1. Introduction
The rapa whelk Rapana venosa (Gastropoda: Muricidae) is a predatory sea snail of particular interest around the world.1 This species has a generation time of one year, individual longevity of over 10 years, annual production of egg cases, and high fecundity, with females producing more than 400,000 eggs per female in one spawning season.2 Their planktonic larval duration is 14-80 days, and adult sizes range from 40 to 70 mm in shell length.3 The native range of Rapana venosa extends throughout the Sea of Japan, the Bohai Sea, the Yellow Sea, and the East China Sea.4,5 The Rapana venosa is a large-sized shellfish of significant economic value.6 Its delicious taste and richness in various trace elements, unsaturated fatty acids, and amino acids make it highly favored by people in China.7 At present, the Rapana venosa available in the market mainly relies on the collection of wild resources. Understanding its reproductive habits, embryonic development stages, and environmental factors affecting reproductive success is crucial for developing effective breeding and management strategies.
Recent studies on rapa whelk include biology,8 physiological,9 artificial culture,10 ecology,11,12 reproductive biology,13 and aquaculture investigations.14 In the artificial breeding and factory farming of Rapana venosa, water quality regulation is the most critical step.15 The effects of different concentrations of water environmental factors such as ammonia nitrogen and nitrite on the growth and development of juveniles are significantly different.16 Therefore, research on the impact of various water quality factors in the water environment on the growth and development of juveniles is particularly important.
Ammonia nitrogen and nitrite are the most common pollutants in the aquatic animal breeding water environment.17 Ammonia nitrogen is the main product in the process of protein metabolism, which is generally produced by direct secretion of aquatic animals, and can also be generated by ammonification of nitrogen-containing organic compounds such as residual bait, animal limbs, aquatic animals’ metabolites, and their excrement in water. This pollutes the aquaculture water environment and affects the healthy growth of aquatic animals.18 Nitrite is generated by ammonia nitrogen in the process of bacterial nitrification or nitrate in the process of denitrification. In the process of aquatic animal breeding, the transformation of ammonia, nitrogen, and nitrite is affected by many factors. In the later stage of aquaculture, the content of ammonia, nitrogen, and nitrite accumulate beyond the standard,19 thus affecting the growth, metabolism, immunity, and survival of aquatic animals.19 Therefore, it is very important to study the toxicity of ammonia nitrogen and nitrite to different species.
Exploring the impact of water quality factors on the successful cultivation of rapa whelk is crucial. Water quality parameters, along with the types of food provided, play a pivotal role in determining the hatching success of egg capsules. However, there are currently few reports about how water quality conditions affect egg capsule hatching. This study investigated the effects of ammonia nitrogen, nitrite, and pH on egg hatching and hatching characteristics. By examining the impact of water quality factors on the hatching of Rapana venosa eggs, this research provides theoretical assistance for improving hatching rates and enhancing the quality of juvenile snails in future artificial breeding efforts. Additionally, through the study of ammonia nitrogen, nitrite, and pH, understanding the mechanisms of these factors offers theoretical guidance for the conservation of wild populations of Rapana venosa and the restoration of their habitats.
2. Materials and methods
2.1. Sample acquisition
Wild rapa whelks were collected from the Yellow Sea (39°N, 122°E) in July 2024. Their shell heights, from spires to ends of siphonal canals, ranged between 12 and 15 cm. The average weight was 251 g (n > 100), and the sex ratio was 1.05 males to 1.0 female. The rapa whelks were stored in tanks at 24℃, pH 8.1, and 30% salinity. They were fed with clams (Ruditapes philippinarum) up to twice per day as needed, and the water in the tanks was changed daily.
