Introduction
The Tarim River system is a crucial water body in China’s arid inland region, known for its long history and complex ecology.1 As China’s longest inland river, it originates in the Tianshan Mountains, flows through the Tarim Basin, and eventually empties into the Tarim Salt Lake.2 The unique geographical and climatic conditions along the river support distinct biodiversity, particularly in fish resources, which are of significant interest for scientific research and ecological restoration. The diverse fish populations in the Tarim River system reflect the area’s ecological characteristics.3 Among them, Schizothorax is famous for its unique biological characteristics and adaptability, and has become a hot topic in academic research. Their importance in the food chain has led researchers to conduct in-depth discussions on the ecological habits, reproductive behavior, and adaptability to the water environment of this group.4
The Tarim River system has bred a variety of fish species, among which the representative species of Schizothoracine fishes, Diptychus maculatus and Aspiorhynchus laticeps, have attracted wide attention5,6. D.maculatus belongs to Cypriniformes and Cyprinidae (Institute of Zoology, Chinese Academy of Sciences, 1979).7 It is a flagship species in the Tarim River system. It is distributed in alpine cold waters and is a rare fish endemic to China.7 Steindachner first recorded and described D.maculatus on the basis of specimens collected at Upper Source Leh on the Indus River, and named it D.maculatus in 1866.8 In recent years, due to the construction of a large number of reservoirs and dams, the breeding habitat and migration channels of D.maculatus have been severely damaged, resulting in a sharp decline in its wild population.9 Due to its endangered status, D. maculatus has attracted wide attention. In 2021, the D. maculatus was listed in the ‘List of National Key Protected Wild Animals (Grade Ⅱ)’.10 According to its ecological characteristics, the research of D.maculatus mainly focuses on distribution, external morphology, physiological ecology, artificial reproduction and disease control. The A. laticeps also belongs to the Cyprinidae, and its habits tend to inhabit large lakes and slow-flowing waters, which has rich economic value.11 The distribution of this fish has been seriously affected by human activities, and there are only a few distribution points left in the area where it was originally widespread.3 The A.laticeps was listed in the ‘List of National Key Protected Wild Animals (Grade Ⅰ)’ and was included in the ‘China red data book of endangered animals (Fish)’ as early as 1988.10,12
D. maculatus is characterized by its prominent spots on the sides of its body, bright colors, and strong adaptability, allowing it to thrive in various aquatic environments. It plays an important role in natural ecosystems and fishery production.8 A. laticeps is widely recognized for its unique kiss-shaped mouth and living habits; its flat snout is adapted for a specific feeding method, yet its population size and distribution are significantly affected by changes in the water environment.13 Although these two fish species have different biological characteristics, they are both vital for the stability and health of the Tarim River ecosystem. Studying their ecological habits and adaptation mechanisms is of great significance for protecting the fragile ecosystem of the Tarim River Basin. Furthermore, protecting the habitat of D. maculatus is crucial for maintaining the ecological balance of A. laticeps and further promoting the protection of regional biodiversity.11,14 Therefore, the comprehensive study of these two fish species can promote the ecological protection of the Tarim River system and help to establish a more stable natural habitat and biodiversity conservation framework.15
To deeply analyze the biological characteristics of the two endangered fishes, effectively protect the germplasm resources of the world’s endemic fishes, and consolidate the basic data system of the biology of the world’s endemic fishes. This study mainly collected and analyzed the morphological characteristics, age and growth characteristics of the two rare fish species distributed in the Tarim River system from 2023 to 2024, and discussed the adaptive behavior of these two rare fish species. It is hoped that the in-depth study of the two endangered fish species will promote the protection of aquatic biodiversity and ecological balance, provide important data and information for the development of biology, ecology, and other related disciplines, and lay the foundation for the conservation of fish germplasm resources.
Materials and Methods
Sample collection
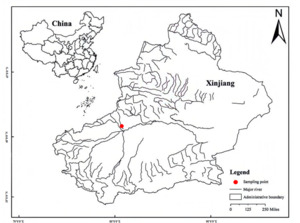
In this study, which was approved by the Science and Technology Ethics Committee of Tarim University (approval code:2023027). From 2023 to 2024, 5 samples of D. maculatus samples and 3 samples of A. laticeps were collected by using gillnets and surface cages (mesh 2a = 20 mm) in the Tarim River (81°57’E, 41°92’N) system, China (Figure 1). Biological determination and gender identification were performed on site, and three pairs of otoliths were removed and stored in 0.2 mL centrifuge tubes; 4-9 vertebrae and anal scale were fixed with 95% ethanol in 2 mL centrifuge tubes for use; and other tissues were fixed with 10% formalin solution and brought back to the laboratory for further treatment.
