Introduction
Otoliths—calcified structure matter in the ear sacs of all teleost fishes—consist of calcium carbonate (~96 %), organic matter, and trace elements. Otolith deposition patterns are distinct1 because of the differing physicochemical properties of calcium carbonate and organic matter.2,3 This pattern results in alternating light and dark bands in otoliths. External environment changes leave characteristic marks on otoliths, making them a valuable source of fish life information. In the study of the early life history of fish, otoliths are crucial for calculating early-stage growth and development, as determined by daily rings. The application of otolith microstructure to identify the age of juvenile fish was first proposed by Brothers et al.4 Subsequently, identifying daily rings in fish otoliths became a significant research method in fish ecology.5–8 Otolith microstructure analysis is widely used to determine the age of juvenile and young fish, as well as to make presumptions about spawning grounds and hatching periods.9–14 Since fish are influenced by various growth environments and genetic factors, otolith shape and ring deposition exhibit interspecific differences. Otolith shape can thus be utilized to identify different geographic groups. To date, scholars have studied otolith shape and ring deposition in various fish species.15,16 Furthermore, otolith microchemical characteristics, such as trace elements and stable isotopes, are closely related to the living environment of the fish. Otolith chemical composition is valuable for age and population identification, providing essential data to understand changes in the growth environment, population size, and resource amounts of the fish.17–22
The Japanese flounder (Osteichthyes, Paralichthyidae, and Paralichthys) is primarily found in the Yellow and Bohai Sea of China, as well as in Korea and Japan and holds economic significance in China. However, in recent years, due to environmental pollution, overfishing, and water conservancy projects, the wild population of Japanese flounder has rapidly declined. Since the 1980s, China has been replenishing the wild population in natural waters by releasing artificially cultivated Japanese flounder larvae into the Bohai Sea to mitigate resource damage caused by anthropogenic actions. Stock enhancement is a vital strategy to improve the aquatic ecological environment, restore fishery resources, protect biodiversity, and promote sustainable development.23–27 Effective assessment of the effects of stock enhancement relies on valid identification of wild and released populations. Currently, in vitro, molecular, and otolith microchemical markers have been established to identify Japanese flounder populations.28 However, a scientific and effective identification technique for identifying proliferating and reproducing fish populations is lacking due to the migratory habits of Japanese flounder. Given the noticeable differences in the early life environment—such as temperature, nutrients, and light cycle—between the wild and released Japanese flounder populations, analyzing the otolith microstructure can trace their early life history and aid in identifying the proliferating population.
Research on the biology of Japanese flounder, both domestically and internationally, mainly focused on gene expression,29,30 feed nutrition evaluation,31,32 genetic breeding,33,34 pathology,35 growth,36 reproduction effects,37 and other related areas. While evaluations of release effects and flounder resource distribution exist, fewer studies explored otolith microstructure, particularly otolith early growth and development in Japanese flounder. Oshima et al.38 measured the otolith daily increment in Japanese flounder along the Pacific coast of Japan, demonstrating a strong correlation between otolith radius growth and body length. This further established that otolith growth increment could reflect body length growth and predict the fish growth trajectory. However, otolith early growth patterns and daily otolith ring characteristics in Japanese flounder are not clear.
Therefore, this study aimed to analyze early otolith microstructure and investigate the relationship between daily otolith increments and the growth of wild and domesticated Japanese flounder parents. Additionally, we aimed to provide essential data for the future use of otoliths to infer the early life history, evaluate the effects of enrichment and release, and facilitate population identification of the Japanese flounder.
Materials and Methods
Sample collection
The experimental samples consisted of young fish hatched from eggs fertilized by artificial insemination of Japanese flounder obtained from the Beidaihe Central Experimental Station of the Chinese Academy of Fisheries Sciences. The parental fish were wild Japanese flounder caught from the Qinhuangdao Sea. The fertilized eggs were artificially induced on March 30, 2022 and placed into a hatchery tank measuring 4 × 6 × 2 m for hatching. Following hatching, the young fish were reared in static water at a temperature of 20 ± 0.5 °C, with air stones for oxygenation and water changes every 2 days. Initially, rotifers were used as the primary food source. After 14 days, the diet of the fish was supplemented with artemia larvae and a specific amount of algal liquid. All fertilized eggs used in this experiment were progeny from the same spawning parent fish. Starting from day 1 after hatching, 15 eggs were randomly sampled daily for the first 30 days, and subsequent sampling occurred at 5-day intervals from 35–50 days after hatching. The photoperiod was not controlled in the whole process of cultivation.
