Introduction
The Chinese soft-shelled turtle, Pelodiscus sinensis, is highly prized for its exceptional nutritional and medicinal properties, as well as its significant contribution to aquaculture production in China.1 The breeding of turtles has accelerated since the early 21st century due to increasing consumer demand, with annual production reaching 497,500 tons in 2023, marking a 33.13% increase compared to 2022 (data from the 2024 China Fisheries Statistical Yearbook). A notable characteristic of this species is its pronounced sexual dimorphism, with males exhibiting faster growth rates and thicker skirts than females. This sexual growth difference provides an opportunity to enhance production through breeding. Gender control technology could increase breeding yields through monosex cultivation. Additionally, analyzing the molecular basis of allometric growth and sex-based growth differences may lead to targeted growth control technologies, offering new ideas for scientific breeding strategies.2 Despite its economic significance, the molecular mechanisms underlying growth regulation in P. sinensis, particularly those driving sexual dimorphism, remain inadequately elucidated. Bridging this knowledge gap is crucial for enhancing both scientific comprehension and practical applications within the aquaculture industry.
Growth is a complex property of aquaculture culture and breeding, and one of the richest traits for enhancement at the genetic level. Animal growth is a complex metabolism process, and its process is subject to different variables like genotype, nutritional level, hormonal regulation, and environment.3 Among these, the insulin-like growth factor (IGF) system, consisting of IGFs, IGF receptors (IGFRs), IGF binding proteins (IGFBPs), and IGF acid-labile subunit (IGFALS), has a significant contribution towards growth and developmental modulation of the animal.4 While IGF1 and IGF2 have been extensively studied for their direct roles in growth modulation, IGFALS, a key regulatory protein within the IGF system, has received comparatively less attention. Nevertheless, its indirect yet crucial contribution to growth regulation through stabilizing circulating IGF levels deserves further recognition.5 IGFALS was first identified and cloned in humans in 1992.6 It forms a trimeric complex with IGF1/2 and IGFBP3/5, thereby significantly prolonging the half-life of IGFs in circulation.7 However, IGF1 within this ternary complex is unable to activate the IGF1 receptor (IGF1R), underscoring IGFALS’s role in regulating the bioavailability of IGFs.8 Studies have linked IGFALS deficiencies to growth disorders, such as idiopathic short stature in children,9 and its genetic variants have been associated with growth-related traits in cattle, including height, milk yield, and milk fat percentage.10,11 The defects in the IGFALS gene result in reduced IGF expression and activity, leading to growth deficiencies, short stature, delayed puberty, and insulin resistance.12 Despite its importance, research on IGFALS has primarily focused on mammals and fish, with limited exploration in reptiles.8,13,14
IGFs serve as pivotal regulators of vertebrate growth, and their expression patterns are frequently associated with sexual dimorphism in animals.1 Considering the essential role of IGFALS in modulating IGF activity, examining its function in P. sinensis could offer significant insights into the mechanisms underlying sex-based growth differences. This study aims to bridge this knowledge gap by cloning the full-length cDNA sequence of the IGFALS gene using the RACE technique, comparing its expression levels between male and female turtles through real-time quantitative PCR (qRT-PCR), and investigating its role in growth regulation. By clarifying the function of IGFALS in P. sinensis, this research will lay the foundation for understanding the action mechanisms of IGFs in the allometric growth and sexual dimorphism of P. sinensis.
Materials and Methods
Experimental materials
In this study, the Chinese soft-shelled turtles were obtained from Changde Hezhou Aquatic Products Co., Ltd. The turtles were maintained under standardized rearing and management conditions and were fed formulated feed twice daily at 9:00 a.m. and 4:00 p.m. The study included both healthy adult and juvenile turtles, all of which were free from any visible injuries.
(1) Tissue Expression Profiling Sampling: Tissue samples from the muscle, intestine, liver, gonads, kidney, spleen, pituitary gland, heart, and lungs of adult soft-shelled turtles were rapidly frozen in liquid nitrogen and stored at -80°C.