Methods
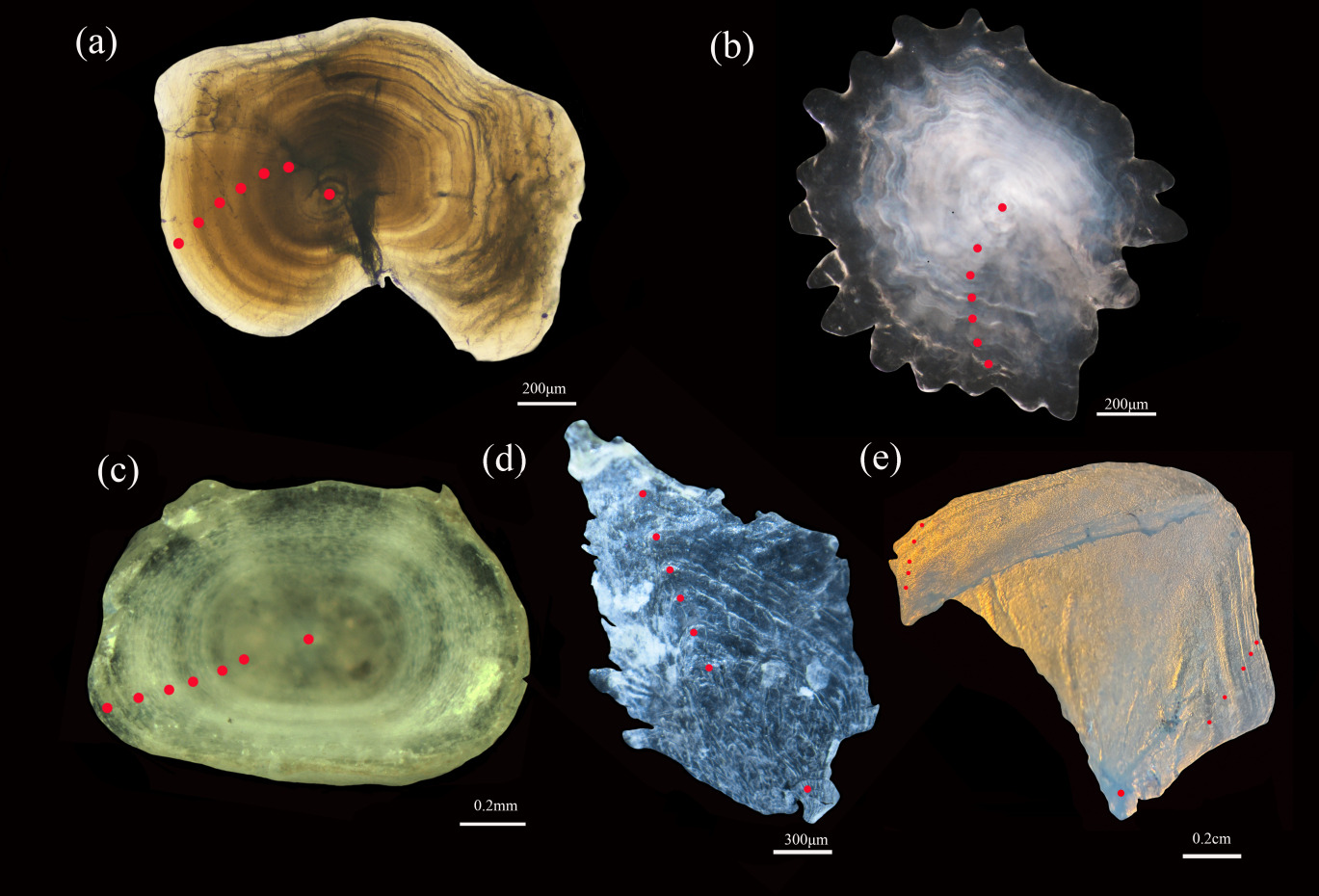
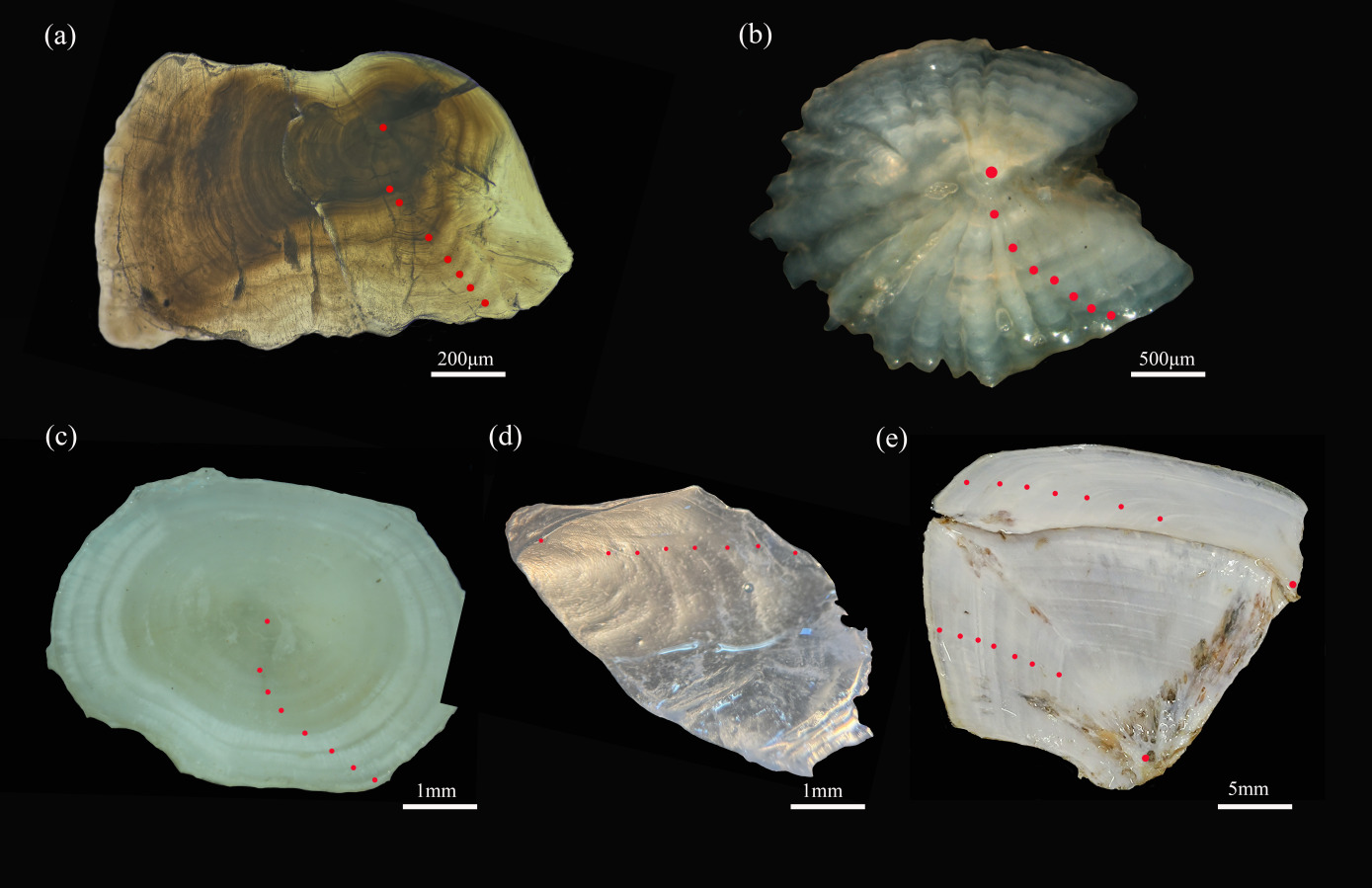
Treatment with 5 kinds of age identification materials, embedding and fixing of lapillus, sandpaper grinding, polishing, acetone dissolution, turning and fixing, continue polishing until the center core is clear; The growth rings were observed with xylene drops in asteriscus and photographed with SMZ 1270i. The vertebra were boiled in water for 5-10 min, the connective tissue was removed, and the xylene was transparent. The opercular bone was boiled for 1 min, and the connective tissue was removed and photographed by microscope. The anal scale was washed with warm water and the mucous membrane was removed.