2.2. Sample treatment
The rapa whelks began to mate starting on the second day of captivity. After mating for 1-2 days, the females began producing elongate egg cases in chrysanthemum shaped clusters on tank walls. Each egg case was about 2.00-2.50 cm long and had a milky yellow or purple-brown appearance (Figure 1). During mating and spawning, all water in tanks was changed twice a day, and medium aeration was supplied. Once enough egg capsules were available for our experiment, rapa whelks were removed from tanks, and the egg cases were collected and inspected by microscopy to confirm that the larvae in egg capsules were at similar development stages. In this experiment, gradients of three different water quality factors were tested for their effects on rapa whelk egg development: ammonia nitrogen (0.25, 0.50, 0.75, 1.00, 1.25, 1.50, 1.75, and 2.00 mg/L), nitrite (0.0125, 0.0250, 0.050, 0.0750, 0.1000, 0.1500, 0.1750, and 0.2000 mg/L), and pH (6.00, 6.50, 7.00, 7.50, 8.50, 9.00, 9.50, and 10.00). Each indicator also had a control group for ammonia nitrogen (0.00 mg/L), nitrite (0.0000 mg/L), and pH (8.00), with three replications each. The experimental group and the control groups used 81 identical 640 ml (10.0 cm × 8.0 cm × 8.0 cm) blue polyethylene squares. The test gradients were established by manipulating natural seawater. Seawater was filtered through a 500 mesh sieve after sediment and sand filtration. Then, each water quality factor was adjusted by adding appropriate chemicals or diluting with seawater using a 1.00 ml capacity syringe until desired concentrations were reached. Experimental and control rapa whelk egg sacs were simultaneously placed in tanks, with three replications per experimental water quality factor level. The morphological and quality characteristics of each batch of eggs are similar, with the mean ± standard deviation being: weights (0.1388 ± 0.0003g), lengths (27.48 ± 0.52 mm), widths (2.57 ± 0.06 mm). Experimental and control tanks were given light shades (500 ± 50 lux), their water was changed once a day at 08:30, and the square water boxes were washed once every three days. There was no significant difference in light intensity, temperature, salinity, dissolved oxygen content, nitrite, and ammonia nitrogen content between the experimental group and the control group during the experiment (P > 0.05).
2.3. Data Measurement
At 07:30 and 19:30 each day, each side of each tank was examined for the presence of larvae. In addition, all egg capsules were observed under a photographic microscope, and 30 larvae were randomly selected for size measurement using Photoshop software. At 08:00 and 10:00, water quality factors (pH, salinity, temperature) were measured before and after the water change using a water analyzer (Thermo Scientific Orion Star A216), and the surface illumination of each tank was measured using an illuminometer (Jiading Union ZDS-10). Ammonia concentration was determined in all tanks every seven days using an enzyme marker for ammonia nitrogen (by hypobromate oxidation) and nitrite (by diazotization coupling colorimetry).
2.4. Data analysis
Normality and homogeneity of variance of larval release times and of the initial size of larvae were tested using SPSS 24.0 software, followed by One-way ANOVA and SNK multiple comparisons to assess differences between factor levels within each water quality indicator. Necrosis rates of egg sacs were not normally distributed, so nonparametric Kruskal-Wallis H tests were used to compare differences between factor levels. The level of significance was set at P < 0.05.
3. Results
3.1. Effects of water quality factors on egg sac necrosis rates
The results of Kruskal-Wallis H test are shown in Table 1. The experimental results demonstrated that the concentration of ammonia nitrogen and nitrite did not have a significant impact on necrosis rates. However, necrosis rates were significantly influenced by pH (P < 0.01). Necrosis reached 100% within the pH ranges of 6.00-7.00 and 9.00-10.00, while it was relatively low within the range of 7.50-8.50. Excluding pH values of 6, 6.5, 9, 9.5, or 10, which all resulted in complete necrosis, there was a significant effect of pH on necrosis within the remaining range of pH 7, 7.5, 8, and 8.5 (P < 0.05). Specifically, as the pH values within this range increased, the necrosis rate decreased (Figure 2).