(2) Sex Steroid Treatment Protocol: The turtles were assigned to three experimental groups: a 17α-Methyltestosterone (MT) group, an estradiol (E2) group, and a control group, each consisting of 45 turtles (mean mass: 35.42±14.25 g). A total of 15 mg of MT and 15 mg of E2 were each dissolved in 0.5 mL absolute ethanol, followed by dilution with 4.5 mL olive oil and storage at 4°C in the dark. The control group received a solution of 0.5 mL absolute ethanol in 4.5 mL olive oil. Prior to injection, all turtles underwent a one-week fasting period and had unrestricted access to water. Each turtle was then injected with 0.1 mL of the prepared solution into the thigh muscle of the hind limb. Post-injection, the turtles were allowed free access to water but were not fed. Liver samples from juvenile turtles were harvested at 0, 12, 24, and 48 hours after injection. These samples were rapidly frozen in liquid nitrogen and stored at -80°C. Additionally, skin tissues were collected for genomic DNA extraction using the FastPure Cell/Tissue DNA Isolation Mini Kit (DC102). Sex identification was performed using sex-specific molecular markers.15
Cloning of IGFALS gene in P. sinensis
RNA was extracted according to the E.Z.N.A.® Total RNA Kit II (Omega, R6934) instructions, and treated with DNase I to remove genomic DNA. RNA quality was assessed by 1.5% agarose gel electrophoresis, and RNA concentration and A260/280 ratios were measured using an ultra-micro UV spectrophotometer (Denovix, DS-11). Total RNA was reverse transcribed into cDNA using the RevertAidTM First Strand cDNA Synthesis Kit (Fermentas, K1621).
Primers targeting intermediate-length repeat expansions in the IGFALS gene were designed with Primer Premier 6.0, using predicted mRNA sequences from P. sinensis genomic data as the template (accession number: XM_014577011.2). Based on these, 5’-RACE and 3’-RACE primers were also designed, and RACE amplification was conducted using the SMARTer® RACE 5’/3’ Kit (TaKaRa). All primers were synthesized by BioSune Biotechnology (Shanghai) Co., Ltd. The detailed sequences of the primers are provided in Table 1. Positive PCR clones were selected, cut, recovered, ligated, transformed, and subsequently sent to BioSune Biotechnology for sequencing. The sequencing data were analyzed and assembled using SeqMan to determine the full-length IGFALS gene cDNA sequence.
Bioinformatics analysis of IGFALS gene in P. sinensis
The open reading frame (ORF) of the target gene was retrieved using NCBI’s ORF Finder and the deduced AA sequence was predicted. This sequence was subjected to BLASTp homology analysis against relevant species in NCBI. The physicochemical properties, signal peptide (SignalP 6.0), structural domains (SMART), transmembrane domains (TMHMM), and protein tertiary structure (BioEdit, Expasy-ProtParam) were predicted using online software, respectively.
MEGA 11.0 software was used to analyze the homology of the IGFALS AA sequences in P. sinensis with related sequences from other species in NCBI. A phylogenetic tree was constructed using the neighbor-joining (NJ) method, and bootstrap analysis with 1000 replicates was performed to assess the robustness of the tree topology, providing confidence values for each node.