CT scanning was performed on the whole fish (Micro CT μ80 microCT instrument). Scanning conditions were as follows: voltage (70 kVp), current (114 μA), 360° rotation scanning, scanning 180 min, resolution 14 μm, average frame 4, Angle gain 0.4°. The skeletal system was reconstructed by pCT Ray v4.0-1.
Data processing and analysis
SPSS 18.0 and ORIGIN 9.0 were used and expressed as Mean and standard deviation (Mean ± S.D.).
Results
Morphological characteristics
D. maculatus is cylindrical anteriorly and slightly flattened posteriorly. Head obtusely conical. Anastomosis prominent. Mouth inferior, transverse or arcuate; mandibles with acute angular margins; right and left lobes of lower lip narrow, with granular surfaces; postlabial sulcus interrupted. Whiskers 1 pair, stout, shorter than or equal to eye diameter. Eyes equal in size, laterally superior. Pharyngeal teeth columnar, apically pointed, hooked. Hypopharyngeal bones narrow and long. Thorax and abdomen naked and scale-free; gluteal scales and lateral line scales larger; lateral body scales arranged above and below lateral line in imbricate or very sparse. Lateral line complete, laterally median (Figure 2, Figure 3). Dorsal fin pattern Ⅱ, 8, anal fin pattern Ⅱ, 5, pectoral fin pattern Ⅰ, 7~8, ventral fin pattern Ⅰ, 7~8;
A. laticeps ellipsoidal, slightly oblate. Big head. Kiss flat, wedge-shaped ; wide mouth, front; the jaw is in the upper collar, and there is no qualitative edge. Must be short, 1 pair. Eyes small, oval, side upper, close to the snout. The hypopharyngeal bone is narrow, 5 ~ 6 times wider than the width of the pharynx. The pharynx is columnar, apically pointed, and hooked. The gill cake is sparse and short. The scale is small, the side line is complete, and the side is medium. The distance from the starting point of dorsal fin to the rostrum is greater than that from the caudal fin base. The anal fin is close to the anus. The starting point of the ventral fin is located below or slightly behind the starting point of the dorsal fin. Caudal fin forked. Body color changes slightly with individual size.Aged individuals over 900 mm in length have brown spots on both sides of the head and each scale. The upper side is thicker and the lower side is lighter. The dorsal fin is brown, and the pectoral, ventral, anal fins and the lower leaves of the caudal fin are bright orange (Figure 4, Figure 5). Dorsal fin pattern Ⅲ, 8, anal fin pattern Ⅱ, 5, pectoral fin pattern Ⅱ, 7~8, ventral fin pattern Ⅰ, 8~9.
Quantifiable character analysis
The standard deviations of body height, body width, head length, snout length, eye diameter, eye distance, caudal stalk length and caudal stalk height were 20.77, 13.79, 22.61, 8.53, 1.78, 8.45, 12.83 and 9.93, respectively. The standard deviation of body mass is 125.19, indicating that the size of body mass is different. The standard deviations of total length and body length were 61.55 and 67.44, respectively, indicating that the population size of D. maculatus covered a wide range (Table 1). The standard deviations of body height, body width, head length, snout length, eye diameter, eye distance, caudal stalk length and caudal stalk height were 5.00, 4.75, 10.02, 2.81, 0.99, 2.43, 5.67 and 0.04, respectively. The standard deviation of body mass is 125.19, indicating that the size of body mass is different. The standard deviations of total length and body length were 22.72 and 19.51, respectively, indicating that the population size of A. laticeps covered a wide range (Table 2).
From the ratio of measurable traits (Table 3 and Table 4), it can be observed that the BL/ED ratio shows the widest variation range among the two fish species, while the HL/CPL and BD/TW ratios exhibit the smallest variation range. Body length ranges from 5.19 to 6.49 times the body height, 3.97 to 4.83 times the head length, and 5.27 to 8.82 times the caudal stalk length. The head length varies from 3.82 to 9.20 times the eye diameter, and the caudal stalk length is 1.48 to 2.79 times the caudal stalk height. These observations indicate that D. maculatus has a long body, a slender structure, a relatively small head, small eyes located at the front of the head, and a slightly rectangular tail stalk.In contrast, the BL/ED and HL/ED ratios of A. laticeps display significantly greater variation compared to those of D. maculatus. This suggests that A. laticeps possesses a long and slightly flattened body, along with a larger head.