Sample processing
After sample collection, each fish was placed on a slide, dried with filter paper, and photographed under a light microscope (Lecia Microsysterms Ltd. DFC7000T&M165FC). The total length (TL) of the fish was measured using the software of the system (accuracy of 0.01 mm). Subsequently, each fish was placed in a centrifuge tube with anhydrous ethanol and labeled for storage.
After photographing the fish, we picked and prepared each sagitta and lapillus under a dissecting microscope. This involved removing the organic film, cleaning with anhydrous ethanol, drying, and finally fixing and sealing them with neutral gum for later examination. Otoliths that were difficult to identify or damaged because of photorefraction or cracks in the otolith rings were excluded from further analysis. Samples that could not be processed immediately were stored in 1.5 ml Eppendorf tubes containing anhydrous ethanol.
During the first 30 days, the otoliths did not require processing, and clear rings were visible. After 30 days, sagittal otolith samples needed to be ground with 2000#, 3000#, and 5000# water-abrasive sandpaper until the cores were visible and the rings clear. Care was taken to avoid over-grinding, with constant observation under the microscope. The ground otoliths were rinsed with water to remove any surface powder, and water stains were gently wiped away with a piece of mirror paper before photographing under a fluorescence microscope. The length and width of the otoliths were measured using Motic Images Plus 2.0 software.
Data collection and statistical analysis
The otolith shape and development images were edited and processed using Adobe Photoshop 2022 without altering the otolith characteristics. Data are presented as mean ± SD and subjected to statistical analysis using a non-parametric test in SPSS Statistics 26.0. A significance level of P < 0.05 indicated a significant difference, while P < 0.01 was considered highly significant. Image analysis and processing were performed using Origin 2022 software.
Results
