Introduction
White shrimp (L. vannamei) is one of the main aquaculture species in Vietnam, generating high profits and significantly contributing to the country’s seafood export values. In 2023, the production of white shrimp reached 632.3 thousand tons, accounting for 67.7% of the total shrimp production in Vietnam.1 Despite this success, shrimp farming still faces numerous challenges, including intense competition from Ecuador and India regarding market price and supply, disease outbreaks, and increasingly polluted farming environments. Although shrimp farming technologies are developing, and some advanced farming technologies have been applied, shrimp production in Vietnam has not significantly increased compared to previous years. This stagnation is largely due to disease outbreaks that result in mass mortality of shrimp.2 Vibrio spp. (such as V. parahaemolyticus, V. cholerae, V. alginolyticus, V. vulnificus, V. harveyi, …), which are gram-negative bacteria, are common pathogens in shrimp farming, and have severely impacted the aquaculture industry in Vietnam as well as globally.3 Among these, acute hepatopancreatic necrosis disease (AHPND) caused by V. parahaemolyticus carrying plasmids containing the PirA and PirB (Photorhabdus insect-related (Pir)) toxin genes, is confirmed to be a common disease, occurring in all shrimp farming areas in Vietnam. AHPND is known for its rapid spread, sudden death, and high mortality rate (nearly 100%) in infected shrimp, causing disease in shrimp from 30 to 35 days after stocking,4 leading to heavy losses for shrimp farmers across the country.
The use of antibiotics is one of the common ways farmers choose to treat diseases caused by Vibrio spp. in shrimp farming3 because antibiotics bring quick desirable results and save costs. However, the high frequency and uncontrolled use of antibiotics has increased the number of antibiotic-resistant bacteria in farming ponds.5 Moreover, antibiotics have many undesirable effects such as negative impacts on the environment and product quality.3 Farmed shrimp products containing antibiotic residues are banned from consumption and export.6 The use of antibiotics in shrimp farming also changes the natural microflora, causing ecological imbalances and harming natural organisms.6
Therefore, to minimize antibiotic use in shrimp farming, medicinal plants are considered a safe and effective solution for preventing and treating diseases caused by bacteria pathogens. In particular, the antibacterial activity of herbal extracts has been studied and applied in aquaculture.7,8 Extracts from the stems and leaves of various plants can resist bacteria such as Staphylococcus aureus, Seratia marcescens, Sarcina lutea, and Escherichia coli in vitro.9 Extracts of leaves and seeds of Rhdomyrtus tomentosa have shown the ability to resist bacteria causing acute hepatopancreatic necrosis disease (V. parahaemolyticus) in white shrimp.10 The methanol extract of Eclipta prostrata has antibacterial activity against 12 strains of Vibrio spp. isolated from the intestine of black tiger shrimp.11 The extract of Piper betle can resist pathogenic bacteria in aquatic animals such as Edwardsiella tarda, Staphylococcus aureus, Pseudomonas aeruginosa, Edwardsiella ictaluri, and Aeromonas hydrophila.12 Furthermore, many studies have shown the effectiveness of using herbal extracts in shrimp and fish farming to improve growth, enhance the immune system, and inhibit pathogenic bacteria.8,13
Syzygium nervosum, also known as myrtle tea or shampoo tea, is widely distributed in tropical countries such as China, Vietnam, Laos, Cambodia, and other tropical regions. In Vietnam, the tree often grows wild or is planted in rural areas to harvest leaves and flower buds for tea and medicine.14 The composition of S. nervosum leaves contains minerals, plant antibiotics, vitamins, and essential oils.15 According to Mai and Chuyen,16 the total polyphenol content of S. nervosum leaves is high (146.6 mg catechin/g of S. nervosum leaves). Tea made from S. nervosum leaves has been reported to have antibacterial, choleretic, and anti-cancer cell growth properties.14 In vivo, ethanol extract of S. nervosum leaves has shown the ability to resist V. parahaemolyticus, which causes acute hepatopancreatic necrosis disease in shrimp.17 The present study aimed to evaluate the effectiveness of supplementing shrimp diets with S. nervosum leaf extract on growth performance, survival rate, and immune parameters in white shrimp (Litopenaeus vannamei). Additionally, it also investigated the extract’s ability to prevent acute hepatopancreatic necrosis disease in shrimp fed supplemented diets.