Age determination
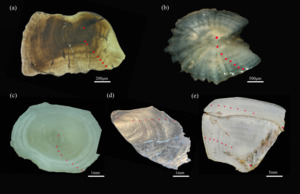
The age of D. maculatus was determined using five age determination materials (Figure 6), 6+ (a) for lapillus, 6+ (b) for asteriscus, 6+ (c) for vertebra, 6+ (d) for anal scale, and 5+ (e) for opercular bone.
The age of A. laticeps was determined using five age determination materials (Figure 7), 7+ (a) for lapillus, 7+ (b) for asteriscus, 7+ (c) for vertebra, 7+ (d) for anal scale, and 7+ (e) for opercular bone.
Anatomical observation
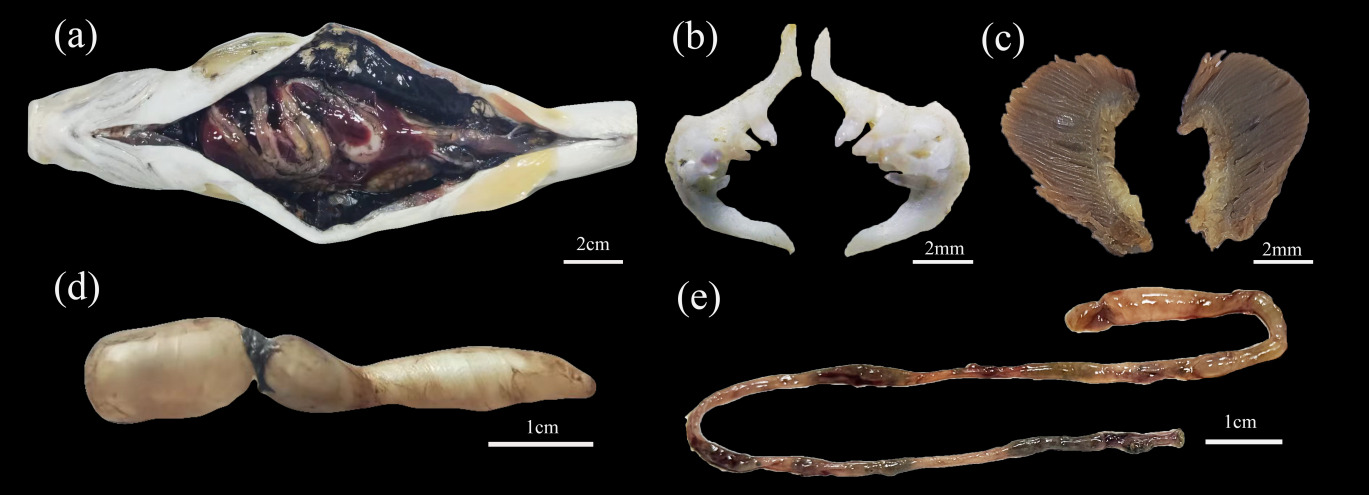
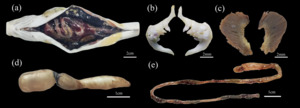
The anus of D. maculatus is not advanced and the abdominal cavity is large, the peritoneal wall is black, and the mesangium keeps the organs in a relatively fixed position in the body cavity (Figure 8-a). There are no gnathic teeth, oral teeth and free tongue in the mouth, but there are pharyngeal teeth in the oropharyngeal cavity, only on the fifth branchial arch (pharyngeal bone), columnar, apical, and hooked, powerful teeth with few grains and distinct grains, teeth of 3·4 - 4·3; It has two rows of pharyngeal teeth on the inside: the first row has the largest four, the second row has three smaller ones. Gill harrows are 14 to 19 for outsiders and 19 to 24 for insiders (Figure 8-b,c). Anterior chamber (bladder body) 37 mm, posterior chamber 10 mm (Figure 8-d). D. maculatus has no stomach, and the intestine is closely connected with the esophagus and coiled in the abdominal cavity. The intestinal recursion area is smaller than other parts, so the intestine is divided into four parts: anterior intestine, midintestine, posterior intestine and rectum. The intestinal length is 205 mm (Figure 8-e).
