INTRODUCTION
Zebrafish Danio rerio (Hamilton 1822) is in the top 30 best selling ornamental fish species.1 This species has been used as a model species in many biomedical and human diseases research. It is most important for biomedical and human diseases research as a model species as well as for the development of aquaculture in developing countries. Rapid development with a short generation, ovipar reproduction and embryo transparency, availability throughout the world, reasonable cost of maintenance, and possessing orthologous genes with humans make them indispensable vertebrate animal model.2–4
Gametogenesis is an energy-consuming process that can be successfully performed only when adequate energy stores are available within the fish. Current studies show that probiotics play a significant role in metabolism and reproduction.5,6 They are used to improve reproduction and growth performance in ornamental zebrafish.7 Of these, Bacillus subtilis (B. subtilis) is a fast growing, Gram positive, aerobic, potential probiotic found naturally in the human gut and in fermented foods. It belongs to the genera Bacillus, and Bacillus produces various extracellular enzymes, antimicrobial substances, and several digestive enzymes, as well as acts as a growth promoter.8 It has the ability to break down proteins and carbohydrates and produces vitamins biotin and cobalamin.9 B. subtilis has been used in many studies as a probiotic and the number of these studies is increasing rapidly.10,11
The effects of Artemia enriched with probiotic, B. subtilis on the reproductive parameters and relative fecundity of ornamental fish, Poecilia latipinna was investigated. Maximal fry production per female and relative fecundity were found to be significantly higher in fish fed with enriched Artemia with B. subtilis than in the other groups.7
Further studies on zebrafish have shown that probiotics have positive effects on zebrafish intestinal health. Probiotics living in water, on the surface, and inside the fish larvae provide protection, increased development, and increased functionality of the intestines. They produce several digestive enzymes and vitamins.12 Effects of probiotics on zebrafish model infected with Aeromonas hyderophila have been investigated.13 The authors suggested using probiotics for the improvement of public health and ornamental fish and fisheries industries.
The effects of probiotic Pediococcus acidilactici on the expression of five quality markers in zebrafish testis were studied.14 The authors indicated that P. acidilactici had a potential use as a probiotic supplement in the zebrafish diet for the improvement of molecular parameters in testicular cells, indicating that probiotic supplementation could affect male reproductive performance.14 Duration of their study was only 10 days and their probiotic was P. acidilactici, in addition thay had excluded females and investigated the effecst of P. acidilactici on males. Age of zebrafish was eight months. The effects of dietary probiotic Lactobacillus rhamnosus administration on six-month-old female zebrafish was investigated.15 Their study has demonstrated the beneficial effects of diet and gut microbes in the maturation of female zebrafish. The duration of the experiment was also 10 days. The authors used L. rhamnosus as a probiotic and excluded males.
In the present study, the duration of the experiment was 100 days, and the experiment started just after the first feeding at two weeks post-fertilization. Probiotic was B. subtilis. Gonads of both, males and females were examined. These were the main differences between the present study and the study by Valcarce et al.14 and Gioacchini et al.15
Research related to probiotics and reproduction, and intestinal health is still scarce in comparison with the amount of research on disease resistance, and more studies on the effects of probiotics on reproduction in aquaculture were suggested by Aydın and Çek-Yalnız.11 Moreover, to our knowledge, there is no study that investigates the effects of probiotic B. subtilis supplemented diet on male and female gametogenesis and intestinal health starting at two weeks post fertilization. In this regard, the primary goal of this study is to evaluate the effect of different inclusion levels of B. subtilis on growth, survival rate, spermatogenesis, and oogenesis of zebrafish. In addition, the effects of probiotic feeding on intestinal health were observed by histopathological method. The results of this study could be beneficial to the reproductive performance improvement strategy in ornamental zebrafish culture.
MATERIALS AND METHODS
Experimental fish, design and feeding
Fish experiments were approved by the Iskenderun Technical University in Turkey (The Project number is 2022LTP-08). Artificial production of experimental zebrafish (Danio rerio, Hamilton, 1822) was done in the aquarium unit of Marine Science and Technology Faculty, İskenderun Technical University, İskenderun, Turkiye. The larvae production method was performed according to Dede and Çek-Yalnız.16 After four days of post-fertilization, 600 larvae with an average weight of 0.014 ± 0.002 g. were selected randomly and assigned to five groups with three replicates, with 40 juveniles in each aquarium. A static water system including 15 acrylic tanks of 29-L capacity (23x35x37 cm) was used. During the experimental studies, the photoperiod was maintained on a 12h light: 12h dark schedule. Water in each aquarium was continuously aerated with 4 cm air stone, and 70% of water was exchanged weekly. Water quality parameters were measured and recorded once a week. Water temperature ranged from 24° to 28°C. The mean temperature was recorded as 27±1˚C. The minimum and maximum dissolved oxygen levels were 6.5 mg/L and 7.5 mg/L, respectively. Ammonium level was <0.03 mg/L, nitrite level was< 0.06 mg/L and nitrate level was recorded as 5.18 mg/L. During the experimental work, fish were fed with two different feeds. The first one was Artemia (Subreme Bay Brand, INC. San Franciso, USA) and the second one was dry feeds containing probiotics. In other words, the fish were given the live prey from the time of the first-feeding until the time of the establishment of the treatment groups at 2-weeks post fertilization, and provided the dry feeds thereafter.
