1. Introduction
The black tiger shrimp (Penaeus monodon) is an economically important aquaculture species along the coast of China, primarily farmed in southern China.1,2 Insufficient dissolved oxygen in water and acute decrease in dissolved oxygen are key factors leading to aquaculture failure in the production process of P. monodon.3,4 Hypoxic environments can lead to multiple effects on P. monodon; firstly, hypoxia reduces the dissolved oxygen content in the water, affecting the shrimp’s ability to breathe through their gills, leading to respiratory distress or even suffocation. Additionally, hypoxia affects shrimp metabolism and physiological functions. As shrimp metabolism relies on oxygen, hypoxia forces a shift from aerobic to anaerobic metabolism, potentially slowing down metabolic rates and affecting energy consumption and growth.5,6 Hypoxia also impairs the immune system, making shrimp more susceptible to diseases.7,8 In summary, hypoxic environments impact shrimp respiration, metabolism, physiological functions, and behavior, potentially adversely affecting their survival and development.
Dissolved oxygen levels in aquatic environments are highly influenced by external factors such as temperature, salinity, water depth, and atmospheric pressure.9 In recent years, eutrophication caused by increased industrial wastewater discharge, climate warming due to the greenhouse effect, and sudden or catastrophic weather events have led to more frequent occurrences of hypoxia. Additionally, hypoxia often occurs during long-distance transportation of aquatic animals.10 Hypoxia not only alters shrimp behavior but also induces physiological and biochemical changes, significantly impacting their vital activities. This study conducted a hypoxia stress experiment to measure enzyme activity in the hepatopancreas of P. monodon and examined changes in tissue structure, aiming to elucidate the physiological impacts of hypoxia on shrimp. The findings may help formulate effective farming management strategies to improve the survival rate and stocking density of P. monodon.
2. Methods
2.1. Experimental materials
The experiment was conducted at the Shenzhen Experimental Base of the South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. The shrimp used in the experiment were a newly approved national breed “Nanhai No. 3” bred by the research team with a body weight of 3.44±0.03g. The shrimp were taken from the cultivation pond and temporarily reared in an 8 cubic meter concrete pool for three days, during which they were fed normally, the feeding feed is commercial feed, purchased from Guangdong Haida Group Co., Ltd (Crude lipid 40%, Crude lipid 6%, Moisture 8%, Ash 12%). Feeding was stopped one day before the experiment.
2.2. Experimental design
A pre-experiment was conducted before the formal experiment, based on this, two experimental groups of hypoxia stress and conventional conditions control were set. The hypoxia group had two concentration gradients, with three replicates per group, totaling nine tanks (80 L), and 20 shrimp per tank. The duration was 4 hours, with sampling every 2 hours. Sixty liters of seawater were added to each of the three tanks: (a, reduce the DO to (2.0±0.2)mg·L-1; b, reduce the DO to (1.0±0.2)mg·L-1;c, aerated normally). In the experiment, a water-dissolved oxygen automatic controller (MEACON, Hangzhou Meikong Instrument Equipment Co., Ltd.) was used to regulate the dissolved oxygen content in the water. Twenty shrimp were placed in each tank, maintaining the temperature at (28.0±0.3)℃. During the experiment, the experimental shrimp were not fed with bait.
2.3. Sample collection
Samples were taken at 2h and 4h, with three shrimp randomly selected from each tank. Gill and hepatopancreas tissues were collected for observation of tissue structure changes and antioxidant enzyme measurements. Gill tissues for sectioning were stored in cryovials containing paraformaldehyde and kept at 4℃ for future use; gill and hepatopancreas tissues for enzyme activity were collected in cryovials, flash-frozen in liquid nitrogen for 24 hours, and then transferred to -80℃ for storage.