IGFALS gene expression analysis in P. sinensis
Specific primers for the IGFALS gene were designed using Primer Premier 6.0, with GAPDH (accession number: NM_001286927.1) serving as the internal reference gene (Table 1). Quantitative real-time PCR (qRT-PCR) was performed using a LightCycler® 480 PCR instrument (Roche, USA). The 10.0 µL reaction mixture included 1.0 µL of cDNA template, 0.25 µL of each primer (10 µmol/L), 5.0 µL SYBR® Premix Ex Taq™ II, and 3.5 µL deionized water. The PCR protocol consisted of pre-denaturation at 95°C for 5 minutes, followed by 95°C for 10 seconds, 58°C for 10 seconds, and 72°C for 10 seconds. Following amplification, melting curve analysis was performed to evaluate the specificity of the amplification products and to check for primer dimers. The melting curve ranged from 65°C to 95°C, increasing at a rate of 0.5°C every 5 seconds. To ensure the reliability and reproducibility of the results, all experiments were conducted with three independent biological replicates. Each biological replicate consisted of tissue samples collected from different individuals under the same experimental conditions. For each biological replicate, technical triplicates were performed. Relative mRNA expression levels of the target and reference genes were calculated using the 2-ΔΔCT method. Data were expressed as mean ± standard error. One-way ANOVA was performed on the relative expression levels under different tissue and hormone treatments using SPSS 22.0 software, with significance tested using Duncan’s multiple comparisons. GraphPad Prism 8.0 was used for plotting.
Results