3.3. Effects of water quality factors on larval release time
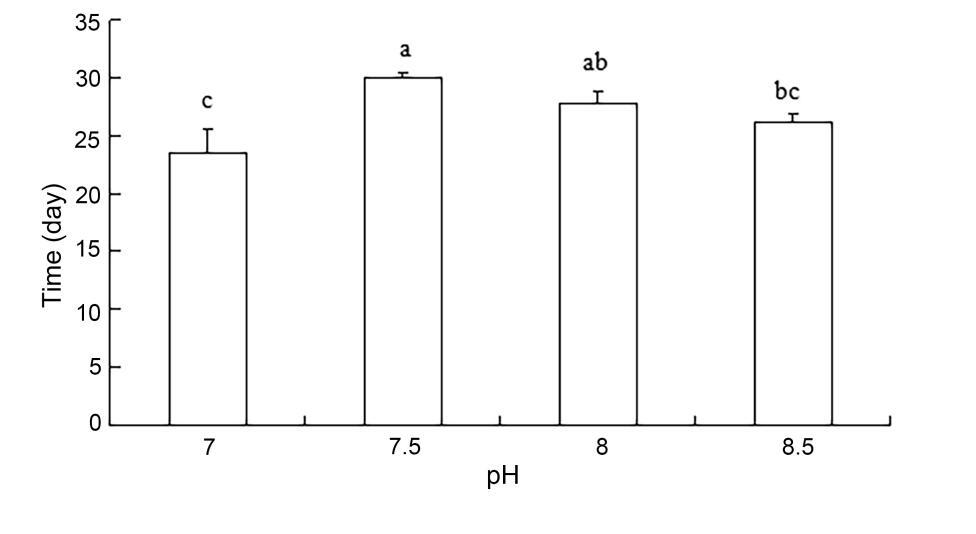
The variance analysis of each factor on the quality of Rapana venosa egg bag release of larvae is shown in Table 2. The experimental results showed that there was no significant effect of ammonia nitrogen or nitrate on larval release times from egg capsules. In contrast, there was a significant effect of pH on larval release times (P < 0.01). Within the pH range of 7.50 to 8.50, the larval release time was shortest at a pH value of 8.5 (P < 0.01). The pH value was 8.5 (26.11 ± 0.84 days) < 8.0 (27.83 ± 1.04 days) < 7.5 (30.00 ± 0.00) (Figure 3).
3.4. Effects of water quality factors on the initial size of rapa whelk larvae
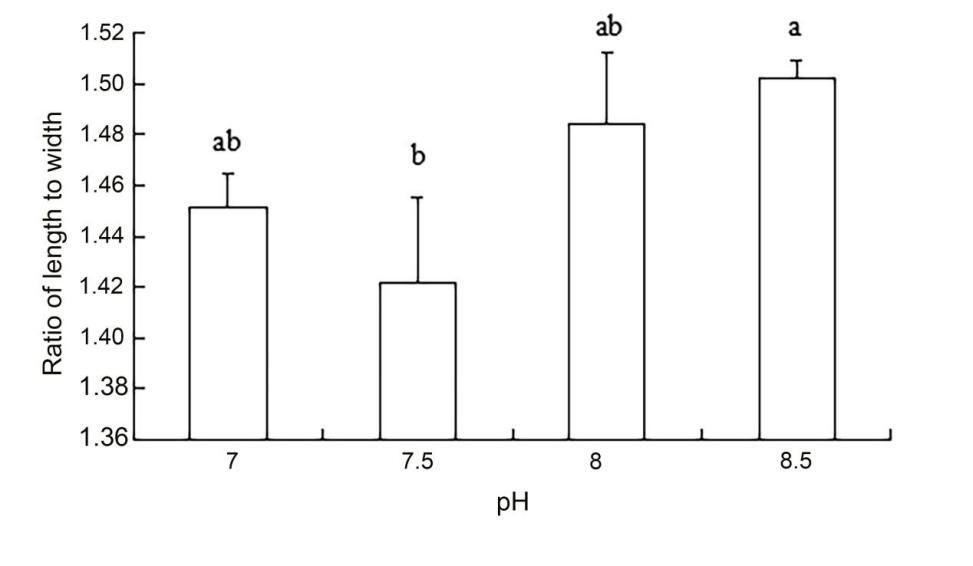
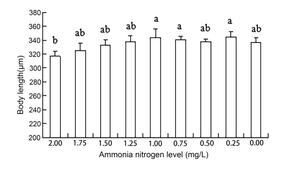
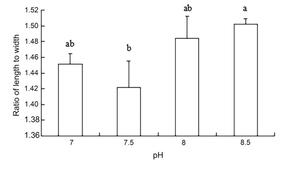
The results of ANOVA showed that ammonia nitrogen had a significant effect on the length and length-to-width ratios of larvae (P < 0.05) but had no significant effect on body width (Figure 4) (Table 3). The decrease in the amount of ammonia nitrogen concentration led to a decrease in the difference of body width and aspect ratio (P < 0.05) (Figure 5). Nitrite had a significant effect on the length-to-width ratio of larvae (P < 0.01), but not on the length or width of larvae (P < 0.01) (Figure 6). There was a significant effect of pH on the body width of larvae (P < 0.01). There was also a significant effect of pH on the ratio of length to width (P < 0.05). With increased pH values, the body width of larvae decreased (Figure 7), and the ratio of length to width increased (Figure 8).