Materials and Methods
Source of S. nervosum leaves and extraction
Fresh S. nervosum leaves were collected from households cultivating these trees in Thua Thien Hue province, Vietnam. The extraction of S. nervosum leaves was carried out following the method described by Phung.18 Briefly, upon arrival at the laboratory, the leaves were thoroughly washed with tap water and dried in an oven at 55°C for 24 hours. Once dried, they were ground into powder using a multi-function blender. The resulting powder was then soaked in 96% ethanol at a ratio of 1:10 for 60 minutes. Afterward, the mixture was filtered using Whatman No.1 filter paper, and the solvent was removed using a rotary vacuum evaporator (Rotavapor R-100, BUCHI, Switzerland) to yield the S. nervosum leaf extract. The extract was stored in the refrigerator at 4°C for further use.
Preparation of experimental diets
The S. nervosum leaf extract was incorporated into industrial shrimp feed (produced by Thang Long Limited Liability Company, Vietnam, which contains nutritional ingredients as follows: moisture < 11%, protein > 37%, lipid > 4%, and fiber < 4%). The extract was added to the feed at concentrations of 12.5, 25.0, and 37.5 g/kg of feed. Once thoroughly mixed with the extract, the feed was coated with squid oil (Vemedim Limited Liability Company, Vietnam) at a 2% dosage and left to dry at room temperature for 30 minutes. A control batch of feed without S. nervosum leaf extract was also coated with squid oil. The prepared feed was stored at 4°C for use during the experiment.
White shrimp
White shrimp (weighing 1.05 ± 0.04 g) were purchased from a local hatchery in Thua Thien Hue province, Vietnam. The shrimp were tested and confirmed to be free of white spot syndrome virus (WSSV) and V. parahaemolyticus using polymerase chain reaction (PCR) at the Department of Livestock and Veterinary Medicine in Thua Thien Hue province. Upon arrival at the laboratory, the shrimp were acclimatized in composite tanks (with a capacity of 1 m³) under controlled conditions: salinity of 22-25‰, temperature of 25-28°C, pH 7.8-8.1, dissolved oxygen (DO) of 5.5-6.0 mg/L, and alkalinity of 80-130 mg CaCO₃/L. The acclimatization period lasted for 15 days before the experiment.
Vibrio parahaemolyticus
The V. parahaemolyticus strain TX07-3/3 was obtained from the bacterial collection of the Department of Aquatic Pathology, Faculty of Fisheries, University of Agriculture and Forestry, Hue University. This strain, which contains the PirA and PirB toxin genes, was originally isolated from white shrimp suffering from acute hepatopancreatic necrosis disease (AHPND) in Thua Thien Hue province, Vietnam.19 The strain was cultured on Tryptone Soya Agar (TSA, Himedia, India) supplemented with 2% NaCl at 28°C for 24 hours. Afterward, bacterial colonies were transferred to Tryptic Soy Broth (TSB, Himedia, India) also supplemented with 2% NaCl, and incubated at 28°C for 24 hours. Bacterial density was determined by measuring optical density (OD) at 600 nm using a UV-VIS spectrophotometer (U2900, Hitachi, Japan), with OD = 1 corresponding to a bacterial density of 10⁹ CFU/mL. This bacterial suspension was then diluted to a final density of 10⁵ CFU/mL for use in the experiment.
Effects of diets supplemented with S. nervosum leaf extract on growth rate and immune parameters of white shrimp
Experiment 1
White shrimp (weighing 1.05 ± 0.04 g) were randomly arranged into 12 plastic tanks (capacity of 120L) at a stocking density of 50 shrimp per tank. The experiment consisted of four treatments, each with triplicates, including the control group (ĐC) where shrimp were fed a diet without supplemented S. nervosum leaf extract. The experimental groups included shrimp-fed diets supplemented with S. nervosum leaf extract at different concentrations of 12.5 g/kg feed (NT1); 25.0 g/kg feed (NT2) and 37.5 g/kg feed (NT3). Shrimp were fed four times daily (7h, 11h, 14h, and 18h), at an amount equivalent to 3% of their total body weight. The feeding trial lasted for 28 days.
Water quality parameters were maintained under experimental conditions: temperature (25-28°C), salinity (22-25‰), dissolved oxygen (5.5-6.0 mg/L), and pH (7.8-8.1). The experimental tanks were siphoned daily to remove waste and leftover feed, with water compensation according to the losses from siphoning and evaporation. Additionally, 20-30% of the water in each tank was replaced weekly to ensure optimal environmental conditions throughout the experiment.
