Introduction
Penaeus monodon, one of the significant aquaculture species worldwide, boasts a broad consumer market and relatively high aquaculture profits.1 With the rapid development of the aquaculture industry, improving aquaculture environments and enhancing aquaculture efficiency have increasingly garnered attention from practitioners. The application of safe, efficient, and environmentally friendly probiotics in aquaculture is regarded as a potentially effective strategy for preventing and controlling diseases and infections in aquaculture.2
Clostridium butyricum is a Gram-positive, obligately anaerobic, and endospore-forming bacterium that exhibits strong tolerance to strong acids, relatively high bile concentrations, and high-temperature, high-pressure environments, making it a good alternative to antibiotics.3 Butyric acid, one of the major metabolites of C. butyricum, serves as a primary nutrient and energy source for the regeneration and repair of intestinal epithelial cells, which undergoes energy metabolism through β-oxidation in intestinal epithelial cells.3 Studies have found that feeding aquatic animals with formulated feeds supplemented with appropriate amounts of butyric acid can effectively maintain the health of their intestines.4 At the cellular level, butyric acid not only preserves cell viability but also increases mucin secretion. In terms of intestinal mucosa, butyric acid promotes intestinal mucosa repair and reduces its permeability, contributing to the restoration of physiological functions.5 The intestinal microbiota performs many critical functions, such as pathogen displacement, competition for nutrients and receptor binding sites, production of antibacterial factors, development of structural factors, and metabolic activities.4 Dysbiosis of the intestinal microbiota can lead to various diseases, and C. butyricum promotes the proliferation of probiotics, such as Bifidobacterium, Lactobacillus, and Bacteroides, while inhibiting the reproduction of harmful bacteria, such as Staphylococcus, Candida, Klebsiella, Campylobacter, Pseudomonas aeruginosa, Escherichia coli, Shigella dysenteriae, Salmonella typhi, and Saprophytic bacteria, thereby reducing the production of harmful substances in organisms.6 Dietary supplementation with a mixture of live C. butyricum, live cells, and fermented supernatant, as well as spray-dried spores, can significantly improve the growth performance of shrimp and induce nonspecific immunity in their serum, providing resistance to Vibrio parahaemolyticus. Additionally, it alters the intestinal microbial community structure and the expression levels of digestion and immune-related genes in the experimental shrimp.7 Therefore, this study aims to investigate the effects of C. butyricum and its metabolites on the growth performance, immune competence, and intestinal microbiota of P. monodon. The findings of this research contribute to enhancing the understanding of the impact of different forms of C. butyricum supplementation in feed on P. monodon, providing basic data and references for the formulation and optimization of feeds for this species.
Materials and Methods
Experimental Materials
Prior to the experiment, P. monodon shrimp were temporarily reared in cement ponds at the Shenzhen Experimental Base of the South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, for one week and then fasted for 24 hours. A total of 360 shrimp with similar vitality and size, with an initial body weight of (2.33 ± 0.05) g, were selected and randomly divided into four groups, with three replicates per group and 30 shrimp per replicate. The groups were as follows: the control group (CG, fed without supplementation), the raw liquid group (YYG, fed with C. butyricum raw liquid supplementation), the supernatant group (SQG, fed with C. butyricum supernatant supplementation), and the bacterial sludge group (JNG, fed with C. butyricum bottom sludge supplementation). The C. butyricum strain was sourced from the South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, and the C. butyricum preparation was produced by Guangzhou Xinhai Lisheng Biotechnology Co., Ltd. The product was a liquid preparation directly packaged from the fermented raw liquid, with a C. butyricum count ≥8 × 108 CFU/mL.