Preparation of Diets
The essential commercial feed(Bacillus subtilis HBB 493®, AHM, Micron, Türkiye) was composed of 43% crude protein, 4% crude lipid, 9% ash, 8% water, and 8% crude fiber. B. subtilis was purchased from Fisher Turkiye, dissolved in sterile distilled deionized water, and sprayed on the commercial feed. 2% fish oil was used as a binding agent and was added to the commercial feed by spray method. Maltodextrin was added as a stabilizer in all experimental diets, including control groups. Diet of all other experimental groups (excluding control groups), were prepared by addition of B. subtilis in 1.5% (6.5x109 cfu/g), 3%(1.3x1010 cfu/g), 6%(3.9x1010 cfu/g ) and 9% (3.9x1010 cfu/g) ratio. Further, experimental feed was gently homogenized in Stainless Stell 3D Tumbler Mixer (Alphie, Hexagon, India) at 80 rotations for 20 minutes. Prepared diets were dried at room temperature (25°C) and stored in the refrigerator (+4°C) until their use. Each diet was fed twice at the visible satiation per day (at 09:00 and 17:00) for 100 days.
Growth parameters
In order to collect growth parameters, 10 fish in each tank were weighed randomly at each sampling period, and all fish present in the tank were counted to get records to assess cumulative mortality across treatments.
Growth parameters data were taken at 0, 20, 40, 60, 80 and 100 days post-fertilization and were calculated as follows:
IW= Initial Body Weight (g)
FW= Final Body Weight (g)
WG= Body Weight Gain (%)
WG (%) = [(FW−IW)/IW]x100
Specific Growth Rate (SGR), (% BWday−1) = [(Ln FW– Ln IW) / (Experimental Period) ]×100
Feed Conversion Ratio (FCR %) = Total Fed (g) /Body Weight Increase (g) ×100
Daily Growth Rate (DGR) = ((FW – IW) x100/ experimental Period x IW)
INf= Initial number of fish
FNf= Final number of fish
Survival Rate (SR%) = (FNf/INf) x100
Histopathology
At the end of the experimental studies, after 100 days, five fish from each aquarium were sampled for histopathological observations. The fish were anesthetized in 2-phenoxethanol (0.4%). Head and tail part of the fish were removed and the middle part of the body was fixed in 10% neutral buffered formalin, dehydrate, embedded in paraffin, sectioned at 5µ, and stained with hematoxylin and eosin for histopathological examination. The developmental stages of gonads were determined for each fish. Classification of testis and ovaries was based on the histological criteria adapted from Çek et al.,17 Çek and Yılmaz.18 The health of the intestine was also observed under the light microscope and photomicrograph was taken.
Statistical analysis
The data are presented as the mean ± standard error (±SD). Differences between groups in terms of weight and length were determined by the one-way ANOVA test. Differences in survival rate, specific growth rate, daily growth rate, and food conversion rate between groups were tested with (SPSS ver 13 for Windows 10, SPSS, Chicago) Duncan’s multiple comparison procedure.19 Effects with a probability of P<0.05 were considered significant.
RESULTS
Effects of Bacillus subtilis on Growth Parameters and Survival Rate
At the beginning of the experiment, there were no significant differences between the initial body weight and length of zebrafish (P>0.05). As the experiment progressed, improved growth parameters were recorded in all B. subtilis fed groups (Table 1, Figure 1). The fish fed with the highest level of B.subtilis diet (V) had significantly higher final weight gain than fish fed diets II, III, and control groups (I). Among the experimental groups, fish fed the highest level of B.subtilis diet (V) had the lowest food conservation ratio, and significant differences were recorded than fish fed diets II, III, IV, and control groups (P<0.05). The specific growth rate gradually increased among the experimental groups, starting from 1.75±0.10 in group II and reaching 2.00±0.08 in group V (Table 1). In the control groups, the lowest body weight gain, lowest specific growth rate, and lowest daily growth rate were measured. The survival rate was highest in dietary B.subtilis group IV. However, there were no significant differences in survival rate between experimental and control groups (P>0.05). The present results indicated that increasing dietary B.subtilis level led to increasingly better daily growth rate, specific growth rate and feed conversion ratio (Table 1).