The effects of diets supplemented with S. nervosum leaf extract on the growth performance, survival rate (SR), and feed conversion ratio (FCR) of white shrimp were assessed after 28 days of the experiment. Additionally, immune parameters such as total haemocyte count (THC), phenoloxidase activity (PO), and lysozyme activity (LYS) were measured at three-time points: day 01 (the start of the feeding trial), day 14, and day 28 of the experiment. These measurements were used to evaluate the fluctuation of the shrimp’s immune response to the dietary treatments over time.
Determining growth performance, survival rate, and feed conversion ratio
Samples of shrimp were randomly collected at the beginning of the feeding trial, as well as on days 14 and 28 of the experiment. Ten shrimp were collected from each tank to assess growth performance and were then returned to their respective tanks. Growth parameters, survival rate, and feed conversion rate were calculated according to Adel et al. (2017).
Daily Growth Rate (DGR): DGR (g/day) = (Wf –Wi)/T
Specific Growth Rate (SGR): SRG (%/day) = 100 * (lnWf – lnWi)/T
Survival Rate (SR):
SR (%) = (the number of shrimp at the end of the experiment/ the number of shrimp at the start of the experiment) x 100
Feed Conversion Ratio (FCR):
FCR = Total amount of feed fed to shrimp/Weight gain of shrimp
Wherein, Wi is the initial weight of shrimp (g), Wf is the final weight of shrimp (g), and T is the period of the experiment (days).
Determining immune parameters
Shrimp sampling
Shrimp samples were randomly collected at the start of the feeding trial (day 1) and on days 14 and 28 of the experiment. Three shrimp were taken from each tank during each sampling and subsequently removed.
Blood sampling and determination of total haemocyte count (THC)
Haemolymph sampling and total haemocyte count (THC) were conducted following the methods described by Liu and Chen.20 Briefly, 0.1 mL of haemolymph was collected from the ventral sinus of each shrimp using a 1 mL syringe (25 gauge) containing 0.9 mL of precooled anticoagulant solution (30 mM trisodium citrate, 0.34 M sodium chloride, 10 mM EDTA, 0.12 M glucose, pH 7.55). Total haemocyte counts were determined by placing a drop of the diluted haemolymph onto a haemacytometer and counting the haemocyte cells under a light microscope at 40X magnification. Each measurement was done in triplicate for accuracy.
Phenoloxidase (PO) assay
Phenoloxidase (PO) activity was determined following the method of Hernández-López et al.,21 which measures dopachrome formation from L-3,4-dihydroxyphenylalanine (L-DOPA) spectrophotometrically at 490 nm. In brief, 100 µL of shrimp hemolymph was diluted with 900 µL of anticoagulant solution and centrifuged at 6,500 rpm for 20 minutes at 4°C (Digisystem Laboratory Instruments Inc., Taiwan). The hemocytes were resuspended in 500 µL of cacodylate-citrate buffer, centrifuged again, and then dissolved in 100 µL of cacodylate buffer. Afterward, the solution was divided into two tubes: one for measuring PO activity and one serving as a control. For the PO activity, 100 µL of hemocyte solution was mixed with 100 µL of 0.1% trypsin and incubated for 10 minutes at 25°C, followed by the addition of 50 µL of L-DOPA (3 mg/mL) and a further 5-minute incubation. Finally, 800 µL of cacodylate buffer was added. In the control tube, trypsin was replaced with cacodylate buffer. PO activity was determined by measuring absorbance at 490 nm.
Lysozyme activity (LYS) assay
Lysozyme activity (LYS) assay was conducted following the procedures of Chiu et al.22 Briefly, 500 µL of diluted hemolymph was centrifuged, and the resulting precipitate was mixed with 1 mL of 0.02% Micrococcus lysodeikticus (Sigma, St. Louis, MO, United States) suspension. The reaction took place at room temperature, and the absorbance at 530 nm was measured at 0.5 and 4.5 minutes. One unit of lysozyme activity (LYS) was defined as the amount of enzyme that produces a decrease in absorbance of 0.01/min. The specific lysozyme activity was expressed as U/g protein. This assay measures the enzyme’s antibacterial activity by quantifying its ability to lyse bacterial cell walls.
Determining the resistance to V. parahaemolyticus of white shrimp fed with diets supplemented with S. nervosum leaf extract
Experiment 2
After 28 days of experimental feeding with supplemented S. nervosum leaf extract (in Experiment 1), 30 shrimp per tank from each treatment group were subjected to a challenge with V. parahaemolyticus. The infection process involved immersing the shrimp in 30L of seawater with a salinity of 22‰, containing V. parahaemolyticus at a density of 105 CFU/mL for 1 hour, following the method of Loc et al.4 The bacterial density of 10⁵ CFU/mL was chosen as it represents the LD50 value of V. parahaemolyticus for white shrimp, as determined in the study (unpublished data). This procedure was conducted to assess the shrimp’s resistance to bacterial infection after being fed diets supplemented with the extract.