Experimental Feed
Based on the experimental requirements, commercial feed (purchased from Guangdong Haida Group Co., Ltd., with a crude protein content of 36%) was selected and supplemented with C. butyricum raw liquid, supernatant separated from C. butyricum preparation, and sludge-separated from C. butyricum preparation, respectively. The supplements were incorporated into the feed through spraying, mixing, and drying. For the CG, the addition of C. butyricum was 0, with an equal volume of distilled water mixed in instead. The YYG was supplemented with 1% C. butyricum raw liquid, which included bacterial spores and fermentation products formed after fermentation. The C. butyricum preparation was centrifuged at 8000 rpm for 10 minutes at 4°C to obtain separated supernatant and precipitate. The SQG was supplemented with the supernatant obtained after high-speed centrifugation of the raw liquid, which mainly contained short-chain fatty acids such as butyric acid, a fermentation product of C. butyricum, with an addition of 1%. The JNG was supplemented with the sludge remaining at the bottom of the centrifuge tube after high-speed centrifugation of the raw liquid, and the sludge was then rinsed with physiological saline and adjusted to the same volume as the raw liquid for addition, with an addition of 1%.
Feeding and Management
The experiment was conducted at the Shenzhen Base of the South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. During the breeding experiment, the shrimp were fed to apparent satiation once at 08:00, 15:00, and 22:00 each day for a total trial duration of 31 days. Residual feed and feces were removed using a siphon method, while shrimp shells and dead shrimp were removed using a net. The daily feeding rate was 3%-8% of the shrimp’s body weight, and one-third of the water was exchanged daily. The seawater used in the experiment was filtered using a sand filtration method. During the experiment, the water temperature was maintained at 27-32°C, salinity at 28-32 ppt, pH at 7.5-8.0, ammonia nitrogen concentration at 0-0.2 mg/L, and dissolved oxygen concentration at 6-7 mg/L.
Sample Collection and Indicator Measurement
After the experiment, all shrimp were fasted for 24 hours before sampling. The surface moisture of the shrimp was dried using a towel. The number of shrimp in each plastic bucket was counted, and the total weight (accurate to 0.01g) was measured to calculate the survival rate, weight gain rate, and specific growth rate of shrimp in each feed group. The calculation formulas are as follows:
Survival Rate (SR, %) = Final Number of Shrimp / Initial Number of Shrimp × 100%
Weight Gain (WG, %) = (Final Weight - Initial Weight) / Initial Weight × 100%
Specific Growth Rate (SGR, %/day) = 100 × (ln(Final Body Weight) - ln(Initial Body Weight)) / Time (in days)
Feed Efficiency Ratio (FER) = Weight Gain (g) / Dry Weight of Feed Consumed (g)
Whole Shrimp Body Composition Analysis
After the experiment, five shrimp were collected from each aquaculture tank and stored at -20°C for subsequent whole shrimp body composition analysis. The moisture content was measured after drying to a constant weight at 105°C. The ash content was determined by burning in a furnace at 550°C for 8 hours. The crude protein content (N × 6.25) was measured using the Kjeldahl method (Kjeltec™ 8400; FOSS, Copenhagen, Denmark). The crude lipid content was measured by Soxhlet extraction using the Soxtec System HT 6 (Tacator, Stockholm, Sweden).8
Measurement of Hepatopancreatic Enzyme Activity
After the experiment, five shrimp were collected from each aquaculture tank. The hepatopancreas and intestine were excised, frozen in liquid nitrogen, and then transferred to -80°C storage for the measurement of antioxidant enzymes. The hepatopancreas and intestine samples were fully homogenized using nine volumes of PBS buffer (1:9 dilution, w:v). The homogenate was then centrifuged for 15 minutes at 4°C and 3500 rpm, and aliquots of the supernatant were taken for analysis of antioxidant, digestive, and immune-related enzymes. Acid phosphatase (ACP), alkaline phosphatase (AKP), total antioxidant capacity (T-AOC), amylase, and chymotrypsin were tested using commercial test kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Analysis of Intestinal Microbial Composition
After the experiment, three shrimp were randomly selected from each aquaculture tank. The intestines were removed on an ice tray, mixed, placed in cryovials, frozen in liquid nitrogen, and then stored at -80°C. Total genomic DNA was extracted from the samples using PCR-amplified universal primers for 16S rRNA or ITS (Internal Transcribed Spacer). Sequencing of the highly variable regions was then performed to identify strains and analyze the diversity of microorganisms in the samples. A taxonomic table for species annotation was obtained by comparing the current ASV sequences with those in the GreenGenes (16S rRNA) database. Based on recent research findings, the microbial diversity of the four experimental animal groups was analyzed by evaluating α-diversity indices and β-diversity metrics (using principal coordinate analysis), with non-metric multi-dimensional scaling (NMDS) used for data statistical analysis. NMDS scatter plots were used to represent differences in microbial community species composition among different treatments.