Fertilized Japanese flounder eggs, which are transparent, spherical, and separate floating, emerged from the membrane at 53 hours of incubation at a water temperature of 20 ± 0.5 °C. The hatchlings were 2.76 ± 0.17 mm in length, transparent, and had fin folds extending from the back of the head around the tail to the back of the yolk sac. Additionally, they had pectoral fins attached to the back of the yolk. By day 30, the entire body of each juvenile was covered with scales, the body color had deepened, the original right eye had shifted to the dorsal side, and all the eyes were located on the same side. At this stage, metamorphosis was complete, and the total length of the juvenile fish measured 13.46 ± 0.92 mm, with all three pairs of otoliths essentially formed.
Morphological and developmental otolith characteristics in Japanese flounder
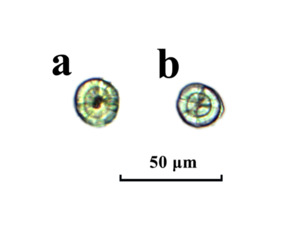
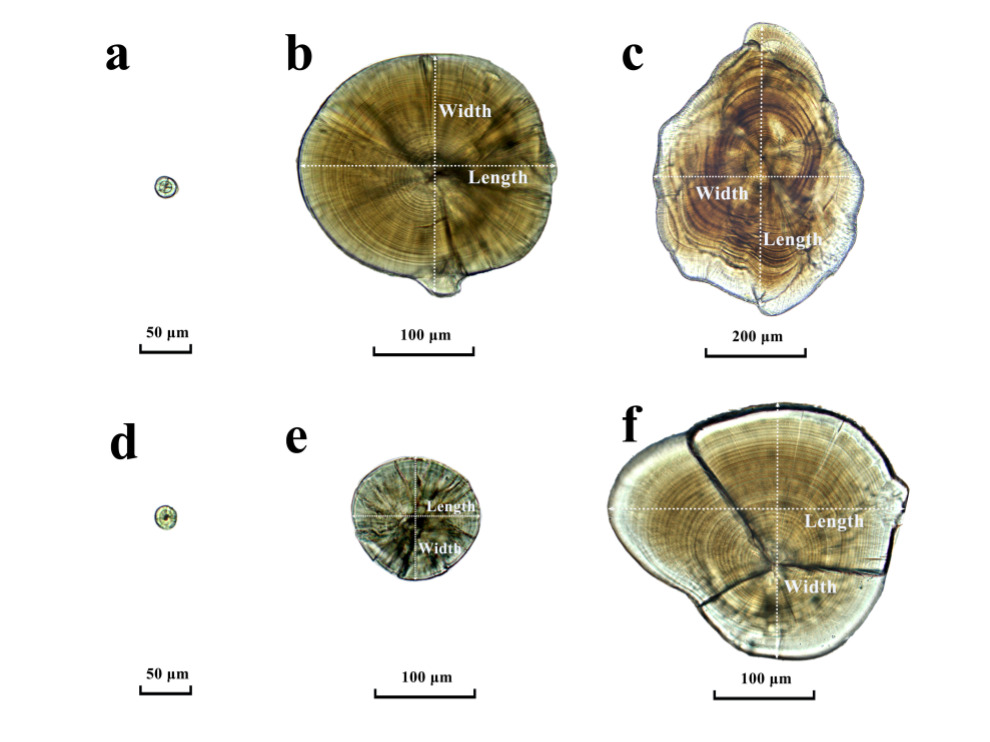
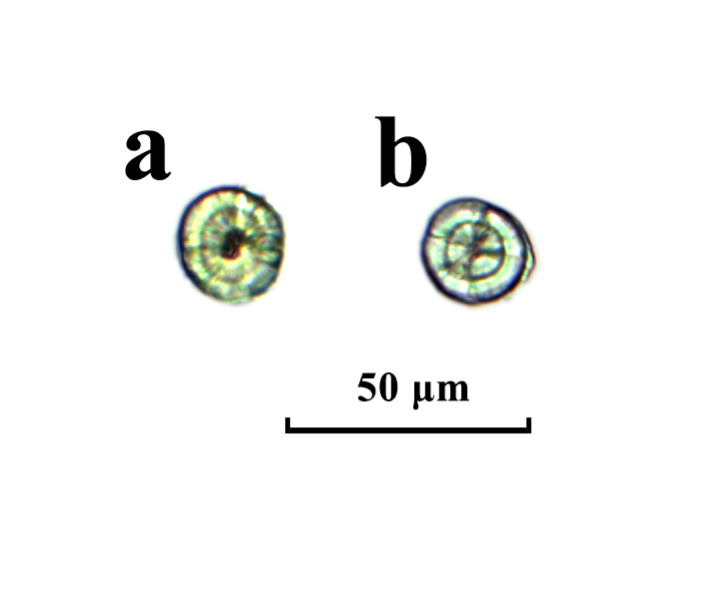
The observation of otolith shape and development revealed that the sagittae and lapillus were formed before hatching (Figure 1a). Initially, the sagittae and lapillus shapes of the newly hatched flounder were quite similar with both being nearly round. On the 3rd day after hatching, the fish opened their mouths. By the 5th day, the eyespots had turned black, the yolk sacs were fully absorbed, and the shapes of the sagittae and lapillus remained round (Figure 1b, Figure 2a and Figure 2d). Around the 7th day post-hatching, two pairs of otoliths became visible in the auditory sac of the fish: a lapillus pair near the eye and a sagitta pair at the back. While both otolith sizes were similar at this point, growth rate differences of the two pairs started to emerge. By day 15, the growth rates of the lapillus length and width exhibited differences. At 20 days, the sagittae diameter exceeded that of the lapillus. By this time, the lapillus shape had begun to shift from its initial subcircular form to a mussel-like shape, with the otolith core relocating to one end. At 26 days, the sagittal otoliths started extending to both ends, changing from subrounded to oval while the lapillus retained their mussel-like form (Figure 2b and Figure 2e). By day 30, all eyes were on the same side, and by the vomeronasal stage, some of the sagittal otoliths had adopted pear or arrow shapes, transitioning completely to these shapes by day 35, while the shape of the lapillus was more like mussel (Figure 2c and Figure 2f). For the initial 30 days post-hatching, otoliths could be observed and counted without any specific treatment.