Sequence characterization of IGFALSX1/2 genes in P. sinensis
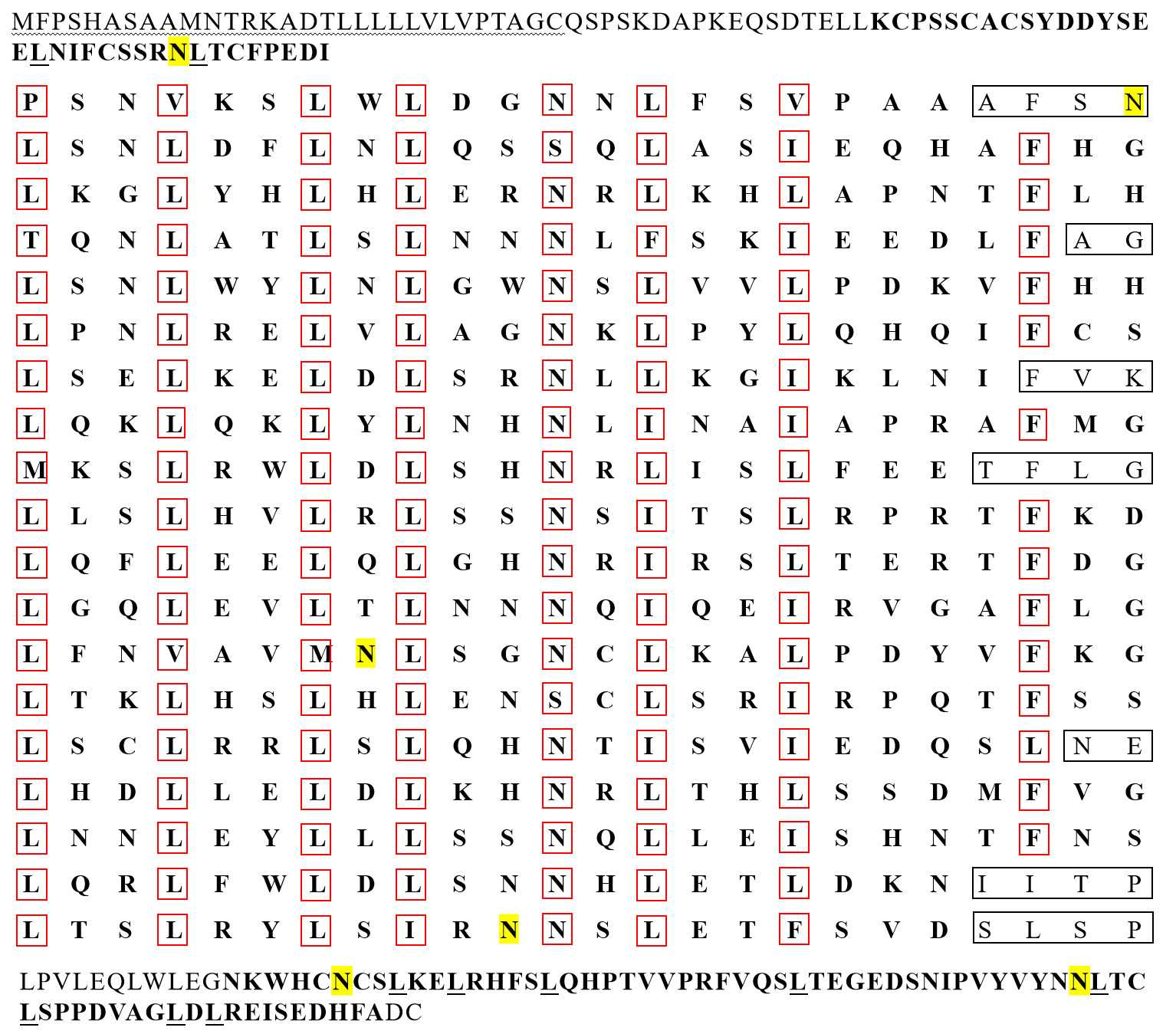
Two transcripts of the IGFALS gene, named IGFALSX1 and IGFALSX2, were obtained from the liver tissues of the Chinese soft-shelled turtle after sequencing and comparison. The full-length cDNA sequences of IGFALSX1 and IGFALSX2 were 2,452 bp (accession number: PV336088) and 2,520 bp (accession number: PV336089), respectively, each containing two exons and one intron. The full-length cDNA of IGFALSX2 included a 5’ UTR of 98 bp, a 3’ UTR of 571 bp, and an ORF of 1,851 bp encoding 616 AAs. IGFALSX1 exhibited a 71 bp deletion at the 5’ end, a 3 bp insertion, and a 1 bp synonymous substitution in the CDS region, with the remaining sequences encoding 617 AAs (Figure 1).
The leucine content was significantly higher than that of other AAs in the AA sequences of IGFALSX1 and IGFALSX2 in P. sinensis. The IGFALS protein is a non-transmembrane protein with a signal peptide cleavage site between AA residues 30-31 in X1 and 29-30 in X2. The protein includes one LRR_NT, 19 LRR, and one LRR C-terminal domain (LRR_C). Among the 19 LRRs, 8 are typical with 24 AAs each (Figure 2). The leucine-enriched sequence is represented as LxxLxxLxLxxNxLxxLxxxxFxx, where “x” denotes a non-conserved AA, and capital letters represent conserved sites. Leucine (L) is predominantly found at positions 1, 4, 7, 9, 14, and 17, while asparagine (N) and phenylalanine (F) are found at positions 12 and 22, respectively (Figure 3).
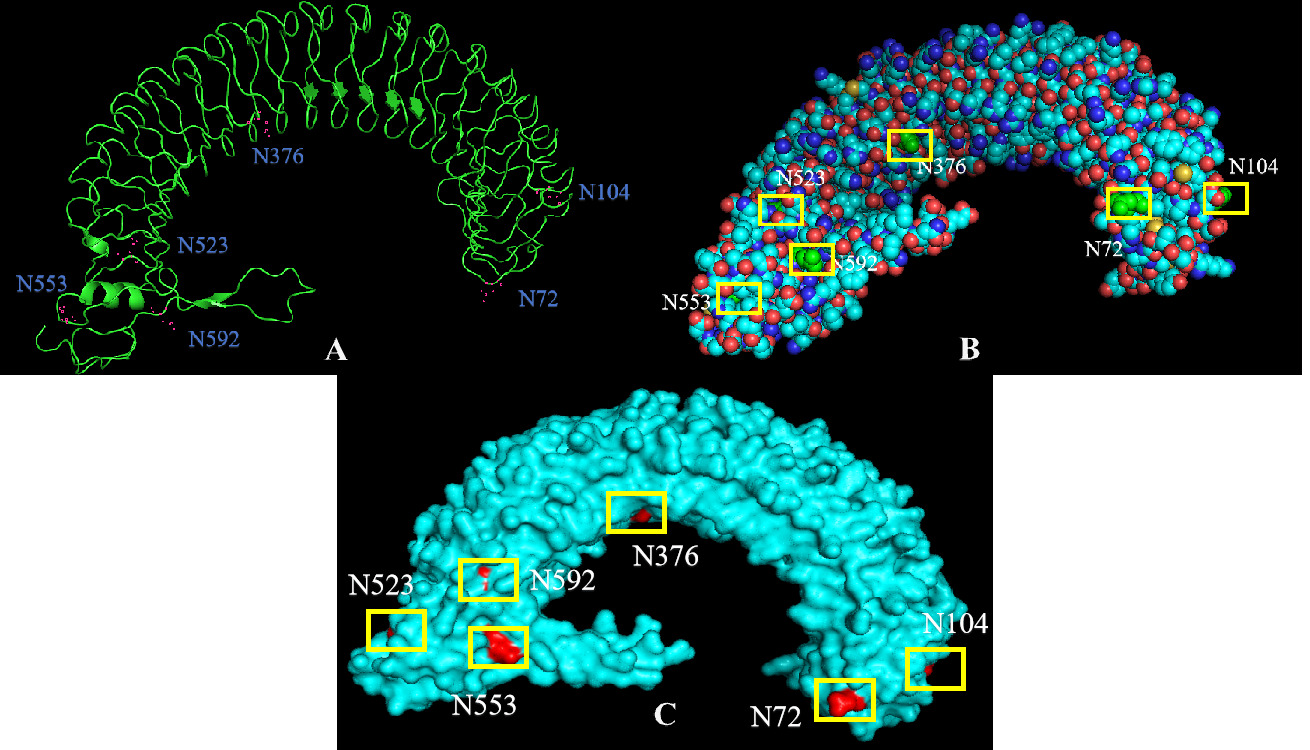