2.4. Enzyme Activity
Hepatopancreas tissue (0.1-0.15g) was accurately weighed and placed in a 2ml centrifuge tube. A 10% tissue homogenate was prepared by adding PBS at a mass-volume ratio of 1:9. Steel beads were then added, and the sample was homogenized for 30 seconds using a tissue homogenizer. After homogenization, the sample was centrifuged at 4℃, 3000 rpm for 10 minutes, and the supernatant was transferred to a 1.5ml centrifuge tube for enzyme activity measurement. Enzyme activity, including acid phosphatase (ACP), alkaline phosphatase (AKP), protein content (CPR), total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), and malondialdehyde (MDA), was measured using commercial assay kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions.
2.5. Tissue Section Preparation
Tissue sections were prepared using the paraffin sectioning method. Samples were first fixed in Bouin’s solution for 24 hours, then dehydrated through a graded ethanol series, cleared with xylene, and embedded in paraffin. Sections were cut continuously at a thickness of 5 μm using a microtome. The sections were then deparaffinized and rehydrated stepwise, stained with hematoxylin-eosin, and finally dehydrated and cleared. The completed sections were mounted with resin, preserved, and observed under a microscope for photography.
2.6. Statistical analysis
The triplicate data of each experimental group were presented as total mean standard deviation (SD), and the effect was tested using one-way analysis of variance (ANOVA). If a significant difference (P<0.05) was observed, the mean values of the groups were further compared using Duncan’s multiple range test. All statistical analyses were performed using SPSS 19.0.
