fasting. It is speculated that CCK secretion in the intestine may come from epithelial secretory cells in the midgut and hindgut, or that CCK participates in the inflammatory response caused by tissue damage to stimulate an increase in secretion. The cumulative distribution of CCK in the intestinal epithelium and hepatopancreas is affected by the degree of fasting and overall CCK secretion. Further experiments can explore this at the subtype and molecular level.
Daily rhythm experiment
The aim of this study was to investigate the potential negative feedback loop between trypsin and CCK in the digestive system of Black bream. Additionally, we explored whether this feedback mechanism would be affected by changes in feeding frequency throughout the day.
Cholecystokinin-releasing peptide, which is secreted by the small intestinal mucosa, is highly sensitive to trypsin. Trypsin, in turn, can inactivate this peptide, thereby regulating the further secretion of CCK and pancreatic enzymes. Kurokawa and Polakof24,25 confirmed the feedback regulation of CCK involved in exogenous feeding. The endogenous rhythm throughout the day was independent of dietary treatment or short-term fasting, which is consistent with the results in Atractoscion nobilis, White seabass.26 The negative feedback loop between CCK and trypsin has also been noted in the development and experiment of Solea senegalensis, Senegalese sole juveniles,15 Atlantic cod larvae,13 and Atlantic herring.11 The fluctuation of trypsin and CCK did not show any significant difference. CCK responds quickly to hunger as a satiety factor, resulting in no obvious synchronous negative feedback. Because the juveniles were in the early stages of development, they were not sensitive to changes in feeding response.
In the early stages, feeding regularly establishes a biological rhythm in the secretion of digestive glands in the individual digestive system. This means that even in a state of complete fasting, CCK will still exhibit a changing trend similar to that after ingestion in the short term. It is worth noting that synchronization was found between the peak of CCK levels and the peak of trypsin activity in each group. This statement is an observation similar to that made by Rojas-García et al.11 CCK regulates the digestive process of juveniles, but the daily rhythm is separated from long-term feeding. No rhythm of CCK secretion was found without food stimulation. It is important to note that there was no significant difference between the initial and final data of CCK and trypsin in the same group. This provides a reliable basis for the subsequent discussion of the closed-loop regulation in the daily rhythm.
Meanwhile, the quality of the feeding may be reflected in the secretion of trypsin. In group C, the trypsin level was the highest. Feeding three times a day was found to be more conducive to the growth and development of Black bream, which is consistent with studies on Arapaima gigas, Arapaima,27 Coreius guichenoti, Largemouth bronze gudgeon,28 Micropterus salmoides, Largemouth black bass,29 and Rainbow trout.30 The trypsin levels in group C exhibited a downward trend at night, which is consistent with the findings of Tillner et al12 after several feedings. Additionally, the C group had higher trypsin levels during the day compared to the other three groups, suggesting that a higher feeding frequency can activate the digestive process more effectively. After a period of fasting, the digestive system may experience functional damage, leading to reduced secretion levels of CCK and trypsin. This reduction can affect the feedback regulation of the two. To maintain normal digestive function, an appropriate feeding strategy is necessary to maintain normal levels of trypsin. Based on the results of this experiment, it is recommended that Black bream juveniles be fed three times a day during this period to maintain normal trypsin secretion levels.
Overall, in the four daily rhythm experiments with different feeding frequencies, there was no significant difference between the initial and final values of CCK and trypsin after 24 hours. This indicates that the short-term change in feeding strategy did not affect the regulation mechanism of trypsin and CCK in juvenile fish. The feedback regulation of CCK in the gut of Black bream was found to be very sensitive in the daily rhythm test, which is consistent with the findings in Trachinotus blochii, Golden pompano.18 The levels of biorhythms for CCK and trypsin change during long-term individual development, but the fluctuations only occur at different times within a single day. Long-term formation of CCK and trypsin in the digestive system is not affected by 24-hour fasting. A single feeding stimulation can make an individual feel hungrier at night than 24-hour fasting. Feeding three times a day may result in better growth for juvenile fish. The digestive system of 64DAH Black bream exhibits a short-term daily rhythm of negative feedback between CCK and trypsin. Altering the frequency of feeding within a day does not impact the long-term circadian closed-loop rhythm of CCK and trypsin.
Conclusion
The feedback between CCK and trypsin was found to be unstable due to short-term fasting. However, fasting mainly damaged the foregut epithelium of Black bream. It can be speculated that CCK participates in the anti-inflammatory response or that the secretion site also exists in the midgut and hindgut, based on the increase of CCK in the later stage of fasting. In contemporaneous circadian rhythm experiments, it was observed that CCK and trypsin had a negative feedback control loop in the daily rhythm. Feeding schedules can impact hunger levels and growth rates. Research suggests that a single morning feeding may cause earlier hunger compared to fasting throughout the day, while feeding three times a day may lead to better growth outcomes.
Acknowledgment
Special thanks go to Chongqing Modern Agricultural Industry Technology System (CQMAITS202315) and Chongqing’s key aquatic science and technology innovation project (No. CQFTIU2024-11) for their support of this project and especially express gratitude to Ms. Qin Li and Ms. Hongyu Tang for providing technical method guidance during the experiment and ideas during the paper writing process and other academic guidance.
Author contribution statement
Conceptualization: Wenyu Li (Equal), Hongyu Tang (Equal), Qin Li (Equal). Methodology: Wenyu Li (Equal), Xiaogang Lin (Equal). Formal Analysis: Wenyu Li (Equal), Hongyu Tang (Equal), Qin Li (Equal). Investigation: Wenyu Li (Equal), Hongyu Tang (Equal), Qin Li (Equal). Writing – original draft: Wenyu Li (Lead). Writing – review & editing: Wenyu Li (Equal), Qin Li (Equal). Resources: Wenyu Li (Equal), Hongyu Tang (Equal). Funding acquisition: Hongyu Tang (Lead). Supervision: Hongyu Tang (Equal), Qin Li (Equal).
Funding
This research was supported by Chongqing Modern Agricultural Industry Technology System (CQMAITS202315), and the Construction and application of key technology systems for the industrialization of integrated ecological rice and fishery planting and breeding in mountainous areas, Chongqing’s key aquatic science and technology innovation project (No. CQFTIU2024-11).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability Statement
The data of the present study are available from the authors upon reasonable request. The first draft of the manuscript has been uploaded to Research Square as a preprint.
Conflicts of Interest Statement
The authors declare no conflict of interest.
Ethical Conduct Approval
The tests and procedures of M. pellegrini in this study were approved by the Laboratory Animal Centre of Southwest University and obtained the qualification of Laboratory Animal Practitioner (SWU _ LAC-20210794).

_of_ftg_and_fcg_within_15_days_of_the_fasting_trial.tif)
_of_ftg_and_fcg_of_the_fasting_study.tiff)




_corre.png)


_of_ftg_and_fcg_within_15_days_of_the_fasting_trial.tif)
_of_ftg_and_fcg_of_the_fasting_study.tiff)



_corre.png)

