Introduction
In 2021, the annual output of oysters in China exceeded 5 million tons, accounting for 70% of the world’s total output. Crassostrea hongkongensis, also known as “white oyster,” is commonly cultured in South China, primarily in the coastal areas of Guangxi, Guangdong, and Fujian Province. The typical breeding methods include intertidal planting culture, hanging raft, trellis pond, and other methods.1 The oyster is one of the main mollusks cultured on the South China coast, with a large scale and high economic value.2
However, the oyster industry is always impacted by diseases, one of which is caused by Polydora sp, a parasitic worm that burrows into the oyster shell and causes significant damage to the shell structure and soft tissue. Due to the limitations of technology, early research on diseases was limited to parasites. After the 1970s, molluscan diseases caused by bacteria and viruses were gradually recognized and valued.3 The Polydora complex, which is affiliated with the Annelidae, Polychaete, Cryptozoospermia, Spionidae, is a collective name for all species of Spionidae that have metamorphosed in the fifth ganglia. Taxonomic studies on the complex of Polydora are mainly based on morphology, while molecular phylogeny is rarely used to classify the complex of Polydora. At present, more than 140 species have been found in the world. It has always been difficult to classify the Polydora complex. Usually, some morphological characteristics of Polydora, such as tentacles, pigmentation patterns on the body, the color of the body of Polydora, the presence or absence of middle tentacles, and the characteristics of hind feet, were used as the classification criteria of Polydora.4 However, due to the uncertainty of the morphological characteristics of Polydora, similar species of Polydora are often misidentified. For example, Polydora cornuta BOSC, 1802, polydora ligni Webster, 1879, and Polydora amarincola Hartman, 1936 are synonyms.5 Therefore, in addition to the identification of morphological characteristics, it is necessary to use molecular biology to further examine the differentiation. Molecular sequences can offer more details on the relationships between Polydora complexes. Rice et al.6 confirmed that the former species P.cornuta BOSC, 1802, contains at least three independent species by comparing the genetic distance of mitochondrial cytochrome oxidase subunit I DNA sequences from three locations in North America.
Polydora can spread globally with ocean currents in the larval stage, and the feeding mechanism of Polydora is flexible, and adaptable to complex and changeable environments, not only in soft sediment or silt layers but also in hard limestone materials, such as marine corals, coral algae, and molluscan shells, such as oysters, abalone, mussels, and scallops.7 Most Polydora inhabits near-shore waters, such as intertidal zones, bays, and estuaries, and some of them are common polychaete parasites on shellfish where they burrow into the shells and produce dark mud bubbles or argillaceous tube holes inside their host shells, commonly known as “mud worms” or “mud bubble worms.” The planktonic larval stage begins to sink when it reaches the sixteenth ganglion planktonic larvae and then parasitizes on the outside of the calcareous shell,8 forming a U-shaped tube with two external openings through its own peristalsis. As the larvae of Polydora continue to creep, the resulting pipe enters the inner surface of the shell, causing the host to produce one or more layers of thin nacre to isolate the pipe on the outside. The larvae continue to expand the pipe burrows under the thin calcareous layer produced by the host, forming “mud bubbles” as the space is filled with debris, dirt, and larval droppings.9 Its irregular shape and darker color affect the appearance of oysters. The Polydora parasitizes in the shell of molluscs, leading to damage and deformation of the host shell, seriously damaging shell components, and even damaging the soft tissue part of molluscs, causing local inflammation, ulceration and atrophy.7 This hinders the growth and development of the host molluscs, leading to a significant decline in quality and often to high mortality in severe cases,10 making this parasite a serious impediment to the development of molluscan culture.
Previous studies have shown that different regions of the mantle may correspond to different biological functions during shell formation.11,12 The formation of nacre inside the shell is closely related to the mantle. Sato-okoshi and Abe13 confirmed that the formation of U-shaped tube is caused by the secretion of a large number of calcium substances from the nacre of the shellfish to wrap the shellfish in order to prevent the shellfish from being deeply chiseled.
The Polydora complex has spread widely and has seriously damaged the oyster culture industry worldwide. Studies on the impacts of Polydora ciliata on scallops, pearls, haliotis diversicolor, and oysters have been reported.14 The infection rates of polychaete parasitic diseases in Fangchenggang City, Beihai City, Shenzhen City, Guangdong Province and Lingshui County, Hainan Province, were all over 76%. The high mortality rate of the Japanese scallop (Mizuhopecten yessoensis) (Jay 1857) in British Columbia, Canada, is attributed to Polydora websteri. For instance, in British Columbia, P. websteri caused up to 84% mortality in scallop grow-out sites from 1989 to 1990, resulting in up to US $449,660 in lost revenue that year.15 Since the 1940s, oyster farms on the east coast of the United States have been plagued by Polydora websteri, resulting in significant losses of oyster farms.16 These examples illustrate the significant impact of the invasion of Polydora on the local aquaculture industry. Studies have shown that the mechanism of the occurrence of parasitic diseases of marine molluscs is complex, and it is relative difficult to control the occurrence and epidemic of diseases completely at present.
In this study, the microstructure and ultrastructure of the mantle and gills of oyster were observed using optical and transmission electron microscopy to observe the physiological basis of the influence of Polydora infection on oysters, which can provide a scientific basis for an in-depth understanding of the effects of Polydora infection on the mantle and gills of oysters.