The challenge experiment was arranged in fifteen plastic tanks (each with a capacity of 120L) and included five treatments, with three replicates for each. Shrimp were stocked at a density of 30 individuals per tank. The treatments consisted of negative control (ĐC1), where shrimp were taken from the control group of experiment 1 and not challenged with V. parahaemolyticus after being fed a diet without S. nervosum leaves extract for 28 days. The positive control (ĐC2) included shrimp from the same control group, but these were challenged with V. parahaemolyticus. Treatment 1 (NTTN1) involved shrimp fed a diet supplemented with 12.5 g/kg of S. nervosum leaf extract for 28 days before being infected with the bacteria. Treatment 2 (NTTN2) used shrimp fed a diet supplemented with 25.0 g/kg of the extract, while Treatment 3 (NTTN3) included shrimp fed a diet supplemented with 37.5 g/kg of the extract, both of which were also challenged with V. parahaemolyticus. This design aimed to assess the effects of varying levels of dietary supplementation on the shrimp’s resistance to V. parahaemolyticus infections.
After the bacterial infection, shrimp were fed diets corresponding to each treatment four times daily at 7:00, 11:00, 14:00, and 18:00, with the feeding amount set at 3% of their total body weight. Throughout the experiment, water quality parameters were monitored and maintained, ensuring temperatures ranged from 25 to 28°C, salinity levels fluctuated from 22 to 25‰, dissolved oxygen at 5.5 to 6.0 mg/L, and pH values from 7.8 to 8.1. Additionally, shrimp were examined daily for pathological signs, and the number of dead shrimps was recorded over 14 days to evaluate the impact of the treatments on the cumulative mortality rate of shrimp.
Determination of shrimp mortality rate
The cumulative mortality rate of shrimp in each treatment was determined by the formula:
\[ \begin{align} &\text { Cumulative mortality rate }(\%)\\ &=\frac{\text { Total cumulative shrimp deaths during } 14 \text { days }}{\text { The total number of experimental shrimp }} \\ &\quad \times 100 \end{align} \]
Data analysis
The survival of shrimp was analyzed using arcsin-transformed data. All data were normal-distributed and homoscedastic. Experimental parameters such as growth performance, survival of shrimp, FCR, THC, PO, and LYS were compared using one-way ANOVA on the SPSS program version 20.0 for Windows, followed by the Duncan test. The cumulative mortality rate of shrimp was evaluated using ANOVA repeated measures in which the experimental factor was the analyzed factor, and the V. parahaemolyticus post-challenging days were an additional factor. Differences were tested at a level of significance of 5%.
Results
Growth performance, survival rate, and feed conversion ratio of white shrimp
After 28 days of the feeding trial, the growth parameters of shrimp, such as final weight (Wf), daily growth rate (DGR), specific growth rate (SGR), and feed conversion ratio (FCR), showed no significant differences between the experimental groups and the control (p>0.05) (Table 1). However, supplementation of diets with S. nervosum leaf extract significantly improved the survival rate of shrimp (p<0.05). In this study, the survival rate of white shrimp ranged from 90% to 94.1%. The survival rate of shrimp in the experimental groups (93.4%-94.1%) was higher than that of the control group (90%) (p<0.05). No significant differences in survival rates were found among the experimental groups (p>0.05). This indicates that the extract did not affect growth parameters, it contributed to improving shrimp survival.
Effects of supplementary diets with S. nervosum leaf extract on immune parameters of white shrimp
Total haemocyte count (THC)
At the start of the experiment, the total haemocyte count (THC) in white shrimp ranged from 15.44 × 10³ cells/mm³ to 15.69 × 10³ cells/mm³, with no significant differences between the treatment groups (p>0.05) (Table 2). After 14 days of the feeding trial, THC values ranged from 15.94 × 10³ cells/mm³ to 17.61 × 10³ cells/mm³, with shrimp in the experimental groups showing significantly higher THC values than the control group (p<0.05). However, there were no significant differences in THC among the experimental groups (p>0.05). A similar pattern was observed on day 28, where THC values in the experimental groups were higher than in the control group (p<0.05), but no differences were found among the experimental groups (p>0.05). Overall, THC values tended to increase across all treatments throughout the feeding trial, with a more rapid increase in the experimental groups.