The tertiary structure of IGFALS in P. sinensis was predicted using SWISS-MODEL online software, based on the structure of the human IGF1/IGFBP3/ALS ternary complex (Figure 4). The N and C termini are close together, resembling a U-shaped magnet. Tertiary structure analysis with PyMOL software revealed that more carbon (C), nitrogen (N), and oxygen (O) atoms, rather than sulfur (S) atoms, were distributed on the protein surface. Aspartic acid residues were less abundant on the protein surface, with N376 and N523 partially covered by nearby AAs, and N104 and N553 located at the outer circle of the protein structure.
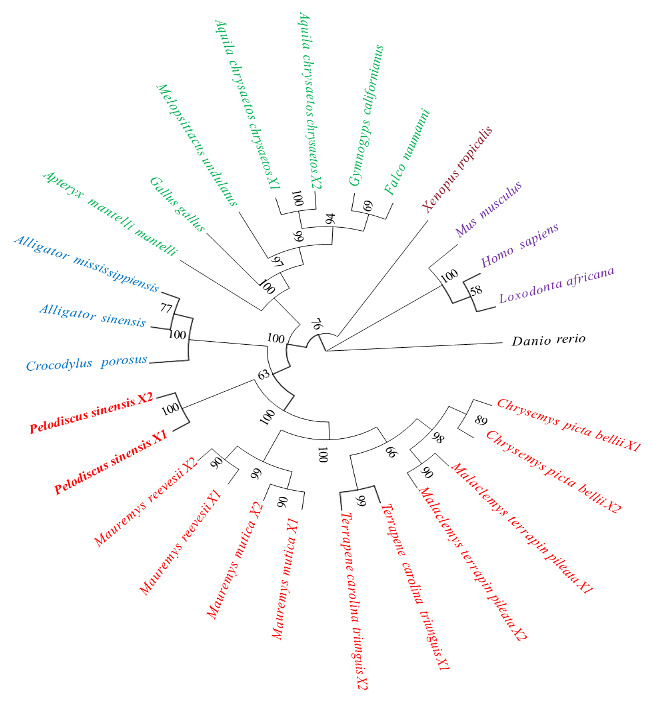
Phylogenetic tree analysis of IGFALSX1/2 in P. sinensis
The homologous alignment analysis of IGFALS AA sequences from several species, as published by NCBI, and IGFALSX1/2 AA sequences from P. sinensis, revealed that the similarity of the IGFALS AA sequence between P. sinensis and Malaclemys terrapin pileata IGFALSX2, and Terrapene carolina triunguis IGFALSX2, was highest at 91.73%. The similarity with zebrafish was the lowest at 56.7% (Table 2). To explore the evolutionary connections of IGFALS among various taxa, a phylogenetic tree was generated employing the neighbor-joining (NJ) approach. The IGFALS proteins were categorized into six separate groups: Chelonia, Crocodylia, Aves, Amphibia, Mammalia, and Fish. These groupings, bolstered by robust bootstrap values, reveal a strong evolutionary link between the Chinese soft-shelled turtle and other Chelonia species, with subsequent associations to Crocodylia, Aves, Amphibia, and Mammalia, while showing the highest genetic differentiation from Fish (Figure 5).