3. Results
3.1. Pre-experimental results
After the pre-experiment, the activity of P. monodon in the rearing tanks was observed. As hypoxia treatment time increased, shrimp activity in the experimental group intensified compared to the control group, with rapid eyestalk movement and fast leg motion. Eventually, due to insufficient dissolved oxygen, some shrimp in the experimental group died, and the remaining live shrimp showed reduced activity and appetite, demonstrating the safety of using Na2SO3 for hypoxia treatment. The DO levels in the water were monitored every 30 minutes using a dissolved oxygen meter, and no significant changes were observed, proving the stability of hypoxia treatment with Na2SO3 and the equipment of the water dissolved oxygen automatic controller.
3.2. Effects of Hypoxia on Phosphatase Activity in the Hepatopancreas of Penaeus monodon
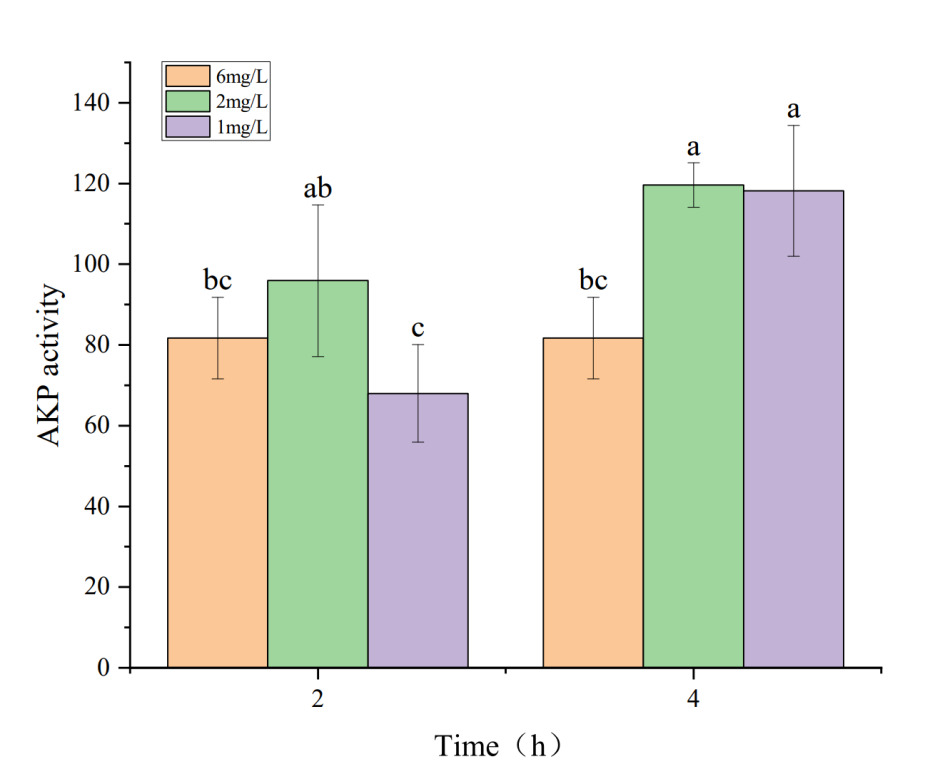
The effects of hypoxia stress on phosphatase activity in the hepatopancreas of P. monodon are shown in Figures 1 and 2. Figures 1 and 2 indicate that under a dissolved oxygen concentration of 6 mg・L-1, AKP and ACP activities did not significantly change over time, suggesting that AKP and ACP activities in P. monodon remain stable under normal conditions. At a dissolved oxygen concentration of 2 mg・L-1, AKP activity initially increased and then decreased with prolonged hypoxia treatment. However, at a dissolved oxygen concentration of 1 mg・L-1, AKP