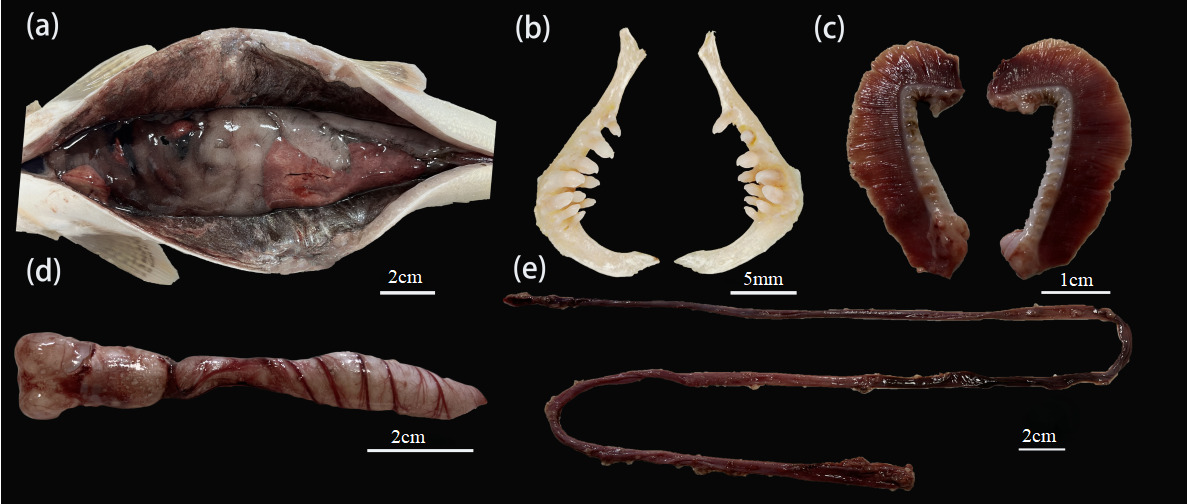
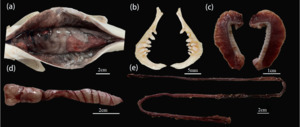
A. laticeps ventral and dorsal scales are small, and the body side scales are larger. There is a line of extra-large anal fins on both sides of the anus and the gluteal fin base, and the gluteal scales are developed (Figure 9-a). Wide mouth, front ; the mandible is longer than the maxilla and has no horny margin. The hypopharyngeal teeth are columnar, and the apex is sharp and slightly curved. There are three rows of pharyngeal teeth, tooth type 2·3·5 ~ 5·3·2, gill raker outer row 11 ~ 14, inner row 15 ~ 18 (Figure 9-b,c). The swim bladder in A. laticeps has two chambers, the anterior chamber is enlarged, and the length of the posterior chamber is about 1.6 times that of the anterior chamber (Figure 9-d). The intestine is short, only 1.2 times the body length (Figure 9-e).
Discussion
Morphological structure
The shape characteristics of fish can be divided into three categories: morphological traits, countable traits and proportional traits.16 We found that the D. maculatus gill harrow was 8~12 for laymen and 12~14 for experts, wich dorsal fin pattern Ⅱ, 8, anal fin pattern Ⅱ, 5. Comparing the morphological characteristics of D. maculatus from other regions, that the D. maculatus from the Ili River gill harrow was 16~20 for laymen and 21~27 for experts, wich dorsal fin fin form Ⅱ, 8~9, anal fin fin form Ⅱ, 5; D. maculatus from the Taxkorgan River gill harrow was 9~13 for laymen and 14~16 for experts, wich dorsal fin type Ⅱ, 8~9, anal fin type Ⅱ, 5. It can be seen that the differences in the number of fin rakers of D. maculatus in the three rivers were not significant (P>0.05),8 but the number of gill rakers of D. maculatus in the Ili River was much larger than that in the Tarim River, and the number of gill rakers of D. maculatus in the Taxkorgan River did not differ much from that of the present study.17,18 Some researchers have investigated intraspecific variation in the morphological traits of Galaxias brevipinnis, G. gollumoides, and G. vulgaris across different hydrological environments, distinguishing between rapid-flowing and slow-flowing habitats.19 Additionally, they compared intraspecific morphological differences between Bryconops caudomaculatus and Biotodoma wavrini in two Neotropical habitats: a river and a lagoon (p < 0.05).20 These findings suggest that the differentiation of morphological traits in fish is closely associated with the environmental conditions in which they live.21 Geographically, the Ili River originates in the western part of the northern slopes of the Tianshan Mountains and eventually flows into Lake Balkhash22; the Taxkorgan River originates in the Karakorum Mountains and is a major tributary of the middle and upper reaches of the Yarkand River in the Tarim River system.23 Therefore, the morphological characteristics of D. maculatus from the Taxkorgan River did not differ much from the present study (P > 0.5), whereas those of D. maculatus from the Ili River were more different from the present study (P < 0.5).24
Compared with most fish, the most significant feature of A.laticeps is the significant enlargement of its head, commonly known as ’ bighead ', which not only gives it a distinctive appearance, but also reveals its unique evolutionary path to adapt to the aquatic environment.25 In contrast, many common fish, such as Cyprinus carpio (Cyprinus carpio),26,27 have round heads and do not exhibit the distinctive head features of A.laticeps. The shape of A.laticeps is slender, the back is slightly protuberant and the abdomen is flat. In addition, the fine and neatly arranged scales are shining with silvery white luster, which together constitute its streamlined shape, effectively reducing water resistance and improving swimming efficiency. This is in stark contrast to the wider and flat fish such as Perca fluviatilis (perch).28 Although the latter has its own unique way of swimming, A.laticeps is better in reducing water resistance. The snout of A.laticeps is flat and broad, which enhances the perception ability and improves the predation accuracy. Compared with predators such as Serrasalminae known for their sharp snout, A.laticeps shows a better predation strategy.29 In addition, the A.laticeps fins, especially the caudal fin, are broad and powerful, providing them with excellent swimming and the ability to evade predators, which is similar to many fish that rely on speed and agility to survive, such as Thunnus, but there are still significant differences in specific morphology and adaptive strategies.30 Furthermore, the characteristics of A.laticeps body color changing with the environment are also rare in fish. This protective color mechanism enables it to be effectively concealed in different water environments and reduce the risk of predation, which echoes the strategy of some coral reef fish to integrate into the surrounding environment by camouflage, but the adaptability of A.laticeps is more extensive and flexible (Ahi et al., 2020; Donohue et al.31).