IGFALS gene expression analysis in various tissues of P. sinensis
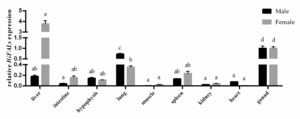
The qRT-PCR was utilized to analyze the tissue distribution of IGFALS gene expression in adult Chinese soft-shelled turtles. The results indicated that IGFALS was expressed in all nine tissues analyzed, with the highest levels found in the liver, lungs, and gonads, followed by the spleen, intestine, and pituitary gland. The lowest expression was found in muscle tissue. In females, the IGFALS gene was most highly expressed in the liver, while in males, it was most highly expressed in the testes (Figure 6).
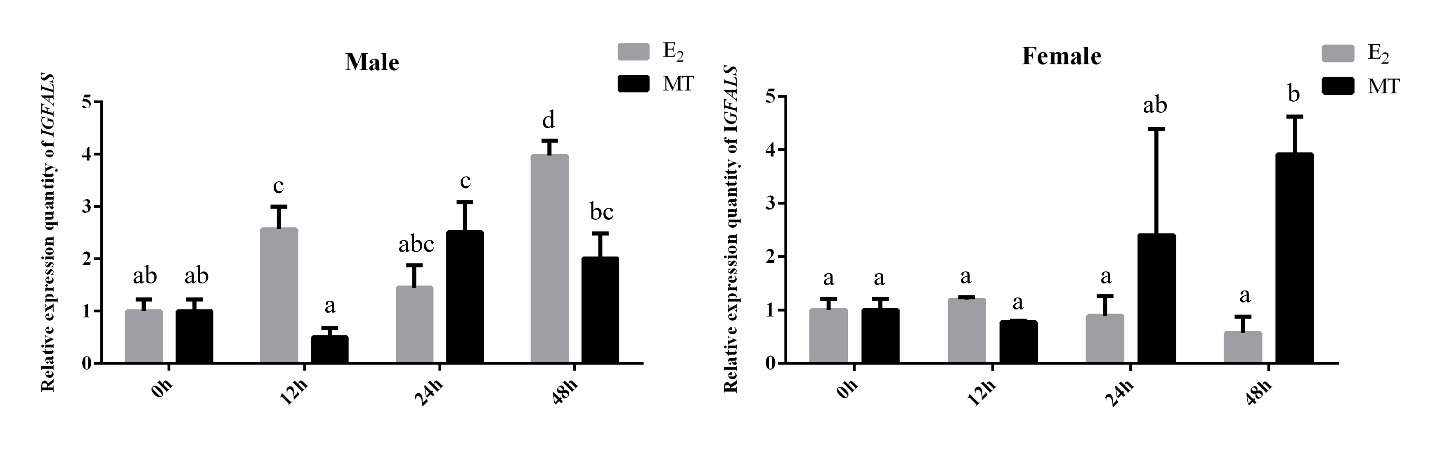
Effects of sex steroid hormone treatment on IGFALS gene expression in liver tissues of P. sinensis
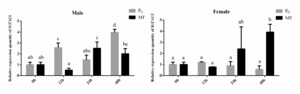
The qRT-PCR was used to detect changes in IGFALS gene expression in the liver tissues of male and female P. sinensis after 0, 12, 24, and 48 hours of sex steroid hormone treatment. As shown in Figure 7, the relative expression of IGFALS in male turtles’ livers exhibited an overall increasing trend after E2 and MT injection. In females, IGFALS gene expression was significantly upregulated at 48 hours under MT treatment (P < 0.05) but downregulated compared to the control group under E2 treatment, with the lowest expression at 12 hours, although no significant differences were observed (P > 0.05).