activity continuously decreased with prolonged hypoxia. Similarly, under a dissolved oxygen concentration of 2 mg・L-1, ACP activity decreased continuously with prolonged hypoxia treatment, while at 1 mg・L-1, ACP activity showed some recovery over time, but its activity was still lower than that of the control group.
3.3. Effects of Hypoxia on Antioxidant Enzyme Activity and MDA Content in the Hepatopancreas of Penaeus monodon
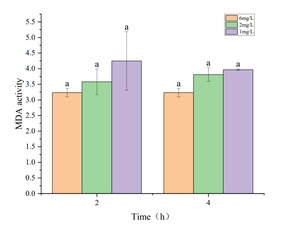
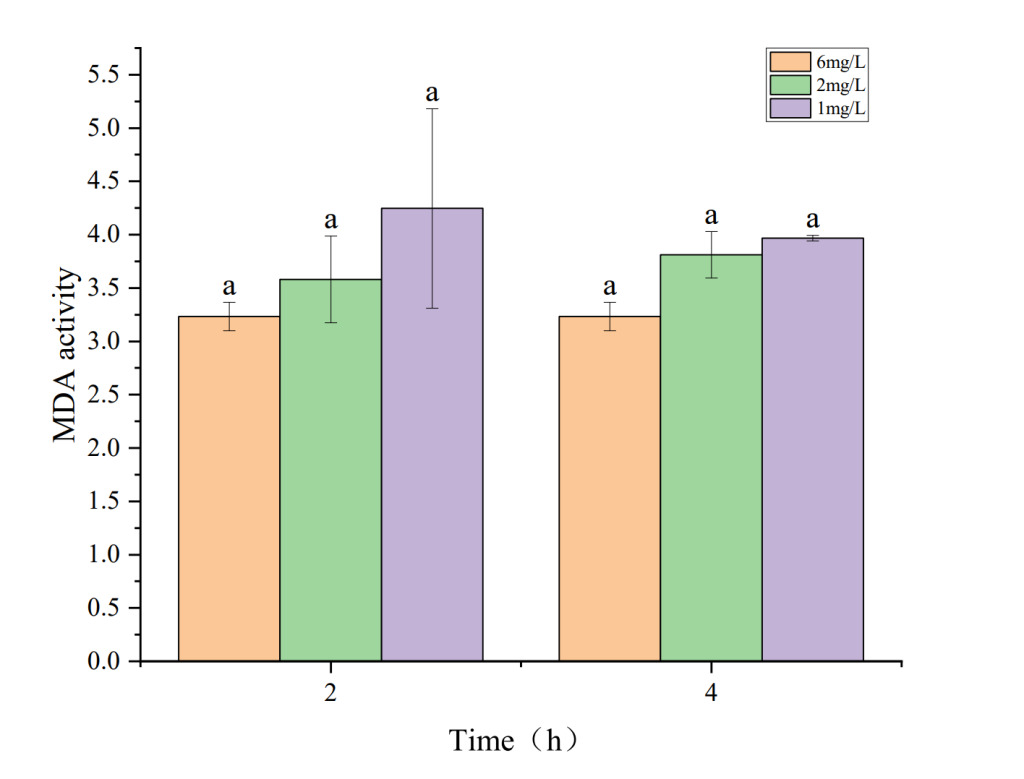
The effects of hypoxia on antioxidant enzyme activity and MDA content in the hepatopancreas of P. monodon are shown in Figures 3 to 5. Figures 3 and 5 show that under hypoxic conditions of 2 mg・L-1 and 1 mg・L-1, both T-AOC and MDA activities in the experimental group were lower than those in the control group and showed a decreasing trend with prolonged hypoxia treatment. The lower the dissolved oxygen concentration, the greater the decline. Figure 4 indicates that under hypoxic conditions of 2 mg・L-1 and 1 mg・L-1, T-SOD activity in the experimental group showed an increasing trend with prolonged hypoxia treatment and was higher than that of the control group.
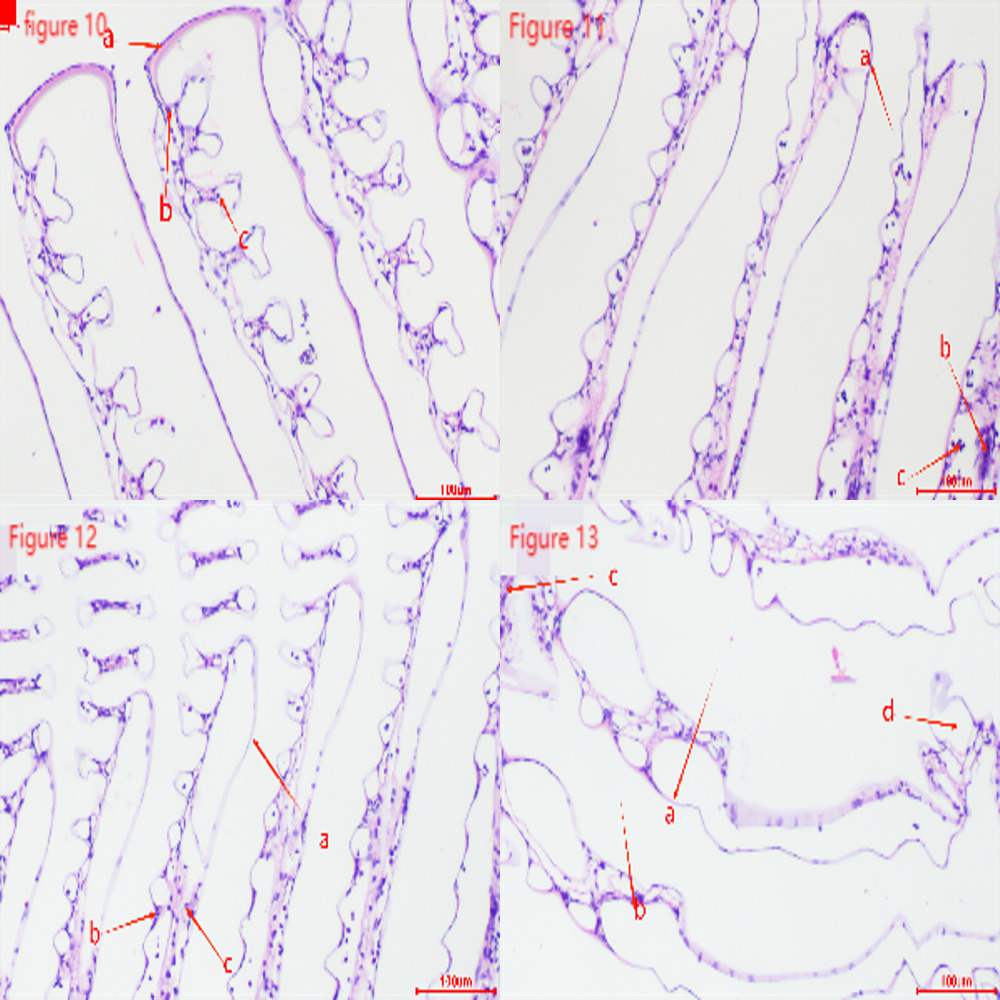
3.4. Effect of hypoxia on the tissue structure of Penaeus monodon
The images show hepatopancreas tissue sections from the control group (dissolved oxygen concentration 6 mg・L-1) and the experimental groups (dissolved oxygen concentration 2 mg・L-1) after 2h and 4h of hypoxia stress, and the experimental group (dissolved oxygen concentration 1 mg・L-1) after 2h of hypoxia stress.