4. Discussion
4.1. Effect of water quality factors on the necrosis rate of the egg capsule of Rapana venosa
Effects of water quality factors on egg capsule necrosis rate: ammonia nitrogen and nitrite had no significant effect on the necrosis rate. The necrosis rate changed significantly with changes in pH (P < 0.01), and the mortality rate was 100% at pH values of 6.00 and 9.00; it was relatively low at pH ranges of 7.50-8.50 (pH 7, 7.50, 8, 8.50). The mortality rate was significantly higher than that of the control group (P < 0.01) (8.0). Variance analysis of the necrosis rate revealed a significant difference between groups (P < 0.05)- that is, different pH concentration gradients had a significant effect on the egg capsule necrosis rate, and as pH increased, the egg capsule necrosis rate differed between the two groups (P < 0.05). The necrosis rate decreased gradually with the increase of pH value.
The hatch rate was 70.1%-85.5% (average 80.8%), in agreement with the results from the experimental treatment groups with variable ammonia nitrogen, nitrous acid, and partial pH. According to Tsi et al., if ammonia nitrogen reaches 0.20 mg/L, D-type larvae do not hatch normally and stay in the single-wheel stage. If ammonia nitrogen reaches 0.15 mg/L, the feeding capacity of larvae is reduced, and their growth is slow. In that experiment, higher concentrations of ammonia nitrogen led to higher necrosis rates, but there was no significant difference in the necrosis rate between different concentration gradients. The effect of the experiment is affected, and this can also be related to the selection and infiltration of the gastropod. Therefore, it is important to further study composition and function. Tsi et al. also reported that the oxygen content in their experimental groups ranged from 0.25-6.58 mg/L, and the concentration of nitrite was lower than in our study. These conditions may have influenced the results of their experiment. Survival of larvae is influenced by pH, and there are many reports that pH affects the physiology of metamorphosis. When the pH of the environment changes, this affects the pH in the blood of aquatic animals, hence affecting the oxygen delivery capacity of the blood. Aquatic animal larvae can be very sensitive to such effects in certain organ tissues, influencing survival rates. Furthermore, it is widely believed that enzymes in organisms are greatly influenced by pH. When pH deviates from an optimum, enzyme activity is gradually reduced. When the pH exceeds the physiological limit of the organism, the enzyme may be severely denatured, resulting in metabolic disorders in larvae. Such conditions can lead to mass death within populations.
4.2. Effects of water quality factors on the release time of larvae
Variance analysis of the effects of different water quality factors on the release time of egg capsule larvae revealed that the different concentrations of ammonia nitrogen and nitrite had no significant effect on the release time of the larvae. A very significant difference was noted in the release time of egg pouch larvae with different pH values, and the release time of larvae with a pH value of 8.5 was the shortest within the range of 7.50 and 8.50. The release time of the egg capsule larvae with a pH value of 8.5 was the shortest in the range of 7.50 and 8.50, respectively. The pH values were 8.5 (26.11 ± 0.84 days) < 8.0 (27.83 ± 1.04 days) < 7.5 (30.00 ± 0.47 days).
Water temperature is an important factor for the normal metamorphosis and growth of larvae. If the water temperature is too low, metamorphosis time is increased, and if the water temperature is too high, larval quality is decreased.20 In addition, within the optimal water temperature range, the rate of embryo development increases with temperature. Furthermore, the incubation and hatching time of fertilized eggs in egg capsules are related to water temperature because of the effect of temperature on chemical and enzymatic reaction rates in cells. Temperatures that are too low or high can cause damage to cells. Bao & You (2004)21 reported that larvae hatched at a pH range of 8.0-8.3 and 21-22℃ at seawater density of 1.030. The incubation rate was 80.9% when the water temperature was 23℃ and 24℃, and the incubation time was 20 days and 26 days, respectively, whereas the incubation rate was only 16 days at 23℃. In our study, where the temperature of each group tested was 24.2 ± 1.2℃, the hatching times of larvae were not consistent with those reported.22 This may be due to large differences in the experimental conditions between studies. Shading was employed for the tanks, resulting in variations of water quality factors between studies.