RNA Extraction and cDNA Synthesis
After the experiment, the hepatopancreas of three shrimp from each tank were collected and immersed in RNALater (Ambion, USA), stored at 4°C for 24 hours, and then transferred to -80°C until further use. RNA extraction was performed using the HiPure Fibrous RNA Plus Kit (Megan, China) according to the manufacturer’s instructions to extract total RNA from all collected samples. Full-length cDNA was prepared from the total RNA, and its purity and quantity were determined by measuring UV absorbance at 260/280 nm using a NanoDrop 2000 spectrophotometer (Thermo, USA). The integrity of the total RNA was assessed by electrophoresis on a 1% agarose gel. According to the instructions of the Evo M-MLV Mix Kit with gDNA Clean for qPCR reverse transcription kit (AG, China), the prepared total RNA was immediately reverse-transcribed to synthesize the corresponding cDNA. The synthesized cDNA was stored at -80°C for further research on the expression of immune-related genes.
Quantitative Relative Expression
qRT-PCR was performed using a Roche Light Cycler® 480 II (Roche) with a 12.5 μL reaction mixture containing 0.5 μL of forward primer, 0.5 μL of reverse primer, 1 μL of cDNA template (50 ng/μL), 6.25 μL of SYBR Green Premix Pro Taq (AG, Guangdong, China), and 4.25 μL of double-distilled water (Table 1). The relative expression levels of Heat Shock Protein 70 (hsp70), Immune Deficiency (Imd), CAT, Toll, Relish, glutathione (GSH), keafl, phenoloxidase (PO), and lys genes were determined using the 2^-ΔΔCT method. One-way ANOVA and Tukey’s multiple range test were used to determine statistical differences. Statistical significance was set at P < 0.05. The data obtained are presented as mean ± standard deviation (SD).
Data Statistics and Analysis
The values of each variable were statistically analyzed and presented as mean ± SD (n=3). Statistical analysis was conducted using SPSS version 17.0 software. Statistical significance was determined through one-way ANOVA. All data were tested for normality, homogeneity, and independence before ANOVA. When significant differences (P < 0.05) were observed, Dunnett’s multiple range test was further conducted to compare the means among groups.
Results
Growth Performance and Specific Growth Rate of P. monodon
Compared with CG, the weight gain (WG), survival rate (SR), specific growth rate (SGR), and feed efficiency ratio (FER) of the experimental groups increased (Table 2). Among them, the WG, SR, SGR, and FER of the YYG were significantly higher than those of the CG (P < 0.05). There were no significant differences in WG, SR, SGR, and FER between the SQG and JNG compared with the CG (P > 0.05). Additionally, there were no significant differences in crude nutrient composition among P. monodon from the CG and the experimental groups (P > 0.05) (Table 3).