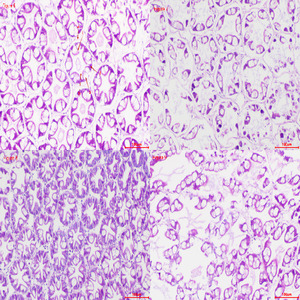
Figures 6-9 show that the hepatopancreas cell structure in the control group (Figure 6) was normal and intact, with complete epithelial cells, a clear lumen, and abundant secretory cells with full transport vesicles. After 2h of hypoxia stress at a dissolved oxygen concentration of 2 mg・L-1 (Figure 7), the number of secretory cells increased, and the volume of transport vesicles enlarged, with the gradual disappearance of internal granules. After 4h of hypoxia stress at 2 mg・L-1 (Figure 8), the lumen significantly shrank, becoming pentagonal or star-shaped, the structure of hepatic tubules reduced, gaps between hepatic tubules widened, and the basement membrane showed damage with the appearance of small granules, and fibrocytes were highly activated. After 2h of hypoxia stress at 1 mg・L-1 (Figure 9), the basement membrane exhibited extensive damage, and the lumen was irregularly torn.
Figures 10-13 show that the gill tissue structure in the control group (Figure 10) was clear and intact, with a distinct central gill chamber, tightly arranged respiratory epithelial cells and lymphocytes, and a continuous cuticle. After 2h of hypoxia stress at a dissolved oxygen concentration of 2 mg・L-1 (Figure 11), the gill filaments began to deform and bend, the cuticle showed slight cracks, and blood cells gathered towards both sides. After 4h of hypoxia stress at 2 mg・L-1 (Figure 12), the arrangement of respiratory epithelial cells became uneven, the cuticle was abraded, and the number of blood cells decreased. After 2h of hypoxia stress at 1 mg・L-1 (Figure 13), most epithelial cells were detached and loose, and the number of blood cells and lymphocytes significantly decreased.