Age
Age is not only the basic parameter of fish growth, reproduction and population structure, but also an important research content of fishery ecology.32,33 Accurate age data of fish are helpful to evaluate population resources and analyze population dynamics. In this study, the age structure of D. maculatus ranged from 2 to 8 years old, and the dominant ages were 6 years old. The age of A. laticeps is 5-8 years old, and the dominant age is 7 years old. Five age-identified materials showed differences in age, with otolith identification being the most accurate, followed by vertebrae, scales, and opercular bones. In many comparative studies of age-identified fish, otolith readings were found to be more accurate than other age-identified materials. Ma34 found that otoliths were the most suitable age material for age identification in three age materials (otoliths, vertebrae, and opercular bones) for Schizothorax o’connori and Racoma waltoni, respectively, and Huo35 found that otoliths were better than vertebrae for age identification in the age materials of Oxygymnocypris stewartii, and that otoliths were also the best age material for the age identification of Gadus morhua and Triplophysa rosa.32,33 Moreover, otoliths are also the best age material for Schizothorax pseudaksaiensis, Gymnocypris selincuoensis, Schizopygopsis malacanthus, and Gymnocypris przewalskii. The reasons for this are that the vertebrae is not easy to determine age because the vertebrae is thickened at the base of the vertebrae and the central whorl is not easy to identify; the scales are prone to wear and tear or stagnation with age, which usually underestimates the age of older and slower-growing individuals, and is only suitable for ageing younger, faster-growing fishes.36
Feeding
During the feeding process of fish, the morphology and structure of feeding organs are closely related to the acquisition and processing of food, in which the first contact with food is the mouth, lips and other external feeding organs.37,38 The size of the mouth slit determines the range of food volume that can be utilized by the fish, and directly affects the bite force. The hypopharyngeal tooth type of D. maculatus was conical, which suggests that D. maculatus has increased the variety of food taken and the degree of omnivory in order to adapt to the colder environment. Numerous studies have found that the intestinal tract of phytophagous fishes is generally narrower and longer, curved and coiled in the abdominal cavity, so that food rich in plant fibers can be fully digested and absorbed by prolonging the retention time of the food in the intestinal tract, whereas carnivorous fishes, whose ingested food has a higher content of protein and fat, is easier to be digested and absorbed, and thus has a shorter intestinal tract. In the present study, D. maculatus had no stomach and a long intestine, consistent with its omnivorous diet.39–41
A.laticeps has a slender body, a micro-bulge on the back, and a flat abdomen. Its streamlined appearance reduces water resistance and improves swimming efficiency, which is crucial for long-term foraging in water.42 The silvery white scales form a subtle reflection in the water, which helps A.laticeps to remain hidden in the underwater environment and thus more effectively approach the prey.43 The flat and broad characteristics of the snout are particularly significant, which enhances the perception ability of A.laticeps, enables it to locate the food source in the water more accurately, and also facilitates its predation.44 A.laticeps uses its broad snout to search for benthic organisms, small fish or aquatic insects in the sediment, showing its unique foraging strategy. The powerful caudal fin also plays an important role in the predation process, helping A.laticeps quickly adjust its direction or accelerate the pursuit of prey. These unique structures make A.laticeps efficient foraging strategies in specific ecological environments.45,46