Materials and Methods
Collection and Temporary Cultivation of Experimental Oysters
Crassostrea honkongensis were collected from Oyster Bay, Tiechong Town, Huidong County, Guangdong Province, China, with a shell weight of 120±0.8g. Seventy Crassostrea honkongensis of similar size were selected and temporarily raised in a laboratory pond. The water temperature was 26 ℃, the salinity was 18 ppt, and continuous aeration was provided. The weight of the soft part and the infection intensity of the soft part and shell of all the oysters were recorded, and the intuitive effect of the infection intensity on the oysters was observed. The weight of the soft part was measured using an electronic balance with an accuracy of 0.1g. The intensity of infection was determined by observing the shell and soft parts, and at the same time, carefully removed the mudworm from the mud pipe with tweezers. A total of 20 mudworms were taken out and placed in a concave glass containing seawater for morphological observation with an anatomical microscope. Then, the mudworms were fixed in alcohol for subsequent molecular identification. Minor infection was characterized by a few smaller mud tubes or recovered mud tubes, with no apparent symptoms in the soft part. Moderate infection was characterized by larger wormholes appearing under the fragile nacre and more mud pipes, with slight atrophying and small lesions in the soft body part. The characteristics of heavy infection were that the shell is almost entirely occupied by mud pipes or wormholes, and the soft body parts are discolored, atrophied and ulcerated. The data are summarized in Table 1.
Molecular identification of Polydora
Polydora were removed from alcohol, rinsed with running water for 1 minute, and ground the tissue of the Polydora directly in a mortar with a grinding rod. DNA was extracted using a marine animal genome extraction kit (TaKaRa MiniBEST Universal Genomic DNA Extraction Kit Ver.5.0). The primer design refers to the taxonomic and histological studies of the Chinese coastal Polydora complex. The upstream primer (5’ CCTWGTCATACACCTRTCWTAATT 3’) and the downstream primer (5’ CCTGTAAATARAGGAATCA 3’) are used for PCR amplification. The amplification conditions were pre denatured at 95 ℃ for 5 min; Denaturation at 94 ℃ for 30 s; Annealing at 55 ℃ for 30 s; 72 ℃ for 2 min; 35 cycles in total; and Large extension at 72 ℃ for 10 min. The amplified products were detected by dyy-12 electrophoresis instrument (Beijing Liuyi Instrument Factory) with 1% agarose gel electrophoresis. After detection, it was sent to the Guangzhou Tianyi Huiyuan company for sequencing.