Phenoloxidase activity (PO)
On the first day of the experiment, phenoloxidase (PO) activity in the blood of white shrimp ranged from 0.032 to 0.136 (Table 3). PO activity increased sharply from day 1 to day 14, then continued to increase gradually until day 28. After 14 days of the experiment, PO activity ranged from 0.197 to 0.227. Shrimp-fed diets supplemented with S. nervosum leaf extract showed significantly higher PO activity compared to the control group (p<0.05), although no differences were observed among the experimental groups (p>0.05). A similar trend was observed on day 28, with PO activity in the experimental groups (NT1, NT2, and NT3) being significantly higher than in the control group (p<0.05). However, no significant differences were found among the experimental groups (p>0.05).
Lysozyme activity (LYS)
On the first day of the experiment, lysozyme activity (LYS) in the blood of white shrimp ranged from 0.631 U/mg protein to 0.639 U/mg protein (Table 4). Overall, LYS tended to increase throughout the experimental period across all treatments. After 14 and 28 days, LYS in the experimental groups (NT1, NT2, NT3) was significantly higher than in the control group (p<0.05). However, no significant differences in LYS were observed among the experimental groups (p>0.05). These results indicated that feeding shrimp with diets supplemented with S. nervosum leaf extract significantly enhanced their lysozyme activity (p<0.05), although varying the concentration of the extract did not lead to any differences in LYS activity among the experimental groups (p>0.05).
Vibrio parahaemolyticus resistance of white shrimp fed diets supplemented with S. nervosum leaf extract.
After 24 hours of infection, typical pathological signs of acute hepatopancreatic disease began to appear in the shrimp infected with V. parahaemolyticus. These signs included yellowing of the hepatopancreas, incomplete feed in the intestines, and soft shells (Fig. 1B). On the contrary, shrimp in the negative control group (ĐC1) remained healthy, showing no signs of infection; hepatopancreas appeared dark brown, and intestines were full of feed (Fig. 1A). For the infected treatments, shrimp displaying pathological signs had V. parahaemolyticus re-isolated from samples collected from hepatopancreas. Then the bacteria were cultured on TCBS medium, identified using the API 20E kit, and confirmed to be V. parahaemolyticus. Based on the observed pathological signs and the results of bacterial isolation, it was concluded that the shrimp in the infected treatments died caused of the V. parahaemolyticus pathogen.
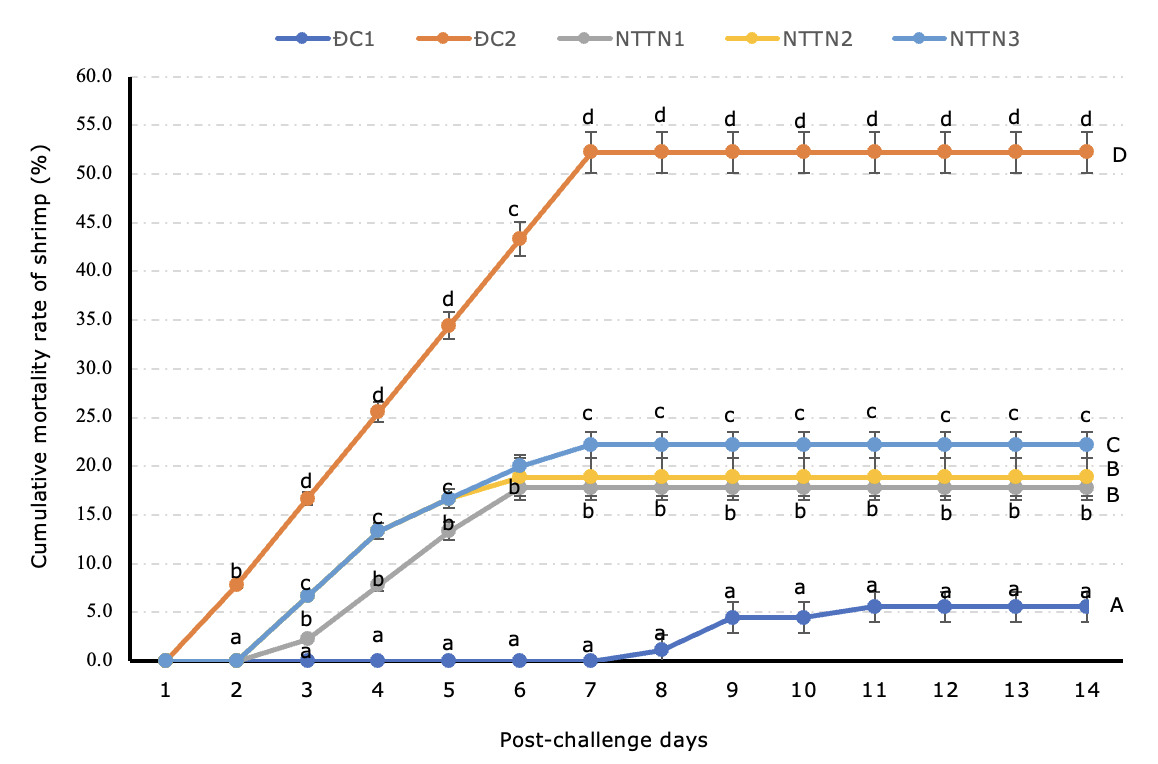
In the present experiment, the mortality of shrimp in the positive control (ĐC2) began one day after infection, while the mortality of shrimp in the experimental treatments started on the second day (Fig. 2). The results of determining the cumulative mortality rate of shrimp in all treatments after 14 days challenging recorded that in the negative control (ĐC1), shrimp did not show any signs of disease throughout the experiment and achieved a survival rate of 94.4% (a mortality rate of 5.6%). In contrast, in the positive control (ĐC2), the mortality rate of shrimp reached 52.2%, which was higher (p<0.05) than those in the experimental groups. In the experimental groups, the mortality rate in NTTN1 (17.8%) and NTTN2 (18.9%) was lower (p<0.05) than in NTTN3 (22.2%) (Figure 2). However, there was no difference in the mortality rate of shrimp in NTTN1 and NTTN2 (p>0.05).