Digestive and Immune Enzyme Activities in P. monodon
In the intestine, compared with the CG, the activities of chymotrypsin and lipase in the YYG were significantly enhanced (P < 0.05) (Table 4). However, there were no significant differences in α-amylase activity between the experimental groups and the CG (P > 0.05). In the hepatopancreas, the activities of trypsin and lipase in the YYG were also significantly increased (P < 0.05), while no significant differences were observed in α-amylase activity between the experimental groups and the CG (P > 0.05). The activity of alkaline phosphatase (AKP) in P. monodon is shown in Table 4. The AKP activity in the YYG was significantly higher than that in the CG (P < 0.05). Similarly, the activity of acid phosphatase (ACP) in the YYG was significantly enhanced compared with the CG (P < 0.05). There were no significant differences in total antioxidant capacity (T-AOC) activity between the control and experimental groups (Table 5).
Analysis of Gut Microbial Diversity in P. monodon
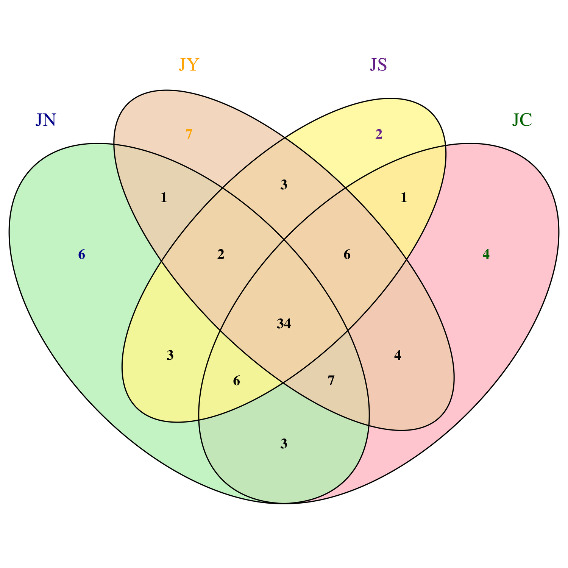
As shown in Figure 1, a total of 34 ASVs (Amplicon Sequence Variants) were obtained through 16S rRNA gene high-throughput sequencing. According to the Venn diagram analysis, 30 ASVs were present across the three experimental groups, with YYG having the highest number of unique ASVs, containing 7 exclusive ASVs. Combined with the Alpha diversity analysis results, the Chao1, Ace, and Shannon indices of the intestinal microbiota of P. monodon in the control group were higher than those in the experimental groups, while the Simpson index was lower in the control group. These findings indicate a decrease in the intestinal microbiota diversity of P. monodon in the experimental groups.
Composition of Gut Microbiota in P. monodon
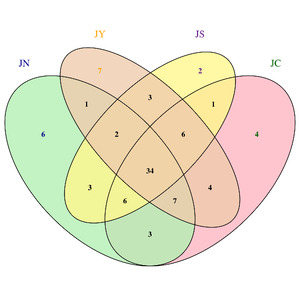
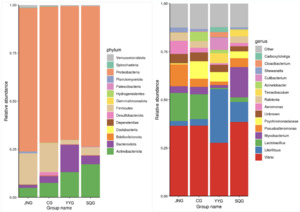
As shown in Figure 2, at the phylum level, Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria were the dominant bacterial taxa. Among them, Proteobacteria was the most abundant phylum in all five groups, accounting for 71%, 69%, 73%, and 75% in the CG, YYG, SQG, and JNG, respectively. As depicted in Figure 2, at the genus level, the Vibrio bacteria in the YYG were significantly reduced, while the proportion of Litorilituus increased. Furthermore, Lactobacillus, Mycobacterium, Pseudoalteromonas, and Aeromonas also comprised a relatively large proportion of the gut microbiota in the YYG.