Light microscope observation
Three healthy oysters and three seriously infected oysters of similar size were chosen. The marginal tissue of the mantle was collected and trimmed into 1cm3 tissue pieces. They were fixed with Davidson fixative, embedded in paraffin, sectioned, stained with HE, embedded with neutral glue, and observed under a light microscope.
Transmission Electron Microscopy
Three healthy and severely infected oysters were thinly cut, and the mantle and gill margins were about 6mm³ tissue blocks. Pre-fixed with glass dishes containing 2.5% glutaraldehyde fixative solution, washed with PBS solution, stored in the dark for 2 hours at room temperature, and overnight at 4 ℃. The fixed samples were transported to Guangzhou Saville Biotechnology Co., Ltd. for routine transmission electron microscope preparation, ultrathin sections (70-90 nm) were stained, and Hitachi ht7700 transmission electron microscope was used for observation.
Results
Clinical Symptoms
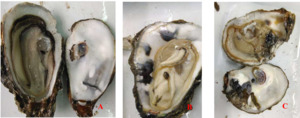
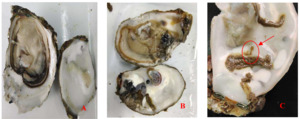
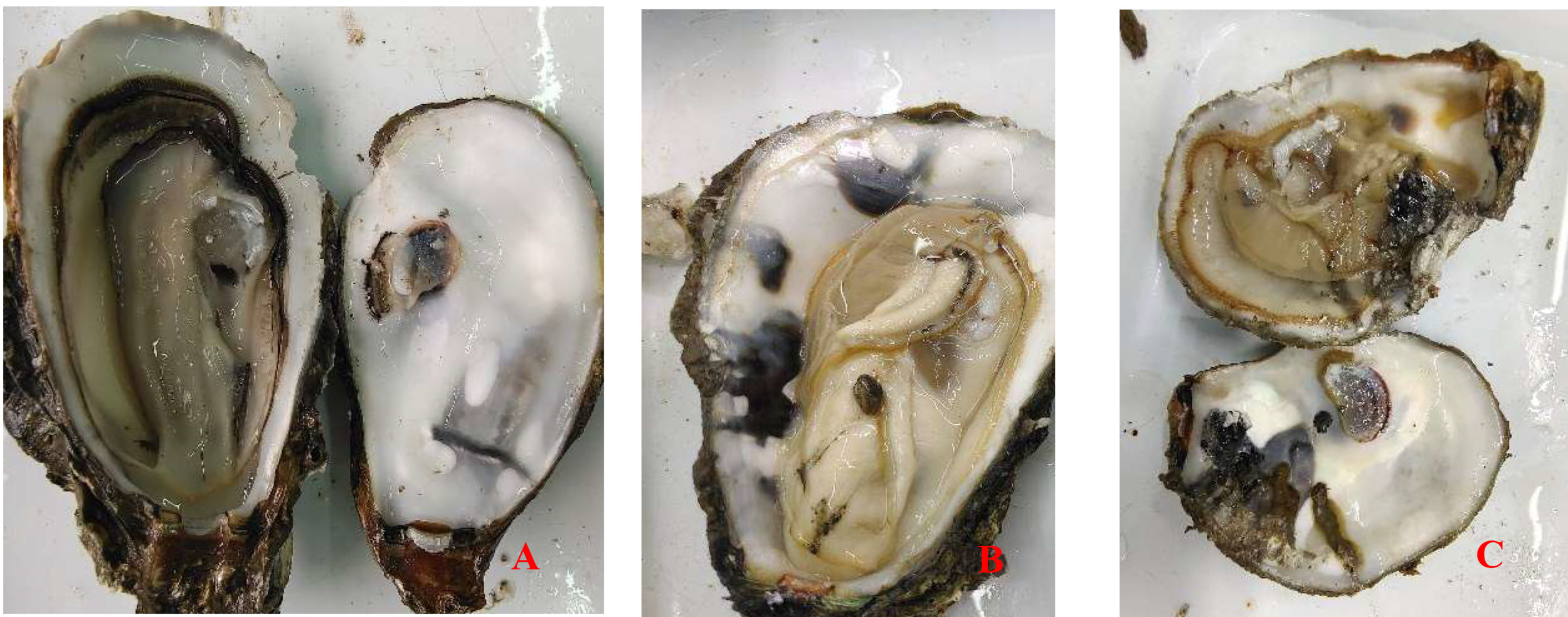
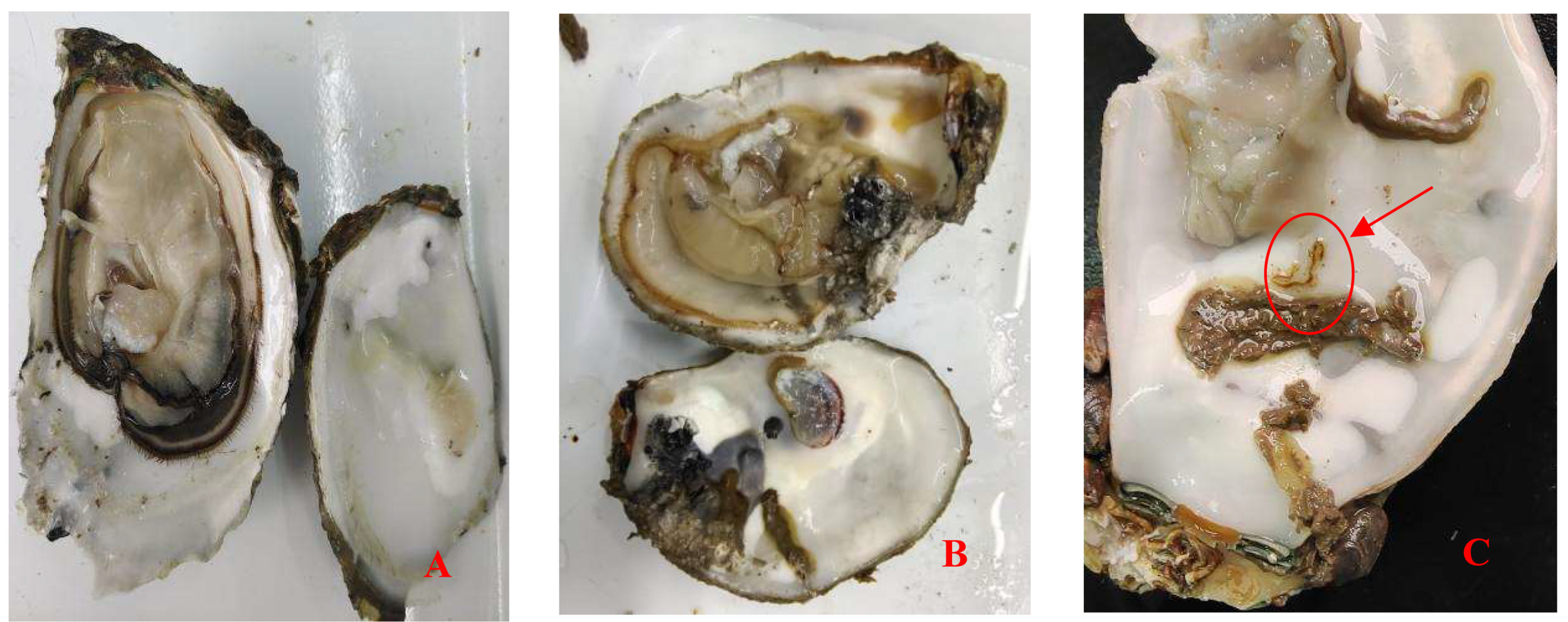
The infection intensity was determined by observing the shell and soft part. The slight infection was manifested by a small number of small mud tubes or mud tubes recovered by pearl layer secreted by oysters, with mud bubbles occupying 5% of the inner surface of the shell and the soft part had no obvious symptoms; Moderate infection is characterized by large wormholes and more mud tubes under the fragile nacre, slight atrophy and small lesions in the soft tissue, with mud bubbles occupying 30% of the inner surface of the shell and obvious yellowing in the soft tissue; The characteristics of heavy infection are that the shell is almost completely occupied by mud pipes or wormholes, with mud bubbles occupying more than 50% of the inner surface of the shell and the soft part is discolored, atrophied, dissolved and festered (Fig. 1).