4. Discussions
4.1. Effect of Hypoxia on the Immune Activity of Penaeus monodon
Under hypoxic conditions, the body generates a large amount of ROS. If not promptly eliminated, these ROS induce oxidative stress due to hypoxia, leading to lipid peroxidation of cell membranes, protein oxidation, and cell apoptosis.11 Excessive ROS production reduces lysosomal membrane permeability, releasing enzymes like AKP and ACP.12 As part of lysosomal enzymes, ACP and AKP play active roles in the immune system and are considered reliable indicators for assessing stress and immune status in biological systems.13
AKP is a phosphatase with the highest activity under alkaline conditions, found in various cells, including liver, intestine, and kidney tissues. Its main functions include hydrolyzing phosphate ester bonds and transferring phosphate groups, participating in energy metabolism, and cell signal transduction processes.14 ACP is an enzyme present in cells and body fluids, requiring an acidic environment for its catalytic process. It has various important functions in the body, such as catalyzing the hydrolysis of phosphate ester substrates into phosphates and organic groups. These substrates can include nucleic acids, sugar acids, and phospholipids. ACP activity is crucial for normal cellular metabolism and physiological functions.15 Under hypoxic conditions, AKP and ACP activities change to adapt to environmental variations. In this study, during hypoxic stress, the AKP content in the hepatopancreas of P. monodon significantly increased after 2h of exposure to 2mg・L-1 dissolved oxygen, indicating that hypoxia stimulated the shrimp’s immune response, enhancing its immunity. This finding is consistent with studies on Odontobutis potamophila16 and Litopenaeus vannamei17 under hypoxic stress. With prolonged hypoxia and decreasing dissolved oxygen levels, AKP content decreased, and ACP content gradually declined, only to increase again after 4h of exposure to 1mg・L-1 dissolved oxygen. Similar to findings in Rachycentron canadum under hypoxic stress, AKP content also showed a decline.18 Based on the experimental results of this experiment, combined with relevant research conclusions of other species, it can be concluded that frequent acute hypoxia stress in the production process of shrimp such as P. monodon can lead to excessive production of ROS, damage to body tissues, and affect immune function, thereby limiting AKP and ACP activity.
4.2. Effect of Hypoxia on the Antioxidant Capacity of Penaeus monodon
Under normal conditions, the production and elimination of ROS in organisms are dynamically balanced, with ROS being generated and simultaneously eliminated by the antioxidant system.19 In a hypoxic environment, the increase in reactive oxygen species (ROS) is significant. These excess free radicals attack the unsaturated fatty acids in biological membranes, causing lipid peroxidation reactions.20 T-SOD is an important antioxidant enzyme that converts superoxide radicals into more stable products. In the process of oxidative damage, T-SOD eliminates excess superoxide radicals through the reaction2O2−+2H+→H2O2+O2, maintaining cellular redox balance and enhancing survival and adaptation to hypoxic conditions.21
In this study, T-SOD activity gradually increased with the duration of hypoxia stress and decreasing dissolved oxygen concentration, peaking at 4h of 2 mg・L-1 hypoxia stress, then slightly declining but remaining higher than the control group. This is because, during initial hypoxia, more free radicals are generated, triggering oxidative stress. To counter this, P. monodon increases T-SOD synthesis and activity to enhance free radical clearance. Prolonged extreme hypoxia may lead to peak T-SOD activity followed by a decline due to functional fatigue or inhibition from long-term overexpression, consistent with findings by Yang22 and Zhao.23
MDA is an indicator of oxidative stress, assessing oxidative damage under hypoxia.24 Increased oxidative damage leads to lipid peroxidation, forming products like MDA. In this study, MDA levels gradually decreased, reaching the lowest at 2h of 1 mg・L-1 hypoxia stress, and slightly increased at 4h but remained much lower than the control group. This could be due to the rising T-SOD levels under hypoxia, reducing superoxide anion production and thus lowering MDA levels.
As hypoxia duration increased, the antioxidant system suffered damage, causing a slight MDA increase, consistent with findings by Wang18 and Zhao.23 T-AOC represents the total antioxidant capacity in the body, indicating the ability to counter free radicals and oxidative stress. There is an interrelationship between T-AOC, T-SOD, and MDA.25 Higher T-AOC enhances cellular antioxidant capacity, reducing free radical-induced damage and lowering MDA levels, while lower T-AOC can lead to insufficient antioxidant defense.