Expression of Immune-Related Genes
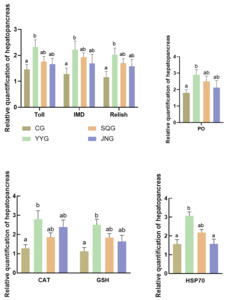
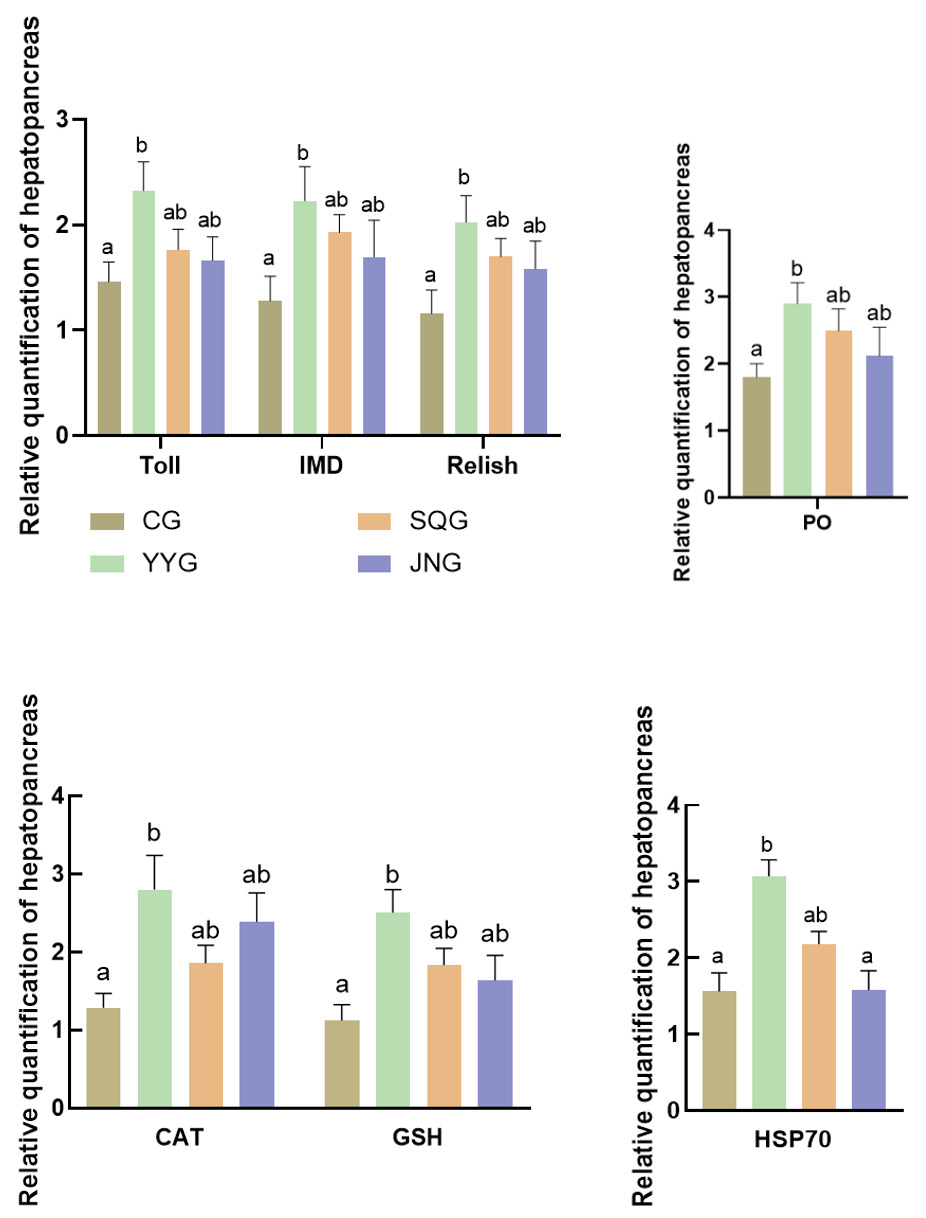
As shown in Figure 3, compared with the CG, the relative expression levels of Toll, IMD, and Relish in the hepatopancreas of P. monodon in the YYG were significantly enhanced (P < 0.05). There were also increases in SQG and JNG, but these were not statistically significant (P > 0.05). As depicted in Figure 3, when compared with the CG, the relative expression levels of CAT, GSH, and hsp70 in the hepatopancreas of the YYG were significantly increased (P < 0.05).