Otolithic primordium and central nucleus
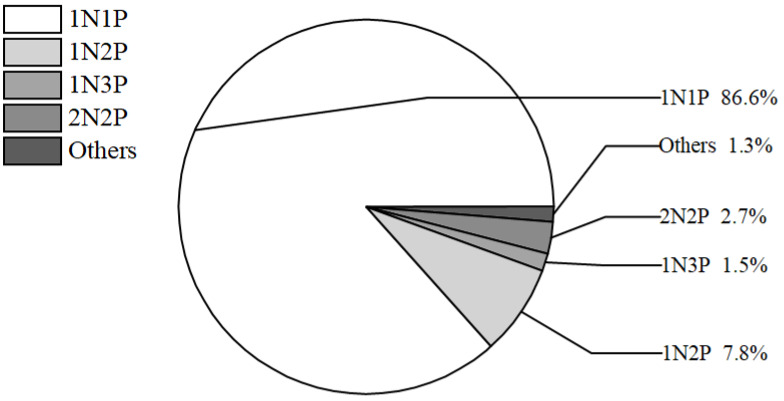
During the otolith growth process of Japanese flounder fries, typically one growth center referred to as the otolith center nucleus (N) was present, along with one otolith primordium (P) at the center. The N, serving as the circular growth center, added rings daily. In the otolith samples, variations were observed, including 1N2P, 2N2P, and 1N3P types in addition to the 1N1P type (Figure 3), with percentages of 7.85, 2.76, and 1.55 %, respectively (n = 1248, Figure 4). A small number of 2N3P, 3N3P, and 2N4P types (1.29 % of the total) were also identified. The primary central nucleus types and protopodium of sagittae and lapillus were consistent with the percentages of total otolith types (1N2P and 2N2P). The percentage of lapillus 1N2P types was 4.22 % higher than that of sagittal otoliths, with types 4N2P and 3N3P occurring exclusively on sagittal otoliths.
Characteristics of daily otolith rings in Japanese flounder
Clear and regular rings were not observed on the otolith surface from 0 to 4 days after hatching. However, a clear first ring did appear on the 5th day when the fish had a total length of 4.27–5.49 mm (Figure 5); subsequently, one ring formed on each day after that. As a result, a straightforward relationship between the age (D) of Japanese flounder and the number of otolith rings (n) was observed, which can be expressed as D = n + 5. Each complete daily ring of Japanese flounder otoliths consisted of one wider growth band and one narrower intermittent band (Figure 6). The growth band was transparent, while the intermittent band had a dull color. The observation revealed that the first daily ring had a notably darker color than the subsequent ones, with 2–4 dark bands of relatively lighter color interspersed between the two darker bands at older ages.
Relationship between total length and incubation days
The TL and age (D) of Japanese flounder larvae raised at 20 ± 0.5 °C exhibited a linear correlation (Figure 7). This relationship can be described by the equation TL = 0.3515 D + 2.9856 (R2 = 0.9472), indicating a daily growth rate of 0.3515 mm/D.
Relationship between length and width of otolith and age
Freshly hatched Japanese flounder litters had a total length of 2.55–2.87 mm and sagittae and lapillus diameters ranging from 16.45 to 23.56 μm, with little difference in size. The otoliths displayed cracks radiating outward from the central nucleus. On the 5th day, the flounder opened their mouths and transitioned from endogenous nutrition to exogenous intake, reaching a total length of 4.27–5.49 mm. At this stage, the sagittae and lapillus had lengths ranging from 23.00 to 30.43 and 20.00 to 27.82 μm, respectively. By the 30th day, when the young had a total length of 12.11–15.07 mm, the sagittae measured between 144.69–253.04 μm in length, and the lapillus ranged from 90.00–27.82 μm in diameter. During this period, the lapillus shape gradually changed from nearly round to oval and eventually to arrow- or pear-shaped by the end of the sampling.