4.3. Effects of Hypoxia on the Hepatopancreas Structure of Penaeus monodon
The liver functions include secreting digestive enzymes, absorbing and storing nutrients, and detoxifying the body.26 It also has defensive functions. The shrimp liver, evolved from the midgut, consists of branched hepatic ducts, which are composed of hepatopancreatic tubules containing various hepatocytes, including columnar secretory cells with multiple or single vacuoles, absorptive cells with granular inclusions, and elongated fibrocytes. Among these, secretory and absorptive cells are predominant.27 With hypoxia stress, liver damage occurs, causing hepatocytes to appear fragmented or the hepatic tubules to become hollow. Under hypoxic conditions, the hepatopancreatic basement membrane shows damage, fibrocytes become highly activated, the volume of transport vacuoles increases, and the number of secretory cells rises. This is because the activity of hypoxia-inducible factor (HIF-1α) in liver cells is enhanced under hypoxic conditions, stimulating the glycolytic pathway. Liver cells produce more lactic acid through glycolysis, providing energy under hypoxic conditions. Due to reduced metabolic activities, the increase in secretory cells and transport vacuoles aids in absorbing stored energy substances within the body. These phenomena have also been observed in studies on Procambarus clarkia,28 Portunus trituberculatus,29 and Macrobrachium nipponense.30
4.4. Effects of Hypoxia on the Gill Structure of Penaeus monodon
Gills are respiratory organs found in aquatic animals, primarily used for breathing, gas exchange, and excreting metabolic waste. In this study, gill tissues showed changes under hypoxic conditions. In the control group, gill filaments were neatly arranged, while in the experimental group, the gill filaments were bent and swollen. Prolonged hypoxia leads to abnormal cell metabolism due to insufficient oxygen, causing cell death and dissolution. Lymphocytes and respiratory epithelial cells significantly decreased, much lower than in the control group. This is similar to the gill tissue changes observed in Cherax quadricarinatus31 under nitrite stress and Macrobrachium nipponense30 under hypoxia stress.
5. Conclusion
This study analyzed the effects of acute hypoxia stress on the antioxidant capacity and tissue damage of P. monodon. The results showed that hypoxia stress caused varying degrees of damage to the hepatopancreas and gill tissues of P. monodon. Gill filaments were bent and swollen, lymphocytes and respiratory epithelial cells significantly decreased, and the cuticle was abraded. Hepatopancreatic cells appeared fragmented, or the hepatic tubules became hollow, with damage to the basement membrane, activation of fibrocytes, enlarged transport vacuoles, and increased secretory cells.
Under hypoxia stress, there were significant changes in the antioxidant enzyme and phosphatase activities in the hepatopancreas of P. monodon. The total superoxide dismutase activity increased under hypoxic stress, but the total antioxidant capacity decreased. This indicates that under hypoxic conditions, P. monodon enhances its antioxidant enzyme activity to cope with environmental changes. However, prolonged hypoxia can damage the antioxidant system. Hypoxia also stimulated the immune system of P. monodon, with increased activities of acid phosphatase and alkaline phosphatase in the hepatopancreas, indicating that the non-specific immunity of P. monodon was affected to some extent.
In summary, acute hypoxic stress causes multifaceted damage to the tissue structure and physiological functions of P. monodon. Understanding these changes is significant for elucidating the mechanisms of hypoxia’s impact on aquatic animals and provides scientific evidence for formulating reasonable aquaculture management measures.
Acknowledgments
This study was supported by National Key R & D Program of China (2022YFD2400104), the earmarked fund for CARS-48, Central Public-interest Scientific Institution Basal Research Fund, CAFS (NO.2023TD34), the earmarked fund for HNARS(HNARS-10-ZJ01).
Data Availability statement
The data that support the findings of this study are available on request from the corresponding author.
Conflict of interest
The authors declare that there is no conflict of interests.
Authors’ Contribution
Conceptualization: Xinyu Qi (Equal), Jieyi Wang (Equal), Falin Zhou (Lead), Qibin Yang (Equal), Song Jiang (Equal). Writing – original draft: Falin Zhou (Equal). Investigation: Jianzhi Shi (Equal), Yangyang Ding (Equal), Yundong Li (Equal), Song Jiang (Equal).
Ethical Approval
The whole experiment was conducted according to the guidelines established by the National Institutes of Health.