Crassostrea honkongensis infected with Polydora exhibit symptoms such as slow response to stimuli, holes and mud pipes on the shell surface, blackening of the inner and outer shell surfaces, mud bubbles filled with Polydora debris and feces, increased secretion of surface mucus, and dirt buildup in organs such as the mantle, gonads, and visceral masses. The soft part of infected oysters appears yellowish brown or light brown (Fig. 2B), the mantle becomes thin and transparent, and the marginal membrane is brownish brown. Visceral masses and gills were grayish green or grayish brown, eroding some gill filaments.
Molecular identification of Polydora complex
The DNA fragment sequence length of the sequenced sample is 794bp, and after BLAST comparison, the result shows that it has the highest similarity with P. websteri, reaching 99%. It is determined that the complex of the genus Polydora in this sample is the P. website.
Histology Analysis, Ultrastructure Analysis, and Pathological Analysis of Healthy and Infected Oyster Mantle
Composition of oyster mantle
Under light microscopy, the marginal membrane of the mantle consists of three parts: shell protrusion, sensory protrusion, and marginal membrane protrusion. The tissue structure includes an outer epithelial layer, inner epithelial layer, muscle fibers, connective tissue, and mucus-secreting cells. Healthy oysters possess three main types of cells in the mantle: columnar epithelial cells, electron-hyaline granulocytes, and electron-dense granulocytes, with columnar epithelial cells further divided into A, B, and C types, with cell sizes between 10 and 15 μm. The nucleus of type A columnar epithelial cells is located in the middle and lower part of the cells, with distinct heterochromatin and euchromatin, most of which contain large nucleoli, complete