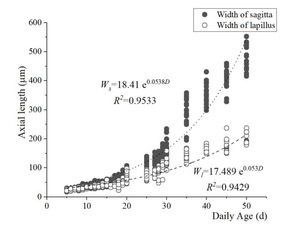
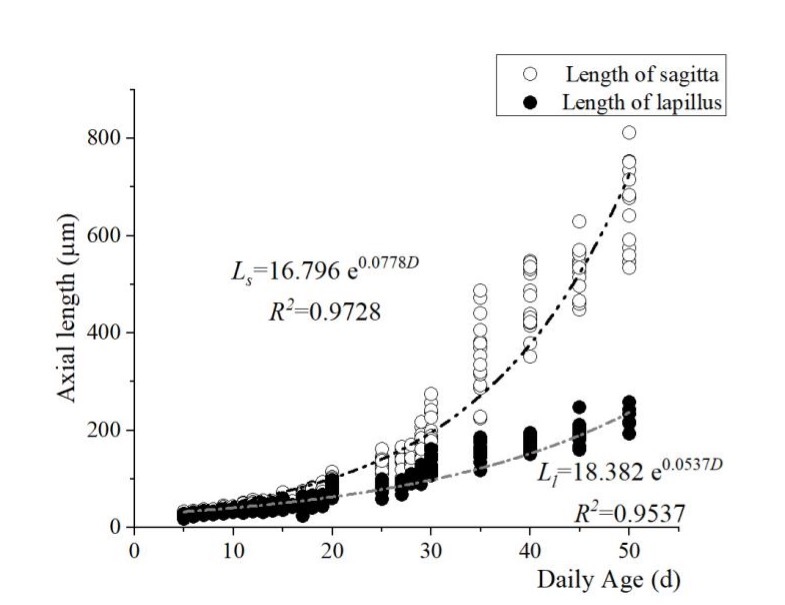
The length and width (L and W) of the two otolith pairs centered on the otolith core were measured, and their relationship to total length and days of incubation were calculated. The results showed that the lengths of the sagittal otoliths (Ls) and the lapillus (Ll) of the flounder young cultured at 20 ± 0.5 °C began to change markedly on the 25th day when the growth rate of the sagittae accelerated significantly. Both the length and width showed an exponential relationship with age (Figure 8, Figure 9):
Ls = 16.796 e0.0778D ( R2 = 0.9728),
Ll = 18.382 e0.0537D (R2 = 0.9537),
Ws = 18.41 e0.0538D (R2 = 0.9533), and
Wl = 17.489 e0.053D (R2 = 0.9429).
Relationship between the length and width of the otolith and its total length
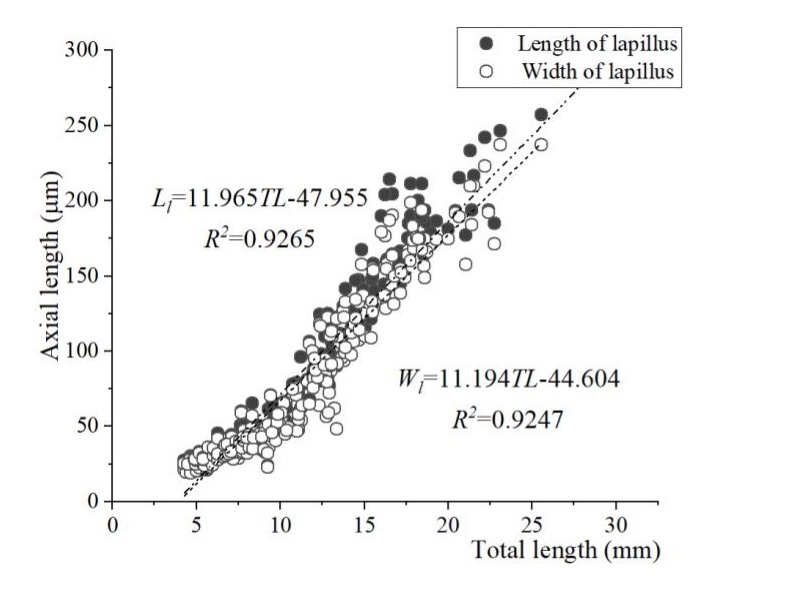
The relationships between the TLs of all the juvenile fish and the length and width of the sagittae and the lapillus were examined. Among these, the length and width of the lapillus displayed the most substantial linear fit to the total length of the juvenile fish. This finding indicates a markedly linear relationship between the total the length and width of the lapillus during the early flounder growth stages (Figure 10). The relationships with the length and width of the otolith, respectively, are as follows:
Ll = 11.965 TL - 47.955 (R2 = 0.9265) and
Wl = 11.194 TL - 44.604 (R2 = 0.9247).