Discussion
C. butyricum belongs to the genus Clostridium and is a strict anaerobic, Gram-positive bacillus. It is a probiotic beneficial to the intestinal tract, capable of inhibiting pathogenic bacteria in vivo to a certain extent and promoting the growth of beneficial bacteria.9 Furthermore, its metabolites include butyric acid and acetic acid, with butyric acid serving as a crucial energy source for the regeneration and repair of intestinal epithelial cells. It possesses the functions of repairing intestinal mucosa and reducing intestinal mucosal permeability.5
The growth performance of aquatic animals is generally indicated by indices such as SR, WG, and SGR. Numerous studies have demonstrated that C. butyricum, as a feed additive, can promote the growth of aquatic animals,10 enhance digestive enzyme activity,11 and boost immunity.12 Some research has shown that, compared with the control group, adding C. butyricum to the feed significantly improves the growth performance of shrimp and reduces the feed conversion ratio.13 This may be due to the ability of C. butyricum added to shrimp feed to synthesize and secrete various digestive enzymes in the shrimp’s intestine. These enzymes help decompose proteins, fats, and complex carbohydrates in the feed, thereby improving feed utilization.12 Additionally, C. butyricum can ferment indigestible starch and polysaccharides in the intestine, mainly producing metabolites such as butyric acid, which provides energy for various metabolic processes such as carbohydrate metabolism, lipid metabolism, and protein metabolism.14 In this study, C. butyricum solution, supernatant separated from C. butyricum, and C. butyricum mud were used as additives mixed into the feed for feeding P. monodon. After the experiment, compared with the control group, SR, WG, and SGR in all treatment groups increased (P > 0.05), with YYG, presumably with C. butyricum incorporated showing a significant enhancement. These results are similar to previous findings, indicating that C. butyricum can improve the growth performance of P. monodon. Compared with the control group, there were no significant differences in nutrient composition among the treatment groups, which may be due to the relatively short breeding period.
In crustaceans, the hepatopancreas is not only an important organ for the absorption and storage of nutrients but also a crucial organ for synthesizing digestive enzymes.15 The activity of these enzymes directly determines the digestive and absorptive capacity of these animals for nutrients.15 Different feeds can affect the digestive enzyme activity of aquatic animals.15 Digestive enzymes play a vital role in the absorption and utilization of nutrients by the body.16 In this experiment, compared with the CG, the digestive enzyme activities in all experimental groups increased, with lipase and protease activities in YYG showing significant enhancements. This is similar to the research results of Duan.17 This phenomenon may be due to the digestive enzymes produced by the probiotics consumed by P. monodon. Simultaneously, C. butyricum can produce short-chain fatty acids, lowering the pH of the intestinal environment and increasing digestive enzyme activity, thereby promoting shrimp growth. T-AOC, AKP, and ACP play important roles in the nonspecific immunity of crustaceans. Among them, T-AOC is a crucial indicator reflecting the organism’s total antioxidant capacity, comprehensively reflecting the antioxidant levels of both enzymatic and non-enzymatic systems in the body. Additionally, increasing the activity of antioxidant enzymes can enhance the resistance of shrimp, while C. butyricum can produce hydrogen and butyric acid in organisms, both of which possess antioxidant properties, providing another explanation for the possible reasons why C. butyricum improves the antioxidant capacity of P. monodon.18 AKP is involved in the regulation of lipid metabolism and affects the absorption of calcium and phosphorus by crustaceans.18 ACP plays a crucial role in the transfer and metabolism of phosphate groups, promoting the absorption and transport of substances.12 Furthermore, it can combine with other enzymes to form phagocytes, effectively destroying and eliminating foreign bodies invading the body.13 These two enzymes are closely related to immune regulation and cellular metabolism, reflecting the immune status of the organism to some extent.12 In this experiment, compared with the control group, T-AOC in the experimental groups increased (P > 0.05), and AKP and ACP in YYG significantly enhanced (P < 0.05), indicating that C. butyricum can increase the immune enzyme activity of P. monodon and enhance the body’s antioxidant defense level to a certain extent.