organelles, and low electron density of secretory granules; the Type B nucleus is located in the middle and upper part of the cell. Most of the nucleoli are not obvious but have high electron density heterochromatin, with large electron-dense granules and few organelles; The number of type C columnar epidermal cells is very small, the nucleus is pyknotic, and the cytoplasm is full of vesicles with different electron densities, lamellar bodies and residual mitochondria, without redundant organelles.17 In healthy individuals, type A cells dominate the marginal membrane, type B cells dominate the central membrane, and columnar epithelial cells are evenly arranged in the marginal membrane. The basement membrane serves as a base for type A cells, with type B cells primarily located underneath. Type A and B basement membranes interpenetrate, forming an irregular and linear “membrane labyrinth” structure.
Histology Analysis
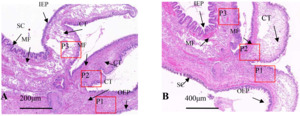
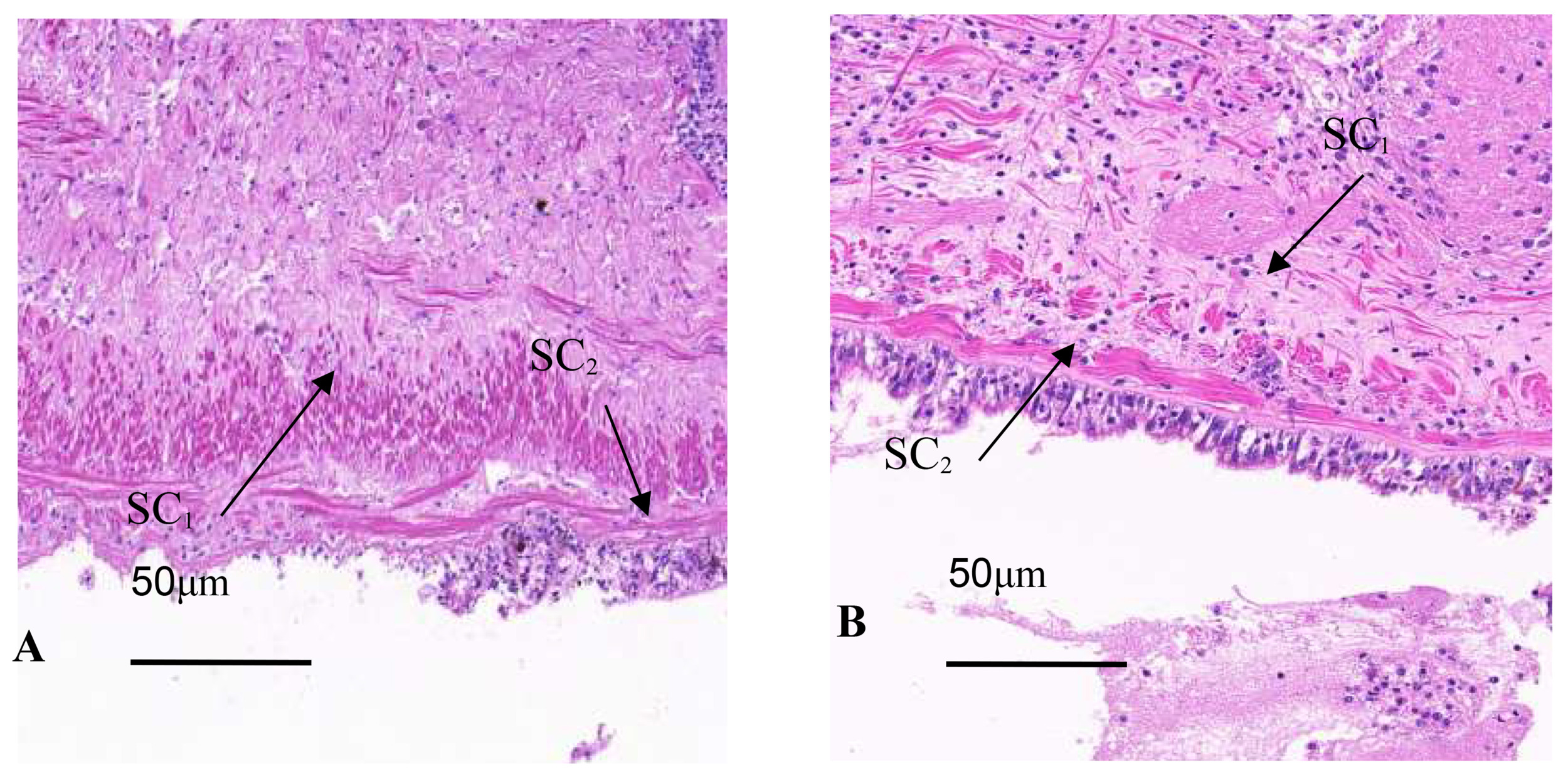
Tissue section staining of infected oyster mantles shows a reduction in crest-like protrusions on the marginal membrane and decreased epithelial cell surface area, with an increase in secretory cells, while there were a large number of basophilic secretory granules in the marginal membrane epithelial layer of the mantle of healthy individuals, dark blue; a few weak basophilic secretory granules were light blue or gray, and in the connective tissue layer adjacent to the epithelial layer, the granules secreted by eosinophils were red or bright red. In infected individuals, the distribution of secretory cells is disordered, with severe tissue tear, nuclear aberrations, partial overflow of secretory granules, mucus masses attached to the outer epithelial surface, and an overall increase in secretory cells, including eosinophils and basophils (Fig. 3A). In healthy individuals, the distribution of secretory cells and granules was orderly, with a layer of secreted mucus evenly distributed over the epithelial layer (Fig. 3B).