The length and width of the sagittae were exponentially related to the total length of the litter (Figure 11). Because the growth rate of the sagittal otoliths in the Japanese flounder changed significantly at the 25th day, the relationship between Ls and TL was divided into two parts using the 25th day as the cut-off point. Both were exponentially related to the length of the litter. The equations defining the relationships are as follows:
Ls = 10.472 e0.1977 TL (R2 = 0.9492) and
Ws = 10.222 e0.211TL (R2 = 0.9543).
Relationship between lapillus and sagittal otoliths
As the TL of Japanese flounder fry increased, notable differences between sagittae and lapillus growth rates became evident after 25 days. Regression analysis of the measured data involving Ls and Ll of 310 fry revealed a linear relationship, expressed by the equation Ll = 0.3028 Ls + 28.534 (R2 = 0.9366) (Figure 12a). Similarly, a linear relationship was observed between the width and the two variables, described by the equation Wl = 0.3791Ws + 21.508 (R2 = 0.9426) (Figure 12b).
Discussion
First daily ring formation of otoliths
The determination of the first daily ring formation of otoliths and the continuity and completeness of daily ring formation are important in the age determination of juvenile fish. Earlier research relied on identifying the time of the first daily ring appearance on sagittal otoliths. Here, we revealed that the initial daily ring on the sagittal otoliths of Japanese flounder materialized on the 5th day. At this juncture, the yolk sacs of the fish had been entirely absorbed, and the fish had commenced reliance on exogenous nutrients for rapid growth, although the otolith growth rate remained at one ring per day. The periodicity of otolith daily ring formation aligns with observations from earlier studies of sagittal otoliths in starry flounders, striped catfish, and horse mackerel.5,6,39 However, the timing of the first daily ring formation exhibits notable variations among species. In certain species, this forms before hatching, with the first daily growth of sagittal otoliths already evident on the day of hatching.40 For instance, in blue tilapia, sagittarian otoliths commence forming 2–3 days before hatching.41 Conversely, in most fish, the first daily ring occurs on or after the day of hatching, and the timing of formation varies. For example, the first otolith ring from European sardine and French grunts is observed on the first day after hatching.42 Contrastingly, species such as haddock, pollock, small and large yellow croaker exhibit the first daily ring on the 2nd day after hatching.43–45 Nevertheless, Joh et al.46 documented the first daily ring forming on the 6th day post-emergence, a timing akin to that in brown sole. The formation of the first daily ring is usually associated with hatching or initial feeding.47 The emergence of the initial daily ring may be linked to embryo development and the initiation of feeding. A swift embryo development leads to the formation of the initial daily ring and daily increment after hatching or once the yolk sac is completely absorbed. Conversely, slow embryo development results in the initial daily ring formation prior to hatching.
Otolith shape and microstructure
The morphological progression of sagitta and lapillus in larval and juvenile Japanese flounder mirrors patterns observed in many other fish species, such as Qingbo and prenant’s schizothoracin.48,49 Initially, sagittal otoliths exhibit a round shape upon emergence from the membrane, laterally expanding and transitioning to an oval shape after 26 days. Subsequently, they gradually adopt a pear- or arrow-shaped form. Conversely, lapillus start as round and slowly transform into a mussel shape after 20 days. This morphological transformation is a common occurrence in the development of otoliths in teleostean fishes. Otolith shape is highly species-specific with their structure remaining relatively stable. Consequently, otoliths are considered suitable experimental materials for studying inter-species or group-level differences in fish.50,51 Furthermore, otolith shape serves as an indicator for seawater environments. Recently, the rapid expansion of acidified waters in the ocean has altered the chemical balance of seawater, posing a major threat to various marine organisms and ecosystems reliant on a stable chemical environment. Wang et al.52 conducted simulations to ascertain the effects of acidification on the otoliths of adult marine Medaka. The study simulated possible levels of acidification in near-shore waters and discovered that seawater acidification did not substantially impact otolith shape but did affect the symmetry of vectorial otolith shape and weight.