At the beginning of the experiment mean larvae weight and length were almost the same among experimental and control groups (P>0.05, Fig. 1). However after 14 weeks, at the termination of the experiment, juveniles in 0.90 B.subtilis groups were significantly larger than control groups (Fig. 1). In other words, the only significant difference in FW was between the control and the fish in the highest probiotic treatment groups.
At the termination of the experiment SGR, WG, FCR, DGR, FW in the fish fed group V were significantly different from those observed in the control, group II, and group III B. subtilis fed groups. Specific growth rate (SGR) and mean weight gain (WG) followed a similar pattern. As the experiment, progressed SGR and WG increased steadily and reached the highest value in group V. Lowest value of SGR and WG were recorded in the control groups. Overall, SGR increased as the level of B. subtilis increased and the highest SGR was recorded in the fish fed diet V groups (9g/Kg) (Table 1).
The mean feed conversion rate was best in group V. The worst feed conversion ratio was recorded in the control group (I). Significant differences were measured between control and group V (P<0.05), (Table 1).
Table 1 shows the survival rates of both control and dietary B. subtilis fed groups. Total survival rates in the control (I) and experimental groups (II, III, IV and V) were uniformly high ranging from 88.3% to 95% (P>0.05). The lowest survival rate was recorded in the fish fed diet II, whereas the highest survival rate was recorded in the fish-fed diet IV groups (6g/Kg). Nevertheless, there were no significant differences between the control (Group I) and the experimental groups (II, III, IV, and V) (P>0.05) (Table 1).
Table 1 shows the daily growth ratio of both control and dietary B. subtilis fed groups. The lowest daily growth ratio was recorded in the control groups, whereas the highest daily growth ratio was recorded in the fish-fed diet V groups (9g/Kg). As a whole, the daily growth ratio increased as the level of B. subtilis increased, and there were significant differences between the control (Group I) and the experimental groups (IV and V) (P<0.05) (Table 1).
Histological results
The formation of spermatozoa in D. rerio was divided into five developmental stages, and the phases of spermatogenesis were distinguishable based on their characteristic nuclear and cytoplasmic morphologies.
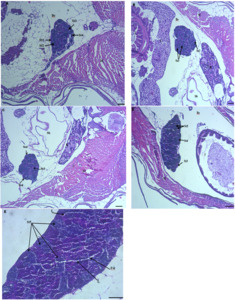
The gonad histology of male zebrafish after feeding with dietary probiotics is provided in Figure 2. At the end of the experimental study, although, all developmental stages of testis were indicated in all experimental groups, in the control group, spermatogonia (St1), primary spermatocytes (St2), and secondary spermatocytes (St3) were most abundant (Fig. 2A). In fish fed with dietary probiotics in group IV and V testis at the stage St5 (spermatozoa) and maturation stage were most abundant (Figs. 2D and E). The testis of fish fed with dietary probiotics in groups III and IV showed a higher proportion of secondary spermatocytes (St3) and spermatids (St4), while fish in group V showed a higher proportion of spermatozoa (St5) (Figs. 2B,C and D). Maturation of testis was detected in the group fed with dietary probiotics 9g/kg. In this group, free sperm cells were detected in the lumen (Fig. 2E). As a whole, the developmental stages of testes increased as the level of B. subtilis increased.
Formation of fertilizable egg in the D. rerio was divided into seven developmental stages and the phases of oogenesis were distinguishable based on the appearance of nuclei, nucleoli, and distribution of cytoplasmic inclusions.
In the control group, the ovaries of D. rerio contained mainly oocytes at stage 1 and 2. In some ovaries, vesicle formation and oocytes at the secondary growth phase were also detected (Fig. 3A). Oocytes at the vesicle formation stage were most abundant in the fish fed diet II (Fig. 3B).
Vesicle and exogenous yolk formation were detected in the ovaries of dietary probiotic supplemented groups III, IV and V (Fig. 3C, D and E). The largest oocytes were stage 7 oocytes (St7) and were completely yolk-filled structures. These stages were most abundant in the fish fed diet V group. Yolk granules (vitellogenin) stained pink with hematoxylin and eosin staining and exogenous yolk formations were not observed in the control group (Fig. 3A). Stages 4, 5, 6 and 7 of oogenesis were not detectable in the control group while these stages including micropyle development, germinal vesicle degeneration and movement of germinal vesicle and fully matured egg (stage 7) were clearly detected in the dietary B. subtilis supplemented group IV and V (Fig. 3D and E).