In infected individuals, the connective tissue was denser and similar to that at the base, with band-like vacuoles in the shell protrusion and secretory cells close to the connective tissue layer that has shed into the connective tissue (Fig. 4A). In healthy individuals, the connective tissue and muscle fibers of the marginal membrane had a certain regularity, with muscle fibers gradually increasing and connective tissue gradually decreasing towards the tip of the protrusion (Fig. 4B). The outer epithelium of healthy individuals’ marginal membrane protrusions had crest-like protrusions with evenly distributed internal structure, while in infected individuals, the outer epithelium of marginal membrane protrusions had no obvious coronal processes, only coronal processes below the bottom of the processes, and muscle fibers and connective tissue was torn and dissolved.
Ultrastructural observations
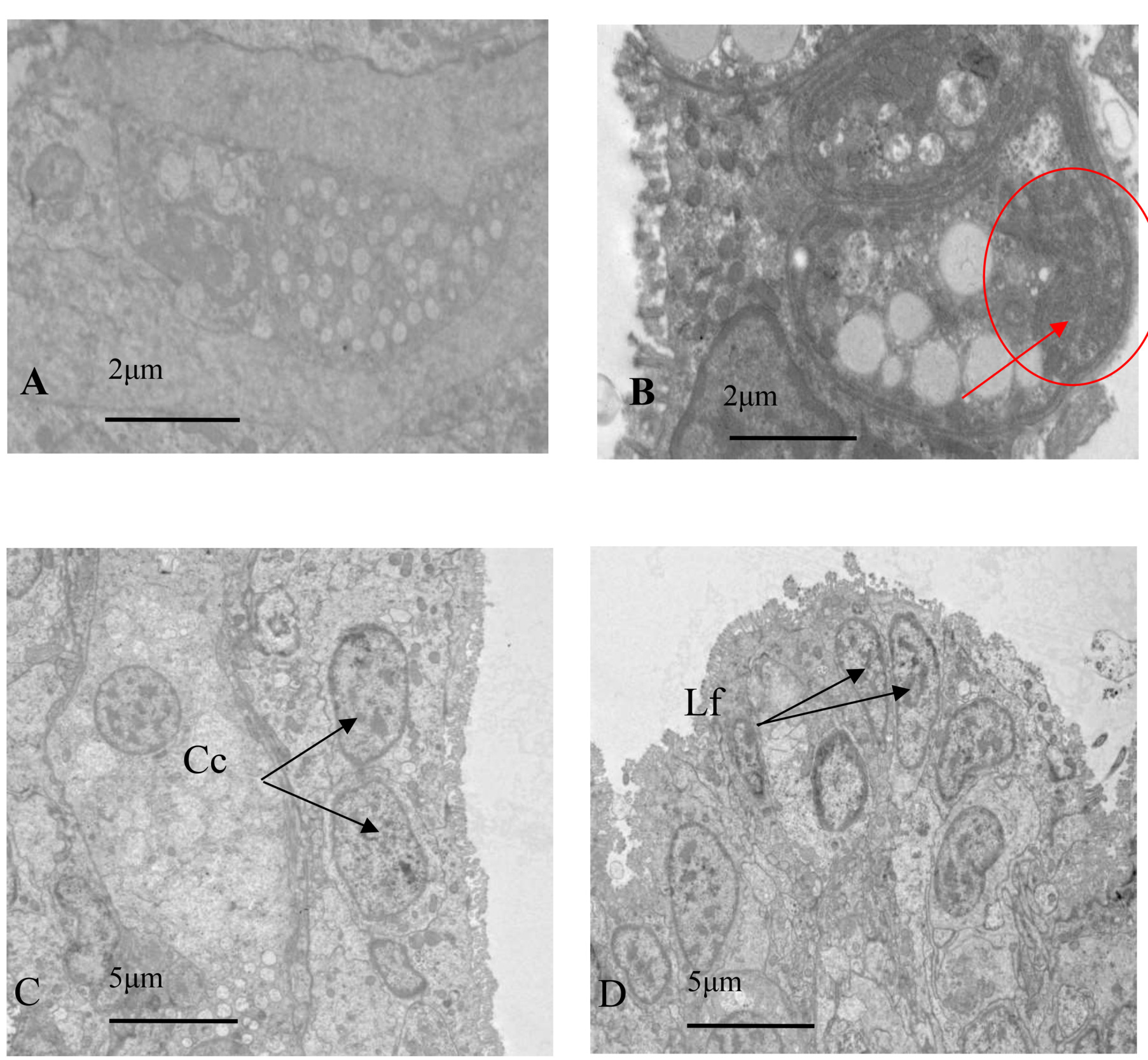
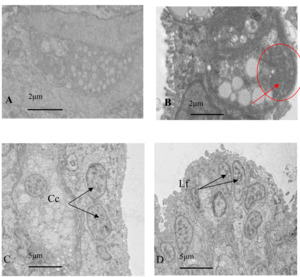
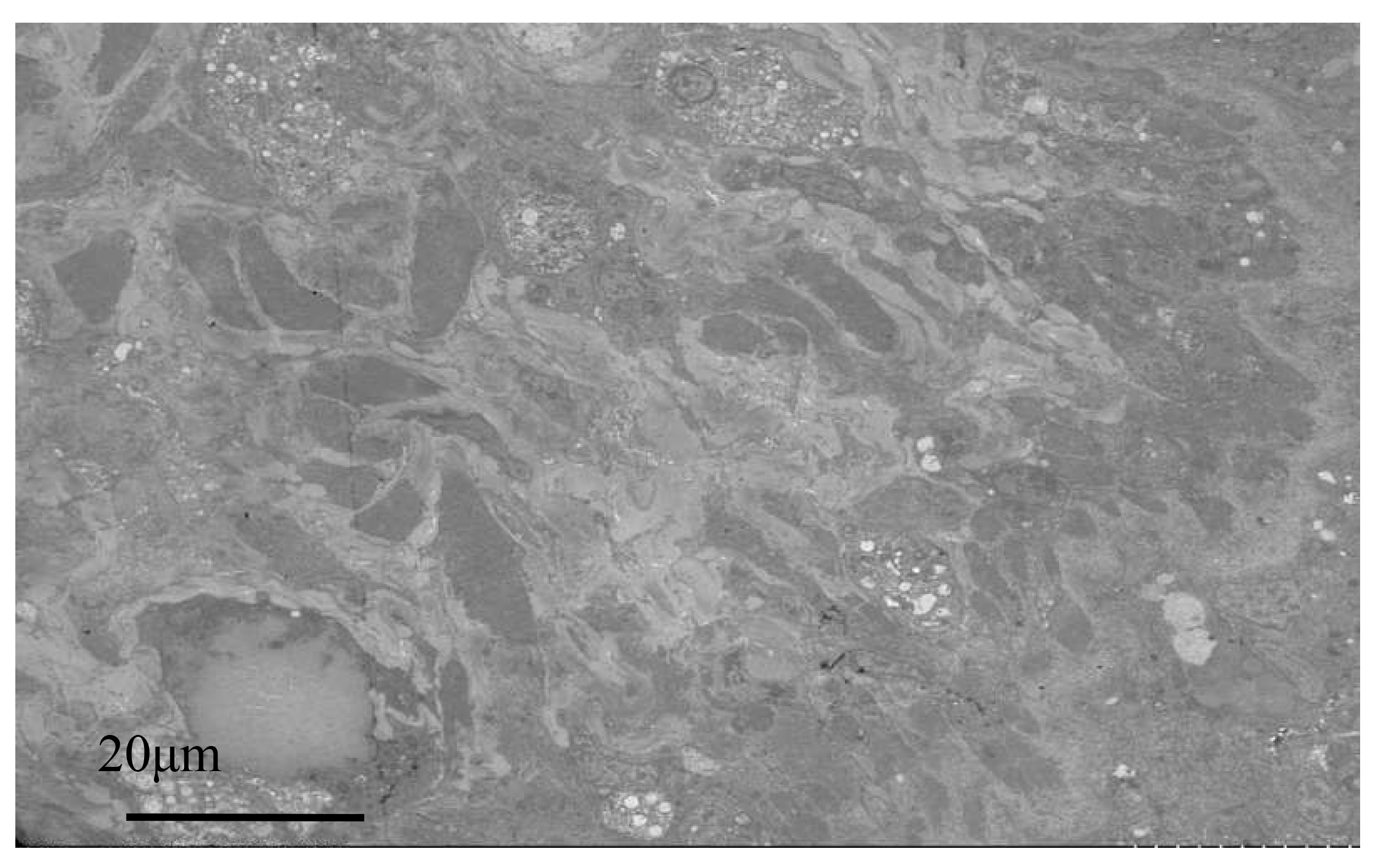
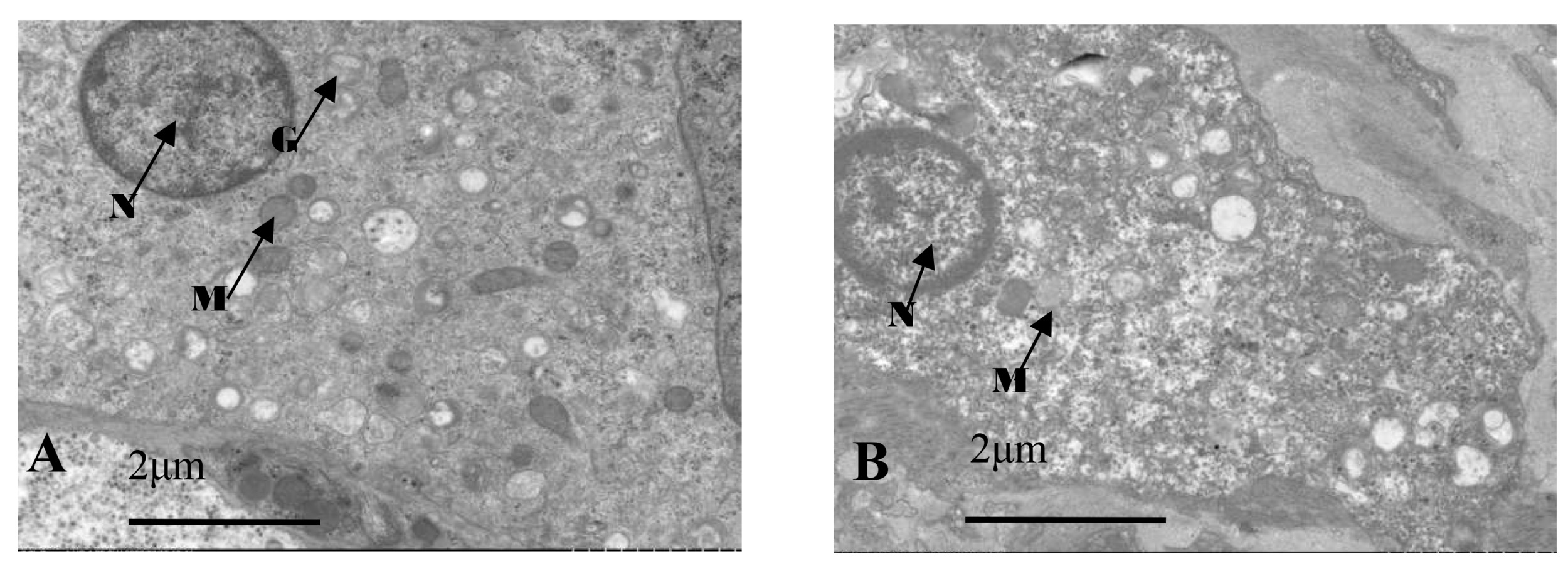
Electron microscopy images of healthy oysters showed that the epithelial layer of the mantle contained a large number of A-type columnar epidermal cells (Fig. 5A), some arranged in a monolayer and some scattered throughout the layer. The cells were about 7 to 14 μm in length, with a nucleus that occupied most of the cell. The nuclei of A-type columnar epidermal cells had relatively distinct euchromatin and heterochromatin, which were mostly located in the middle and lower parts of the cells. The organelles were relatively intact. After infection