Discussion
Growth performance and survival rate of shrimp
Many studies have reported that the use of pure plant extracts stimulates appetite, promotes weight gain (WG), and improves the specific growth rate (SGR) of aquatic species.23 Herbal extracts have been reported to create flavor and stimulate appetite and digestive secretions (amylase, protease, and lipase), leading to an increase in feed efficiency, which in turn improves growth, and enhances the health and survival of shrimp.24 Herbal products extracted from Hydnophytum formicarum, Zingiber officinale, Solanum procumbens, Andrographis paniculata, Psoralea corylifolia, Eclipta prostrata, Ocimum gratissimum, Phyllanthus urinaria, and Tinospora cordifolia have been reported to enhance the health and survival rate of shrimp.25 According to Hoa et al.,26 a diet supplemented with Punica granatum leaf extract significantly improved the survival rate of white shrimp (L. vannamei). Additionally, the use of a diet supplemented with emodin extract has been reported to improve the growth performance of freshwater prawns (M. rosenbergii).27
On the contrary, in our study, the addition of S. nervosum leaf extract to the diet did not enhance the growth rate of white shrimp. This may be because the extract lacks an attractive flavor, thus failing to stimulate shrimp to consume the feed. Additionally, the extract may not promote the secretion of digestive enzymes (further research is required to confirm its effect on shrimp digestive enzyme activity). As a result, there was no improvement in digestion, as evidenced by the lack of a significant difference in FCR (Table 1), nor in feed absorption by the shrimp, ultimately leading to no enhancement in shrimp growth. However, it is noteworthy that the supplementation of S. nervosum leaf extract at concentrations of 12.5–37.5 g/kg feed did not have any negative effects on shrimp growth. Previous study by Kavitha et al.28 have emphasized the importance of selecting appropriate dosages of herbal extracts to achieve beneficial effects, noting that inappropriate doses could result in toxic outcomes. Nonetheless, the addition of S. nervosum leaf extract to shrimp feed significantly improved the survival rate of shrimp. This finding aligns with the results of Huyen et al.29 who reported that the supplementation of diets with Phyllanthus urinaria and Terminalia catappa leaves extracts at concentrations of 1% and 2% did not enhance the growth rate of white shrimp. However, a diet supplemented with Terminalia catappa leaf extract at a 1% concentration significantly improved the shrimp’s survival rate. Similarly, To et al.30 observed no significant differences in growth performances in white shrimp fed a diet supplemented with a Solanum procumbens extract. These findings suggest that while the growth parameters were not significantly affected by the dietary inclusion of S. nervosum leaf extract, the enhanced survival rates indicate its potential as a beneficial supplement for improving shrimp health and resilience.