The gut structure of different treatment groups showed several differences, and some structural anomalies were detected in the control groups (Fig. 4A). These differences were in goblet cells, villus health, and length. The villus ruptured and disposed of, and eventually, a large lumen occurred in the control group (Fig. 4A). The intestinal health of the fish fed the B. subtilis diets increased gradually. In these groups, villus and goblet cells were intact and were clearly detected. Structural anomalies such as excessive hypertrophy, fusion of villus, and degeneration of the mucosal lining were not observed (Fig. 4B, C, D, and E). Moreover, intestinal villus lengths of the fish fed diets containing B. subtilus tend to be higher than that of the control groups (Fig. 4A, and 4C, E).
DISCUSSION
The goal of the present study was to find the effects of dietary incorporation of B. subtilis on growth performance, survival rate, gametogenesis, and intestinal health in D. rerio.
Results show that B. subtilis was beneficial at all dose levels in increasing body weight, length, specific and daily growth rate, feed conversion ratio, gametogenesis, and intestinal health. Survival rate was high in control and all treatment groups; there were no statistical differences between control and fish-fed dietary B. subtilis groups. These results are consistent with the reported results from another study on zebrafish. A previous study examining probiotic effects on growth performance and feed utilization of zebrafish showed the improvement of growth performance and protection of zebrafish against A.hydrophila, a gram-negative bacterium, by improving their mucosal immunity and modulating inflammatory responses.20 In another study, administration of B. subtilis showed the improvement of growth and reproductive parameters and feed utilization of ornamental fish , Poecilia latipinna, after 60 days and able to regulate the activity of digestive enzymes and intestinal microflora.7 Feeding striped catfish (Pangasius hypophthalmus) with probiotic Bacillus subtilis supplementation for eight weeks demonstrated improved growth performance across all dietary groups. Growth consistently increased with higher levels of B. subtilis in the diet compared to the control group.11
In the present study, after feeding the zebrafish with experimental diets for 100 days, spermatogenesis, including maturation phases, was detected in groups II, III, IV, and V. Whereas St4, St5, and maturation phases were absent in the control. These results are consistent with the results of Enzeline.6 The authors investigated the effects of Bacillus sp. supplementation in the diet of African catfish Clarias gariepinus for a duration of 60 days. The results showed that the dietary probiotic supplementation of Bacillus sp. could accelerate the spermatogenesis process in the male African catfish compared to the control. The authors offered an exact amount of 108cfu/g probiotic Bacillus sp. and recommended it as the starting point for the improvement of the reproductive performance in African catfish. The reproductive physiology of silver catfish, Rhamdia queen, was improved by the dietary inclusion of probiotics.5 In the same study, the inclusion of 0.60g/kg of the probiotic could be recommended for the improvement of the reproductive performance of R. quelen. The authors included the histological observation of the gonads, and measured sexual hormone levels.
Saeidi and his colleagues used Lactobacillus plantarum as a dietary supplement.21 The authors investigated its effect on ornamental zebrafish reproduction parameters. It is important that the authors suggested an exact amount of probiotics, 3%, and concluded that L. plantarum should be used as a probiotic supplement in the diets of ornamental zebrafish.
Ghosh and his colleagues carried out the pioneer study on the effect of B.subtilis on the reproduction performance of four ornamental fish species, namely Poecilia reticulate, Poecilia sphenops, Xiphophorus and Xiphophorus maculatus. The authors excluded males and found out that supplementation of B.subtilis in the diet of female ornamental fish positively affected their reproductive performance and increased their survival rate.22
While the finding of authors5,6,21 and,22 are not contradictory to our results, there is a lack of information in the literature on the mechanism, clearance, and pathways of B. subtilis in Zebrafish and other fish species. In the present study, incorporation of vitellogenin was clearly detected in the B.subtilis dietary groups IV and V. For the most vertebrate fish species, including zebrafish, during the oogenesis processes GtH releases from the pituitary gland of the fish and moves into the ovary and induces the theca cells. In the theca cells, cholesterol is converted into the sexual hormone, testosterone that then moves into granulosa cells, through a series of enzymatic conversions, testosterone is converted into estradiol-17β by aromatase, p450 encoded by cyp19a gene, following this conversion, the estradiol-17β moves into liver and makes hepatocyte cells to work and release the protein called, vitellogenin. Thereafter vitellogenin moves into oocytes by blood cells. How the B.subtilis affects these phases remains to be elucidated. In other words, is there any connection between B.subtilis and the pituitary gland, GtH, androgen, and estrogen release needs to be investigated.