by Polydora (Fig. 5B), the overall image of the oyster mantle appeared darker than that of healthy individuals, with a particularly prominent staining of the epithelial layer area. This was due to the abnormal increase of secretion granules and pigment particles in the epithelial layer caused by the Polydora infection. The HE staining also confirmed this, as the pigment particles and secretion granules in the epithelial layer area of the marginal membrane protrusion of the infected individual were significantly increased, and the internal connective tissue area was significantly darker than that of healthy individuals.
Below the basement membrane, there was some disordered white zona pellucida (Fig. 6), consisting of electron-transparent granulocytes and secretory cells, that flooded between the epithelium and connective tissue layers. The overall structure inside the marginal membrane of healthy oysters was clear, with a normal nucleus morphology, complete nuclear membrane, and no chromatin shrinkage or vacuolization inside the nucleus. The outer mitochondrial membrane was clear, and the inner crest was relatively clear. Lysosomes were small, with a clear outer membrane and homogeneous internal substance, and the endoplasmic reticulum was laminated and relatively normal (Fig. 7A). In infected individuals, cells showed obvious pathological changes, with small fractures in the nuclear membrane, chromatin shrinkage, and vacuolization in the nucleus. A large part of lysosomes, the Golgi apparatus, endoplasmic reticulum, and mitochondria had dissolved and disappeared, and the only remaining morphological structure of the endoplasmic reticulum and cellular mitochondria was not obvious, showing semi-degenerate and dissolved forms. The cytoplasm also appeared lysed and there was severe vacuolization (Fig. 7B).