Improvement of shrimp immune parameters
Previous studies have confirmed that medicinal plants containing bioactive compounds can enhance non-specific immunity by acting as immunostimulants when they enter the shrimp’s intestinal system.31 This leads to an increase in total hemocyte count (THC) and promotes key immune responses such as phagocytosis, phenoloxidase (PO) activity, and lysozyme activity.31 These enhancements ultimately improve the antipathogenic capabilities of aquatic species.32 Diets supplemented with various plant extracts have been shown to enhance immunological parameters in different fish species, such as THC, PO, LYS, phagocytic activity, respiratory burst, and complement activities.23 However, the efficacy of medicinal plants in boosting the immune systems and disease resistance of aquatic organisms depends on several factors, including dosage, plant species, active compounds, and the size or developmental stage of the aquatic species.
In our study, the findings revealed that incorporating S. nervosum leaf extract into feed at concentrations ranging from 12.5 g/kg to 37.5 g/kg significantly enhanced immune parameters, including THC, PO, and LYS in white shrimp (Tables 2, 3, and 4). The enhancements in THC, PO, and LYS in white shrimp observed in this study may be attributed to bioactive compounds present in Syzygium nervosum leaf extract, including oleanane- and ursane-type triterpenoids,33 C-methylated flavonoids,34 and polycyclic phloroglucinols.35 Specially, C-methylated chalcones have been identified as the major bioactive constituents responsible for the pharmacological effects of this medicinal plant.15 These compounds are thought to support the shrimp’s immune system by stimulating the production of immune cells, thereby enhancing immune parameters and strengthening the shrimp’s ability to defend against pathogens. Researchers have shown that the increase in THC observed in shrimps fed with diets containing Astragalus polysaccharides and chlorogenic acid can be attributed to the immunostimulants’ ability to enhance cell proliferation in hematopoietic tissue. This mechanism results in a higher number of THC rather than directly stimulating the mitotic function of hemocytes.36,37 Notably, hemocytes play a pivotal role in the immune response of crustaceans, executing essential functions such as pathogen recognition, phagocytosis, melanization, cytotoxicity, and cell communication. These cells are critical for immune defense, particularly against V. parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) in shrimp.38 Meanwhile, phenoloxidase (PO) activity, a crucial component of the crustacean humoral immune system, is activated by bacterial cell wall components such as peptidoglycan, β-1,3-glucan, and lipopolysaccharides. This activation converts prophenoloxidase (proPO) to phenoloxidase (PO), which is stored in granular and semi-granular cells in shrimp blood. In this study, S. nervosum leaf extract, when introduced into the shrimp’s body, may initially induce degranulation of hemocytes, resulting in the release of the proPO system into the medium in vitro or the plasma in vivo. Bioactive compounds in the herbal formula, such as C-methylated flavonoids39 may then activate this system by producing a “degranulating factor” that amplifies the secretion of the proPO system. The proteins generated upon activation of the proPO system can coat pathogens, enhancing the shrimp’s phagocytic capacity. This mechanism could account for the observed increase in PO activity in our data (Table 3).
Supporting this, Harlina et al.40 found that diets supplemented with Chromolaena odorata leaves flour increased shrimp THC by 242.9% and pro-PO activity by 566.7% compared to the control. Additionally, To et al.30 reported that white shrimp fed Solanum procumbens extract-supplemented diet at 1.0% and 1.5% concentrations significantly increased THC and reduced cumulative mortality of V. parahaemolyticus challenged shrimp by 47.6%. Moreover, dietary supplementation with 2% Punica granatum leaf extract significantly enhanced innate immunity, including THC, PO, and superoxide dismutase (SOD) activity in white shrimp.41 Similarly, white shrimp fed a diet supplemented with 1% Terminalia catappa extract showed significant improvement in immune parameters and survival rates after V. parahaemolyticus challenge.42
Lysozyme is another critical non-specific immune parameter that plays a key role in combating pathogens. Formed through the hydrolysis of glycosidic bonds in bacterial cell walls,43 lysozyme is instrumental in breaking down the polysaccharide walls of bacteria, making it a widely recognized and essential humoral immune factor.44 Moreover, lysozyme exhibits lytic activity against both Gram-positive and Gram-negative bacteria, including certain Vibrio species known to cause diseases in penaeid shrimp. Numerous studies have demonstrated that the ingestion of immunostimulants, such as zingerone, guava leaves, and Solanum nigrum extract, can significantly enhance lysozyme activity in shrimp.31,37,45