The gut microbiota refers to the collective entity of various microorganisms residing within the animal intestinal tract, upon which animals rely for the digestion and absorption of ingested food and the execution of various physiological and biochemical functions.13 Changes in the feed of aquatic animals may exert certain influences on the intestinal microbiota. Intestinal probiotics can facilitate the digestion and absorption of food, and maintain the ecological balance of the organism by providing nutrition, competitively inhibiting the growth of intestinal harmful microorganisms or secreting bacteriocins to prevent the growth of pathogens, thereby promoting the healthy growth of the organism and enhancing the survival rate of aquatic animals.19 Furthermore, probiotics can influence the interactions among intestinal bacterial populations, enabling beneficial microorganisms to dominate within the body, further promoting the balanced state of the host’s intestinal microbiota, maintaining and adjusting the healthy microbial environment in the body, and thus enhancing the intestinal nutrient absorption capacity.20 At the end of the experiment, the dominant intestinal bacterial species in shrimp was Proteobacteria. Studies have shown that Proteobacteria is the dominant phylum in the shrimp intestine at various growth stages.21 Some members of this phylum are involved in nitrogen cycling and the mineralization of organic compounds,12 playing a crucial role in the growth and development of organisms. In this study, Proteobacteria was the most abundant bacterium among the five groups, accounting for 71%, 69%, 73%, and 75% in CG, YYG, SQG, and JNG, respectively. Additionally, the proportion of Bacteroidetes increased in YYG.
Crustaceans primarily rely on their innate immune system for self-protection, which mainly encompasses cellular immunity and humoral immunity, with immune signaling pathways playing a vital role.22 Furthermore, probiotics can positively stimulate the cellular and humoral immunity of the body, such as enhancing the activity of interferons and macrophages and stimulating the production of nonspecific immune factors. Currently, three major signaling pathways identified in the innate immune system of shrimp are the JAK/STAT signaling pathway, the IMD signaling pathway, and the Toll signaling pathway. The JAK/STAT signaling pathway is primarily responsible for immune responses to viral infections, while the Toll and IMD pathways are key signaling pathways controlling the expression of antimicrobial peptide genes. Specifically, the Toll pathway plays a crucial role in response to Gram-positive bacteria and fungi. Moreover, the Toll signaling pathway can mediate innate immune responses triggered by bacterial and viral infections. The IMD signal is mainly responsible for the innate immune response to Gram-negative bacteria,23 primarily by regulating the production of antimicrobial peptides to resist bacterial infections. In invertebrates, the Toll receptor was initially discovered in Drosophila melanogaster. The activated signaling pathway prompts the nuclear transcription factor NF-κB to enter the nucleus, ultimately triggering the expression of immune effector molecules such as antimicrobial peptides, while Relish is a member of the NF-κB family.24 That is, Relish is the downstream nuclear transcription factor of the IMD pathway. Recent scientific research reveals that the Toll and IMD pathways in Drosophila exhibit synergistic effects when activated. When the Toll pathway is activated, it may increase the expression level of the Relish gene, thereby enhancing the organism’s immune capacity against bacteria.25 The results of this study showed that compared with the control group, the expression of IMD, Toll, and Relish genes was significantly upregulated (P<0.05) in the group supplemented with C. butyricum stock solution, which is similar to previous research findings.17 Thus, it is inferred that the activation of the Toll pathway by C. butyricum in P. monodon leads to the active expression of IMD pathway molecules, further upregulating the expression of Relish in P. monodon. Prophenoloxidase (PO), which exists as a zymogen in crustaceans as a