Gioacchini and his colleagues 15 investigated the effects of probiotics on the reproduction of ornamental zebrafish for a 10-day experiment. A significant increase in the gonadosomatic index was found, and the authors concluded that gut microbes have a pivotal role in the reproduction of zebrafish.
In our study, the beneficial effects of B. subtilis on intestinal health were clearly detected. It was emphasized that B. subtilis is a beneficial dietary supplement for ornamental zebrafish intestinal health. Some other researchers have reported similar findings. Such as, the effect of dietary supplementation of potential probiotic Lacticaseibacillus casei on intestinal microbiota and gut histology of ornamental zebrafish was investigated.20 Based on the histological observations, the authors suggested using L. casei as a supplementary probiotic for enhancing the intestinal health of zebrafish. Whereas, the probiotic had no significant effect on the rate of colonization of Lactobacillus in the gastrointestinal tract of zebrafish.
After feeding the zebrafish experimental diets for 10 days, enhancement of the gut microbiota was detected.15 High levels of colonization of Lactobacillus rhamnosus in the gut of probiotic treated zebrafish were revealed. They suggested that L. rhamnosus could populate the gastrointestinal tract of zebrafish and likely influence host biological functions via interactions with the host mucosal interface. Nevertheless, the colonization of the suggested probiotic in the fish intestinal tract may not be possible in just 10 days. The suggested probiotic may be temporally colonized, and therefore, it should not be recommended for use in zebrafish culture. Because some studies indicate and state that colonization of probiotics in the intestinal tract may not be possible in one week.23,24 In addition, it is extremely important to examine the intestinal tract to see if there is any inflammation resulting from the applied probiotic-supplemented diet. It may not cause any inflammation in such a short duration (10 days); intestinal tract must be examined in the long term. In our study, we examined the intestinal tract at the termination of the experiment (after feeding with B. subtilis supplemented diet for 100 days) and did not detect any inflammation.
Some missing points exist in our study where only a histological-based method was used. However, in order to clearly assess the level of host and probiotic interaction, further studies are indispensable using real-time PCR, cell culture-based methods, electron microscopy, and/or histochemistry. Moreover, investigations are vital to determine whether intestinal health is directly related to colonization of probiotics or not. As it maybe, populations in the gastrointestinal tract may only persist with regular probiotic feeding and increasing intestinal health.
It is crucial to determine the optimal levels of Bacillus subtilis supplementation for zebrafish to prevent underdosing, which may reduce effectiveness, and overdosing, which could lead to unnecessary costs and potential adverse effects. In our study, four different levels of B. subtilis were used, and it suggested that 3.9x1010 cfu/g was the most effective for enhanced growth performance, survival rate, gonadal development, and intestinal health of zebrafish.
Conclusion
In conclusion, the current study highlights the positive effects of B. subtilis on growth parameters, survival rate, gametogenesis, and intestinal health of zebrafish. Gametogenesis and health of the intestine of the fish fed diets supplemented with B. subtilis increased gradually. Vitellogenin incorporation was evident. Villus and goblet cells were intact. It is suggested that the dietary supplementation of 3.9x1010 cfu/g probiotic B. subtilis should be used for the enhancement of growth parameters, survival rate, gametogenesis, and intestinal health in ornamental zebrafish.
Acknowledgements
We would like to thank Yavuz MAZLUM for performing statistical analysis, to Metin YAZCISI for diet preparation, and to Koray Umut YARAŞ for taking care and sampling of fish. We are grateful to Ersin Bahçeci for allowing us to use his laboratory. We also would like to thank to Iskenderun Technical University Center for Science and Technology Studies and Research (ISTE-CSTSR).
Author Contribution
Investigation: Kemal Dede (Lead). Formal Analysis: Kemal Dede (Lead). Software: Kemal Dede (Lead). Conceptualization: Sehriban Cek (Lead). Writing – original draft: Sehriban Cek (Lead). Writing – review & editing: Sehriban Cek (Lead).
Funding
This article is part of Kemal Dede’ s PhD. thesis. Thesis was funded by a grant from the Iskenderun Technical University (The Project number is 2022LTP-08).
Ethics Statement
Fish experiments were approved by the Iskenderun Technical University in Turkey and were conducted in agreement with the guidelines of Republic of Turkey of Iskenderun Technical Laboratory Animal Ethics Committee.
Data Availability
The data sets (More and original figures) that support this study’s findings are available and will be provided by the authors upon reasonable request.
Conflict of Interest
The authors declare that they have no conflict of interest.
Consent to participate
All authors agree to participate
Consent for Publication
All authors agree to publish.