Enhancement of disease resistance of white shrimp
According to Reverter et al.,46 herbal extracts can enhance immune response, protecting the host against pathogen infections. These extracts have demonstrated high antibacterial activity, immune system enhancement, minimal environmental impact, and no harmful effects on aquatic animals.47 Several studies have confirmed the antibacterial properties of medicinal plants against both Gram-positive and Gram-negative bacteria.24 Specifically, the herbal extracts of Ricinus communis, Vernonia amygdalina, Moringa oleifera, Acanthus ilicifolius, and Wedelia calendulacea have shown antibacterial efficacy against V. harveyi and V. parahaemolyticus.29 The extract of Terminalia catappa leaves contains compounds such as 1-degalloyl-eugeniin, chebulagic acid, gentisic acid, corilagin, geraniin, granatin B, kaempferol, punicalagin, quercetin, tercatain, tergallagin, terflavin A, and terflavin B, which enhance immunity and increase survival rates against V. parahaemolyticus.42 Additionally, Chromolaena odorata leaf flour has been documented to increase resistance to bacterial diseases, reducing mortality in white shrimp farms.40 Therefore, the use of herbs as an alternative to antibiotics for shrimp is gaining popularity.48
In the present study, the addition of S. nervosum leaf extract to shrimp feed resulted in significant reductions in shrimp mortality: 130% reduction at 12.5 g/kg feed, 117% at 25.0 g/kg feed, and 85% at 37.5 g/kg feed. The higher survival rates observed in the experimental groups may be associated with increased total hemocyte count (THC), phenoloxidase (PO), and lysozyme (LYS) activity in the shrimp. Enhanced PO and LYS activities in the blood of white shrimp significantly improved resistance against V. parahaemolyticus infection.49 Furthermore, S. nervosum extract contains numerous antibacterial compounds, including flavonoids, coumarins, tannins, organic acids, free sugars, and sterols.15 These compounds serve as natural antibiotics, aiding shrimp in combating pathogenic bacteria. For instance, flavonoids act as catalysts that inhibit oxidation reactions15 and exhibit antibacterial activity against various pathogenic microorganisms.17 Additionally, coumarins and tannins possess notable antibacterial and anti-inflammatory properties.16
In conclusion, our findings demonstrate that supplementing shrimp diets with S. nervosum leaf extract significantly enhances immune parameters, including total haemocyte count (THC), phenoloxidase (PO) activity, and lysozyme (LYS) activity, which are critical indicators of improved immune function. These enhancements directly contributed to the increased survival rates observed in the supplemented groups. Importantly, the addition of S. nervosum did not negatively affect the growth rate or feed conversion ratio (FCR) of the shrimp, confirming its viability as a supplement for promoting shrimp health without compromising growth performance. Furthermore, the results highlight the extract’s potential as an effective preventive strategy against acute hepatopancreatic necrosis disease (AHPND), as it significantly reduced cumulative mortality in shrimp challenged with V. parahaemolyticus. The study suggests that supplementing shrimp diets with S. nervosum leaf extract at a concentration of 12.5 g/kg is optimal for practical application in white shrimp farming.
Acknowledgments
This study was conducted with financial support from the Hue University project: “Effects of Syzygium nervosum leaf extract on the growth rate, immunity and disease resistance of white shrimp (Litopenaeus vannamei) to Vibrio parahaemolyticus”. Code: DHH2023-02-168.
Authors’ Contribution
Conceptualization: Hoa T. Truong (Equal), Ha N. Tran (Equal), Manh N. Hoang (Equal). Data curation: Hoa T. Truong (Equal), Tung T. Ho (Equal), Van Q. K. Tran (Equal). Formal Analysis: Hoa T. Truong (Equal), Manh N. Hoang (Equal). Funding acquisition: Hoa T. Truong (Equal), Tung T. Ho (Equal), Van Q. K. Tran (Equal). Methodology: Hoa T. Truong (Equal), Tung T. Ho (Equal), Van Q. K. Tran (Equal). Project administration: Hoa T. Truong (Lead). Writing – original draft: Hoa T. Truong (Equal), Manh N. Hoang (Equal). Writing – review & editing: Hoa T. Truong (Equal), Ha N. Tran (Equal), Manh N. Hoang (Equal). Supervision: Manh N. Hoang (Lead).
Competing of Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
This study was conducted according to the ethical standards of the UK Home Office, based on guidance received from our colleague who had done his studies at the Institute of Aquaculture, University of Stirling, Stirling, UK.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.