Discussion
IGFALS, a key member of the leucine-rich repeat (LRR) protein superfamily, plays a crucial role in regulating the activity of IGF1 and IGF2 by extending their half-life and modulating their bioavailability. In this study, the cDNA sequences of the IGFALSX1/2 genes were cloned from the Chinese soft-shelled turtle, encoding proteins of 616 and 617 AA residues, respectively. Sequence analysis revealed that IGFALSX1/2 share structural similarities with IGFALS proteins in other species, including conserved N- and C-terminal domains and 19 LRRs, indicating that the IGFALS protein structure is highly conserved throughout evolution and likely performs similar functions in diverse species. Over 75% of circulating IGF in organisms is carried in the ternary complex of IGF-IGFBP3/5-IGFALS, with the IGF-IGFBP binary complex crossing the capillary endothelial barrier. However, binding to IGFALS can limit IGF delivery to target tissues.16 The L-rich motifs in IGFALS facilitate protein-protein interactions, promoting the formation of polymers with IGF/IGFBP proteins.17 Ternary complex formation is influenced by the glycosylation of IGFALS, with aspartic acid residues playing a key role in binding glycosyl groups.18 Although the IGFALS protein sequence contains 53 aspartic acid residues, only six are glycosylation sites linked to aspartic phthalamine residues. In this study, a predictive model for the tertiary structure of the IGFALS protein from P. sinensis revealed that all six aspartate glycosylation sites are exposed on the protein surface, facilitating glycosylation. Notably, these sites are aligned on the same plane, consistent with findings by Zheng19 and Janosi et al.20 The interaction between IGFALS and IGFBP3 does not rely on any single glycosylation site, suggesting a complex binding mechanism. Future studies should focus on comprehensive modeling and analysis of the interactions among IGF, IGFBP3, and IGFALS to identify specific binding sites and elucidate their regulatory mechanisms. From an evolutionary perspective, turtles represent one of the most morphologically specialized groups of reptiles with a long evolutionary history. A phylogenetic tree constructed from IGFALS amino acid sequences revealed that the Chinese soft-shelled turtle is most closely related to other turtle species, followed by crocodiles and birds, while amphibians, mammals, and fish show more distant relationships. This finding supports the hypothesis that turtles may be the sister group to the common ancestor of crocodiles and birds.21 These insights into the structural and evolutionary conservation of IGFALS highlight its critical role in growth regulation and provide a foundation for further research into its functional mechanisms in reptiles and other vertebrates.