Regarding the microstructure of otoliths of Japanese flounder, the typical occurrence involves a single central nucleus and primordia. However, instances of multiple primordia and central cores exist. We also found The percentage of lapillus 1N2P types was 4.22 % higher than that of sagittal otoliths of Japanese flounder. According to the former studies, some fishes had the otoliths with multiple primordia, such as Gymnocypris potanini, Grass Carp, Silver Carp, Lost River sucker and shortnose sucker, yet the reasons for their presence were not thoroughly explored.53,54 Furthermore, otoliths with multiple primordia or central cores were not investigated in comparison to fish with single primordia and central cores concerning growth, variations, and survival rates. Further investigations are necessary to delve into these aspects. In addition to interspecific variability in otolith shape, disparities in the size and shape of the central nucleus can serve as an effective means to differentiate between wild and cultured populations. Studies of silver and grass carp larvae revealed significantly smaller diameters of otolith center nuclei in artificially propagated populations compared to those in wild populations.54
Otoliths and growth
Here, when the long and short diameters of the otoliths were examined, characteristic growth patterns resembling ring-like structures were revealed. Lapillus growth exhibited a linear relationship with the fish total growth, while sagittal otoliths exhibited an exponential relationship. Lapillus pairs proved to be more suitable for the early identification of juvenile fish, and notable differences in shape and growth between sagittal otoliths and lapillus were observed after 25 days. By this point, sagittal otoliths had become thicker and necessitated polishing for clear observation, which, in turn, could risk damaging the ring pattern. Consequently, lapillus were deemed more appropriate for age identification than other otolith pairs. For those considering using sagittal otoliths for growth predictions in Japanese flounder, valuable insights could be derived from the results presented by Kang et al.55 As an age identification method for yellow croaker otoliths, different sections can be utilized for ring identification, effectively addressing challenges posed by larger otoliths that may not fit entirely within the field of view of the microscope. Additionally, this approach helps tackle the issue of increased otolith thickness with advancing age, which can lead to the focal point not being confined to a single plane, resulting in overlapping, blurred, or entirely absent rings.
By analyzing the relationship between the length and width of the otolith and the total length, we found that when the relationship between Ls and Ws of the sagittae and TL is exponential, R2 shows the highest fitting degree, i.e., 0.9492 and 0.9543, respectively, and both values were above 0.9. The linear relationship of Ls and Ws of the sagittae and TL was Ls = 36.419 TL - 220.22 (R2 = 0.8425), Ws = 27.811 TL - 158.26 (R2 = 0.8726), and both R2 values were below 0.9. Therefore, the relationship between the length, width and total length of sagittae is fit to be an exponential relationship. When the relationship between the Ll and Wl of the lapillus and TL is linear, R2 values were 0.9265 and 0.9247, respectively. The exponential relationship between Ll and Wl of the lapillus and TL was Ll = 13.234 e0.1448 TL (R2 = 0.9324), Wl = 12.58 e0.1432 TL (R2 = 0.9255), R2 between the linear and exponential relationships was very small, and we observed that the growth of the lapillus was faster in the early stage and leveled off in the late stage. Hence, we preferred to use the linear relationship to describe the relationship between the Ll and Wl of the lapillus and TL.
To explore the connection between fish otoliths and growth—aside from this study that employed the relationship between otolith length and body length—researchers associated otolith mass, daily increment, and radius with body length, thereby providing additional compelling evidence that underscores the utility of otoliths as a favorable experimental material for the assessment of fish growth and age. For example, Oshima et al.38 meticulously measured the daily increment of lapillar otoliths in early Japanese flounder from the Pacific coast of Japan and established a strong correlation between the daily radius of otolith increments and body length. Jawad et al.56 delved into the relationship between body length and otoliths in four fish species in the southeastern Yellow Sea, using measurements of otolith weights and lengths. In a comparison and fitting of the equations that described the relationships between otolith mass and age, as well as body length and age for Leuciscus waleckii, Kang et al.55 discovered that the correlation between otolith mass and age outperformed that between body length and age. This insight led them to utilize otolith mass for backward extrapolation of age, resulting in an impressive 94 % agreement rate between the inferred and counted age, all within a one-year age bracket. This outcome lays a robust foundation for the application of otolith mass in fish age identification. Otolith quality linearly improves with age, and this method offers a more efficient and expedient alternative to age identification compared to that of the conventional method which is reliant on otolith rings.