Salinity affects water pressure, cell osmotic pressure, and nutrient absorption for organisms in their environment. In addition, pH can affect respiration in animals, cell membrane charge, activity of enzymes, solubility of nutrients in the growing environment, the degree of dissociation, and the rate of decomposition.23 In a dissolved oxygen-rich water environment, nitrate ions, iron ions, and sulfate ions, among other ions, are stable. However, if there is no oxygen in the water, these are reduced to other forms such as ammonium ions, ferrous ions, and reduced sulfur compounds. Dissolved oxygen content will also affect the composition of organic decomposition products. When dissolved oxygen content is sufficiently high, the reproduction of oxygen-demanding bacteria will increase, as will oxidative decomposition of the organic matter used by oxygen-demanding bacteria. The final products after decomposition are carbon dioxide and ions, such as sulfate and nitrate ions. These products are harmless to rapa whelk larvae but indirectly affect several water indices. For example, carbon dioxide content will affect pH of water. However, in hypoxic or oxygen-deficient water layers, anaerobic bacteria in the sediment are dominant, and their decomposition rate is slow compared with that of aerobic bacteria. Decomposition products of aerobic bacteria, such as hydrogen sulfide, ammonium ions, and certain amines and fats are mostly harmful to the larva. Therefore, to be safe for rapa whelk larvae, dissolved oxygen content in water should be kept above 5 mg/L. In our experiment, the dissolved oxygen content in each group was within 6.46-6.66 mg/L, which would be expected to limit the toxic effects of ammonia nitrogen and nitrite. Al-Hakim et al.24 reported that low pH can significantly affect the growth and development of aquatic organisms. Specifically, low pH can both reduce animal energy intake and increase animal energy loss to excretion. This may also contribute to why low pH causes the growth and development of rapa whelks to slow down in egg cases or even cause death. Furthermore, the metamorphosis rate of phase II free-swimming larvae increased with higher pH and reached a maximum at a pH of 8.0. As the pH value continued to increase beyond 8.0, the rate of metamorphosis gradually decreased.25 Similar to these results, within the range of 7.50-8.50 pH, the larval release time was shortest at pH 8.5 for rapa whelks in our pH gradient experiment. The pH value is 8.5 (26.11 ± 0.84 days) < 8.0 (27.83 ± 1.04 days) < 7.5 (30.00 ± 0.47 days).
4.3. Effect of water quality factors on the size of the hatched larvae of Rapana venosa
A significant effect was noted on water quality factors on the length and the ratio of length to width of the newly hatched larvae (P < 0.05), but no significant effect was observed on body width. With the decrease in the concentration of ammonia nitrogen, there was a significant difference in the body length and the ratio of length to width of the larvae (P < 0.05). The length of larvae increased gradually, and the ratio of length to width gradually increased after hatching. Nitrite concentration had no effect on the lengths and widths of the larvae but had a significant effect on the ratio of length to width (P < 0.01). The pH values of different gradients had a very significant effect on the body width and the ratio of length to width (P < 0.01) and on the ratio of length to width (P < 0. 01) and on the ratio of length to width (P < 0.01). With the increase in pH value, the body width of newly hatched larvae decreased, and the ratio of length to width gradually increased.
Wang et al. (2003)26 reported that larvae hatch from egg capsules in a salinity range of 21-39.5%, and when salinity is 23-39.5%, eggs in egg cases hatched completely within 10 days. At 29.5% salinity, the maximum shell height of hatched larvae was 341 µm, while under 23%, 35.5%, and 39.5% salinity, the maximum shell height of hatched larvae was 284 µm and 298 µm, respectively. A salinity of 29.5-35.5% is suitable for the survival and growth of zooplankton hatched from egg capsules, and the optimum salinity for hatching and growth of zooplankton is about 29.5%. Above and below this salinity range, zooplankton will die within nine days.