Previous research initially identified IGFALS as a liver-specific protein.6 As the study progressed, the presence of the IGFALS gene and protein also being present in the other tissues and organs. A study utilizing the zebrafish identified the presence of the IGFALS being widespread in the expression tissues, the expression being the predominant one being the liver.19 The study here identified the presence of IGFALS being present in all the tissues of the Chinese soft-shelled turtle, suggesting the gene possibly being responsible for the functioning of the various cells of tissues. Additionally, IGFALS gene expression exhibited significant sexual dimorphism in liver tissues, where the expression is higher in the female soft-shelled turtles when compared to the expression observed in the males. This sexual dimorphism suggests gender-specific regulatory mechanisms of IGFALS in this species. Given that IGFs are known to modulate gonadal development and maturation through autocrine, paracrine, and endocrine pathways,22 the elevated IGFALS expression in gonadal tissues implies its potential regulatory role in reproductive organ development. Additionally, we detected significant IGFALS expressions in lung tissues, which may indicate a distinct physiological function within the respiratory system of P. sinensis. Although these findings offer novel insights into the distribution and potential functions of IGFALS, the precise molecular mechanisms underlying its tissue-specific expression and physiological roles warrant further investigation.
Significant growth dimorphism exists between male and female P. sinensis, with males demonstrating accelerated growth rates under identical rearing conditions. This sexual dimorphism in growth patterns may be closely associated with differential sex steroid hormone profiles.23 To explore the role of IGFALS in mediating these growth differences, we examined hepatic IGFALS expression patterns following sex steroid hormone administration (E2 and MT) in both sexes. The data showed IGFALS expression in the livers of males and females changed significantly under E2 and MT treatment, suggesting IGFALS may play a role in regulating the growth and development of P. sinensis in response to steroid hormone modulation. The observed overall decrease of IGFALS expression in female turtles’ livers under E2 treatment replicated what has been encountered in Oreochromis niloticus24 and Channa maculata.25 This may be caused by increased levels of estrogen, which suppress the hepatic expression of the IGF and the growth factors like GH and IGF1. However, we observed a significant upregulation of IGFALS expression in the liver of male juvenile turtles following E2 stimulation. This may be attributed to the low endogenous estrogen levels in male individuals, which rendered them more responsive to exogenous E2 treatment. Consequently, this treatment markedly altered the sex steroid hormone profile in male juvenile turtles, leading to more complex physiological responses. The precise mechanisms underlying these observations warrant further investigation. “Bidirectional regulation of androgen hypothesis” by John-Alder and Cox26 implies the double action by the androgens towards the growth of the males, and in male-larger sexual size dimorphism populations, androgens exhibit growth-promoting effects. Expression by the IGFALS increased strongly with the treatment by MT for the two sexes of the turtles, suggesting the responsiveness by the IGFALS gene towards exogenous treatment by enhanced growth. These results collectively suggest that IGFALS may serve as a crucial mediator in the steroid hormone-regulated growth pathway, though the precise molecular mechanisms require further elucidation.
Conclusion
Two IGFALS gene transcripts, IGFALSX1 and IGFALSX2, were identified and characterized for the first time, revealing structural similarities with IGFALS proteins in other species. Tissue-specific expression analysis demonstrated that IGFALS is widely expressed, with significant sexual dimorphism observed in liver expression, suggesting a gender-specific regulatory role in growth and development. Furthermore, the study highlights IGFALS’s responsiveness to steroid hormone modulation, indicating its potential role in mediating sex-based growth differences. This research advances our understanding of the IGF system in reptiles and provides a foundation for developing gender control technologies and selective breeding strategies to enhance aquaculture productivity.
Acknowledgments
This work was supported by Hunan Provincial Education Department Outstanding Youth Project (Grant No. 24B0625) and University Student Innovation and Entrepreneurship Initiative (S202410549013).
Authors’ Contribution
Writing – original draft: Yulei Zhu (Lead). Methodology: Lin Li (Equal), Yangyang Tu (Equal), Feng Yan (Equal). Validation: Xincheng Chen (Equal), Mengying Chen (Equal). Writing – review & editing: Dan Zeng (Lead).
Competing of Interest – COPE
The authors declare that they have no competing interests.
Ethical Conduct Approval – IACUC
Ethical approval for the animal experiments was granted by the Animal Ethics Committee of Hunan University of Arts and Science (HUAS-2022-0032).
Informed Consent Statement
All listed authors have approved the manuscript for publication.
Data Availability Statement
All are available upon reasonable request.