Nonetheless, certain limitations in using otolith quality for age identification persist, given that the formation mechanism of otoliths is still under investigation. Questions remain such as whether variations in the growth environment markedly affect otolith quality, potentially impacting the age identification accuracy. Additionally, uncertainties arise regarding the potential implications of extremely low otolith quality and any anisotropic growth patterns during specific fish development stages. Furthermore, the applicability of otolith quality to represent a broader age and length range of fish for age identification requires further investigation. In the foreseeable future, a combined approach that integrates ring counting and otolith quality could address issues related to low otolith quality in age identification while simultaneously improving ring counting efficiency in the later life stages of fish. While the utilization of otolith mass for age identification can provide an initial estimate at a yearly level, achieving precision to the exact day necessitates the continued use of the ring counting method.
Leveraging otolith information for early growth research in fish offers insights into their early life history and aids in reconstructing the ecological background during that phase. This understanding helps researchers discern fish spawning grounds and hatching periods. Consequently, comprehending early otolith growth and development patterns establishes a foundation for exploring the ring information encapsulated within otoliths. It also furnishes essential data for future applications, such as reconstructing the early life history of Japanese flounder, assessing the impacts of stocking and releasing Japanese flounder, and distinguishing between different groups. Furthermore, this study highlights lapillus as advantageous for otolith studies of juvenile fish, given their ease of processing and clearer otolith ring patterns compared to sagittal otoliths, suggesting that lapillus should take precedence in future juvenile fish otolith research.
Conclusion
Fish age identification is the basis of fishery resource assessment, and otolith is one of the main fish age identification materials. Currently, no unified age identification standard for Japanese flounder exists. To study the growth and life history of Japanese flounder in natural waters, the otolith daily increment formation and early growth of Japanese flounder were studied in this study. We found that sagittal otoliths and lapillus began forming even before the fertilized eggs hatched. The initial daily ring on the sagittae and lapillus materialized on the 5th day, when the yolk sacs of the fish had been entirely absorbed. The sagittal otoliths changed from subrounded to oval at 26 days, and had adopted pear or arrow shapes by day 35. The lapillus shape had begun to shift from its initial subcircular form to a mussel-like shape. After 25 days, the growth difference between the sagittae and lapillus became larger, the sagittae became thicker, and the observation process of the daily ring became more complicated. We also found that the otoliths had characteristic growth patterns resembling ring-like structures in the long and short diameters, and exponential or linear correlations were present between otolith and age and length, and hence, the growth and life history of Japanese flounder in natural waters can be studied by the daily ring on the otolith.
Acknowledgments
This work was supported by the Project of Yellow River Fisheries Resources and Environment Investigation from the MARA, P.R. China (HHDC-2022-0405) and Scientific Research Foundation of Hebei Normal University of Science & Technology (2019YB018, 2022YB036).
Authors’ Contribution
Conceptualization: Jiabao Tang (Equal), Qinglin Wang (Equal). Methodology: Jiabao Tang (Equal), Zhaohui Sun (Equal). Writing – original draft: Jiabao Tang (Equal), Shanshan Yu (Equal), Fei Si (Equal). Formal Analysis: Jiangong Ren (Equal). Investigation: Jiangong Ren (Equal). Funding acquisition: Shanshan Yu (Equal), Fei Si (Equal). Writing – review & editing: Fei Si (Equal). Supervision: Fei Si (Lead).
Competing of Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
Japanese flounder was used in this study following the Guidelines for the Care and Use of Laboratory Animals by the Chinese Association for Laboratory Animal Sciences (No. 2011–2). The Animal Care and Use Committee of Beidaihe Central Experiment Station, Chinese Academy of Fishery Sciences, approved the experiments.
Informed Consent Statement
All the authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.

_during_the_fertilized_floun.png)








_and_width_(b)_of_sagitta_and_lapillus.png)
_during_the_fertilized_floun.png)





_and_width_(b)_of_sagitta_and_lapillus.png)