O’Connor et al. (2007)17 showed that larval growth was positively correlated with temperature and negatively correlated with acidity within the acceptable temperature range, indicating that a decrease in pH would make larvae smaller. Therefore, higher temperatures within the acceptable range can counteract the negative effects of acidification to some extent.
In egg cases, rapa whelks hatch into disk larvae, which live as free-swimming organisms before metamorphosing into juvenile snails. Hatching from egg cases after embryos develop into disc larvae can protect the developing embryos of invertebrates living in the ocean to varying degrees, for example, by mitigating the effects of adverse environments and resisting predator attacks. Free-living zooplankton larvae have the advantage of temporarily avoiding competition with adults for food, reducing mortality on the seafloor and the possibility of future inbreeding. Hence, free-living forms have more adaptability to the environment. Furthermore, continuous replenishment of zooplankton larvae from other populations can increase rates of evolution. Overall, the biological function of the egg capsule is to provide protection from unfavorable environmental conditions. Under this premise, the occurrences of variable degrees of environmental danger are consistent with our results, as this is also the main reason for variation in larval size under different water quality conditions.
5. Conclusion
The optimal survival pH for Rapana venosa juveniles is 9, while the lowest is 7. At pH 8.5, the hatching time for Rapana venosa juveniles is the shortest, making it the best pH for hatching. Ammonia nitrogen and pH have the greatest impact on the body size traits of Rapana venosa juveniles. This study provides an overview of the effects of ammonia nitrogen, nitrite, and pH on the necrosis rate of Rapana venosa egg masses, the hatching time of larvae, and the size of newly hatched larvae. Environmental factors significantly influence the growth and development of Rapana venosa in both aquaculture and natural environments. However, nutritional factors also play an important role that should not be overlooked. The growth condition of Rapana venosa juveniles is the result of the combined effects of these factors.27
In future experiments, we will explore the growth and development mechanisms of Rapana venosa juveniles more comprehensively by considering differences in both environmental factors and nutritional conditions. In the aquatic environment, these environmental factors also interact with each other. Changes in one factor can lead to changes in others. Therefore, when conducting artificial breeding of Rapana venosa, it is important to take into account all the influencing factors. Due to various limitations, the study on the growth and development of Rapana venosa juveniles presented in this paper is not yet comprehensive. The concentration gradients were set relatively large, and the experiments were conducted in small laboratory water bodies as preliminary tests. In future experiments, we will refine the concentration gradients of different factors, simulate the conditions of industrial aquaculture, and design more rigorous experiments. This will allow us to further explore the optimal growth range and tolerance range of Rapana venosa juveniles, providing more reasonable theoretical guidance for their artificial breeding.
ACKNOWLEDGMENTS
This research was supported by the Major Science and Technology Project of Liaoning (2024JH1/11700010), the Science and Technology Major Projects in Liaoning Province (2025), the National Natural Science Foundation of China (42076101), and the Central Government Subsidy Project for Liaoning." Addition "the Major Science and Technology Project of Liaoning (2024JH1/11700010).
AUTHORS’ CONTRIBUTION
Methodology: Menghao Jia (Lead). Writing – original draft: Menghao Jia (Lead). Writing – review & editing: Menghao Jia (Equal), Ying Tian, Zhenlin Hao (Equal). Investigation: Linxuan Cai (Equal), Wenjia Li (Equal), Ying Tian (Lead). Formal Analysis: Ying Tian (Lead). Conceptualization: Zhenlin Hao (Equal). Project administration: Zhenlin Hao (Lead).
ETHICAL CONDUCT APPROVAL - IACUC
The article adheres to the Convention on Biological Diversity and the Convention on Trade in Endangered Species of Wild Fauna and Flora Research.
CONFLICT OF INTEREST - COPE
The authors declare that they have no conflict of interest.
INFORMED CONSENT STATEMENT
All authors and institutions have confirmed this manuscript for publication.
DATA AVAILABILITY STATEMENT
The data supporting the results of this study can be acquired from the corresponding author on rational request.

_rapa_whelk_egg_cases_and_(b)_mating_rapa_whelks_(c)_larval_shell_(d)_ma.png)





_rapa_whelk_egg_cases_and_(b)_mating_rapa_whelks_(c)_larval_shell_(d)_ma.png)