defensive enzyme, reflects the immune status of the organism and has the function of recognizing foreign substances.26 It is a key indicator for measuring the immune capacity of shrimp and plays an important role in recognizing and defending against foreign substances in the shrimp’s defense system.27 In this experiment, the expression of PO was significantly upregulated after the addition of C. butyricum stock solution, indicating that C. butyricum can activate the PO prophenoloxidase system of P. monodon, leading to an increase in PO activity, which will enhance the shrimp’s ability to recognize and defend against foreign substances, thereby strengthening their immune capacity and improving their resilience to adversity. In this study, compared with the CG, the relative expression levels of all immune-related genes (PO, Imd, Toll, and Relish) in the hepatopancreas of P. monodon significantly increased after the addition of C. butyricum stock solution. Among them, the group supplemented with C. butyricum stock solution exhibited the most significant effect. In contrast, although the separated supernatant and bacterial mud could also stimulate the upregulation of immune-related genes, their effects were inferior to that of the group supplemented with C. butyricum stock solution. This may be due to the optimal probiotic effect of C. butyricum and its secreted products on P. monodon. Further research is needed to investigate the impact of these three addition methods on the differential expression of immune-related genes in P. monodon. Heat shock protein (HSP) is a highly conserved protein that is highly induced and expressed in stress cells of almost all organisms.23 Among them, HSP-70 is a molecular chaperone that protects proteins from denaturation and assists in the repair of damaged proteins, playing a crucial role in maintaining intracellular stability and enhancing the organism’s stress resistance.28 In this study, the expression of HSP-70 in P. monodon fed with C. butyricum stock solution was significantly upregulated, indicating that C. butyricum improved the antioxidant status of the hepatopancreas of P. monodon, thereby enhancing its defense capability against oxidative stress. During the normal physiological activities of organisms, various biochemical reactions generate free radicals, causing oxidative damage to the body.29 At this point, antioxidant enzymes such as SOD, CAT, and GSH in animals form an antioxidant defense system to maintain the organism’s oxidative stability and reduce oxidative damage to cells66. In this study, the addition of C. butyricum stock solution increased the expression of CAT and GSH in the hepatopancreas of P. monodon, thereby enhancing its antioxidant capacity and disease resistance, and promoting healthy growth, and C. butyricum may jointly regulate oxidative stress by increasing antioxidant enzyme activity and reducing reactive oxygen metabolite production.
Acknowledgments
This study was supported by National Key R & D Program of China (2022YFD2400104), the earmarked fund for CARS-48, Central Public-interest Scientific Institution Basal Research Fund, CAFS (NO.2023TD34), the earmarked fund for HNARS(HNARS-10-ZJ01), the Central Public-Interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (2024XT01).
Data Availability statement
The data that support the findings of this study are available on request from the corresponding author.
Conflict of interest
The authors declare that there is no conflict of interests.
Authors’ Contribution
Conceptualization: Jingyan Li (Equal), Falin Zhou (Equal), Guoliang Wen (Equal), Keng Yang (Equal), Song Jiang (Equal). Writing – original draft: Jingyan Li (Equal), Falin Zhou (Equal), Jianshe Zhang (Equal). Writing – review & editing: Guoliang Wen (Equal), Keng Yang (Equal), Jianzhi Shi (Equal), Yangyang Ding (Equal), Jianshe Zhang (Equal), Song Jiang (Equal). Investigation: Yundong Li (Supporting), Jianzhi Shi (Supporting), Song Jiang (Equal). Data curation: Jianzhi Shi (Equal). Formal Analysis: Jianzhi Shi (Equal). Methodology: Yangyang Ding (Supporting).
Ethical Approval
The whole experiment was conducted according to the guidelines established by the National Institutes of Health.