Conservation status
According to genetic studies, D. maculatus may have migrated from central China to the Xinjiang region. This fish is mostly found in the upper reaches of rivers at high altitudes, and is a cold-water fish that prefers to inhabit rivers below 20°C. Due to the high requirements of the living environment and the increase of pollution from human activities in recent years, the population of D. maculatus has declined, and in 2021 the D. maculatus was listed in the ‘List of National Key Protected Wild Animals (Grade Ⅱ)’. Therefore, it is extremely important to strengthen the research on its ecological habits and reproduction patterns to provide a basis for its protection, management and sustainable utilization.47,48
As a unique rare aquatic wild animal in Xinjiang, the protection status of A.laticeps has attracted much attention. Due to the limitations of its own biological characteristics, such as mature age, low fecundity, and the interference of environmental changes and human activities, A.laticeps was once on the verge of extinction. Since the 1980s, A.laticeps has been listed as a first-class protected animal in China and included in the ’ Redbook of Endangered Wildlife ’ and the ’ National Key Protected Wildlife List of China.49–51 In recent years, in order to protect A.laticeps, a number of measures have been taken in Xinjiang, including the establishment of aquatic wildlife rescue centers and protection bases, artificial breeding and proliferation.52 The scientific research team successfully hatched and cultivated a large number of healthy fry by selecting mature gonadal broodstock and implementing artificial spawning. At the same time, the proliferation and release activities have also achieved remarkable results. A.laticeps has re-formed its population in waters such as Bosten Lake, and the population has recovered. However, the protection of A.laticeps still faces many challenges, such as the continuous change of ecological environment and the invasion of alien species.53 Therefore, it is necessary to continue to increase protection, strengthen scientific research, and explore more effective protection methods and technologies.54–56
The study results showed that the morphological features and age structure of fishes are closely related to their living environment. Regarding morphological features, D. maculatus and A. laticeps demonstrate unique adaptations. These features include the number of gill rakers, body size, and changes in body color, which are strategies developed by fish to adapt to different ecological environments. Regarding the age structure, otoliths are the most accurate material for identifying the age of fishes due to their resistance to abrasion and clear annual rings. The study of feeding organs shows that the size of the mouth slit, the type of pharyngeal teeth, and the structure of the digestive system are all closely related to the food acquisition and processing ability of fishes.
In terms of conservation biology, both D. maculatus and A. laticeps are facing the challenges of ecological changes, including pollution and invasive alien species. Population recovery and ecosystem stabilization of these endangered species can be facilitated by strengthening scientific research and developing effective conservation strategies. This is important for the conservation of biodiversity and the sustainable use of ecosystems.
Acknowledgments
This research was supported by the Tianshan Talent Training Project of Xinjiang(No.2023TSYCCX0128).
Authors Contribution
Conceptualization: Linghui Hu (Equal), Aizhi Han (Equal), Liting Yang (Equal), Gulden Serekbol (Equal). Formal Analysis: Yong Song (Equal), Jiaxuan Liu (Equal). Investigation: Yong Song (Equal), Jiaxuan Liu (Equal). Methodology: Liting Yang (Equal), Gulden Serekbol (Equal). Writing – original draft: Liting Yang (Lead). Supervision: Bin Huo (Equal), Daoquan Ren (Equal). Writing – review & editing: Chengxin Wang (Equal), Shengao Chen (Equal).
Competing of Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
All experimental protocols were approved by the Science and Technology Ethics Committee of Tarim University (approval code:2023027) and adhered to animal welfare laws, guidelines and policies.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.

.png)

.png)



.png)

.png)