Ultrastructural and Pathological Analysis of Healthy and Infected Oyster Gills
The gills of healthy oysters had densely packed cells and contain normal levels of secretions without any other contaminants (Fig. 8A). Electron microscopy showed the three main cell types in oyster gills: cubic cells, ciliated cells, and glandular cells. Ciliated cells had abundant mitochondria, rough endoplasmic reticulum, and a developed Golgi apparatus. The mitochondria were round or oval and mainly located near the nucleus and tip of the cytoplasm, and had a high number of microvilli and some visible microtubule structures. The surface of cubic cells was covered with dense microvilli, and the nucleus takes up most of the cell. Glandular cells had several cell types, and their nucleus was at the base of the cell, with many transparent dense particles and vacuole-like substances in the cytoplasm. Meanwhile, an aggregated bacterial colony was found in the glandular cells of infected oysters, and the outer layer was surrounded (Fig. 9).
The gill tissue of infected oysters was nearly dissolved and collapsing, and the damaged tissue was surrounded by interstitial fluid containing various secretory cells, organelles, and microvilli. The nucleus of pre-gill leaf cilia cells, gill leaf cubic cells, and gill epidermal cells had abnormal nuclear karyotype, multiple invaginations of nuclear membranes, partial nuclear solidification, and ruptures of nuclear membranes, and some organelles such as the Golgi apparatus were missing. Some cells also had dense microvilli that had collapsed (Fig. 8B).
Discussion
The Polydora’s parasitism in molluscs and the resulting decline or death is linked to specific cell types within the cells. For instance, the damaged organelles and cell types, such as the mitochondria, chlorine cells, and glandular cells, in diseased Haliotis diversicolor individuals result in reduced energy provision, imbalanced Na+ and K+ distribution, causing intracellular Na+ stores, which allow excess extracellular water to enter the cell, causing cell swelling, organelle structure abnormalities and even death.14 The present experiment showed that the glandular cells, chlorine cells, and various organelles in oyster tissue infected by the Polydora were abnormal, hindering normal physiological functions.
There are conflicting opinions on the pathogenicity and lethality of microorganisms that parasitize marine molluscs.18 While some reports suggest that the death of pathogenic molluscs is linked to infection by pathogenic organisms, others argue that pathogenic organisms cause minor or no damage to host tissue cells. The results of this experiment showed that there was no significant change in the soft part of oysters with mild infection, and slight pathological changes were observed by HE staining, while severely infected individuals had obvious pathological changes, serious tissue cell destruction, and lost some physiological functions, resulting in death. Hence, the harm caused to oysters by the Polydora infection depends on the degree of infection, and severe infection can cause serious pathological damage or death to the host.
Environmental conditions (mainly temperature and salinity) play an important role in regulating parasite pathogenicity and host disease resistance.19 Liu et al.20 identified a new Crassostrea hongkongensis parasite, Thraustochytrium pachydermum, at salinities between 15 and ~25 ppt. The salinity of the environment affects the proliferation and size of T. caudivorum in Crassostrea hongkongensis, with the highest number of zoospores produced at 25 ppt salinity.20 Similarly, Tsu et al.21 found that salinity affects the yield and motility of zoospores in four Mangrove Thraustochytrid isolates, each of which had its optimal range of proliferative salinity,21 indicating that salinity is an important factor affecting parasite proliferation and infection pathogenicity. The increase of silt in the environment can also increase the susceptibility of oysters to Polydora,8 while a decrease in pH value in water can weaken it.22 The number of oysters infected with Polydora also shows a cyclical periodicity. Recently, through collection and dissection experiments of Crassostrea hongkongensis, it was found that the number of oysters infected with Polydora showed cyclical periodicity, and the number of infected Polydora in each batch of collected oysters fluctuated. The less infected individuals did not have obvious abnormalities. In order to ensure the accuracy of the experiment, this experiment synthesized the results of several sampling, and selected the oyster with the highest degree of infection for experimental analysis.
The Polydora complex’s parasitism seriously destroys cultured shellfish’s immune system. At the same time, it is easy to cause secondary infection of bacteria, viruses, mycoplasma, chlamydia, and other microorganisms, causing harmful microorganisms to escape from the natural protection system of shellfish and directly infect their soft parts, causing corresponding tissue lesions. The Polydora penetrates the shell and parasitizes the shell, making the shell surface covered with a large number of pipes, which not only makes the shell easy to break, but also its metabolites are directly excreted in the shell, making it more vulnerable to infection by harmful microorganisms in the excretion, which will at least reduce the quality of the shell, and even lead to the death of the shell.23
Most of the organelles in mitochondria, lysosomes, and endoplasmic reticulum in all diseased oysters’ envelope and gill tissues were irreversibly damaged to varying degrees. The damage to organelles will inevitably lead to abnormal cell function. For example, mitochondrial damage will affect the energy supply, destroy the ion balance in cells, cause cell edema, intracellular organelles, and mitochondrial membrane rupture and degeneration; the secretion function of lysosome decreases, and the harmful substances in the capsule cannot be removed in time, causing the accumulation of inflammatory substances in cells, leading to the collapse of cells near death, further leading to the pathological changes of the whole tissue, the ulceration and atrophy of the diseased oyster muscle, loss of eating and movement ability, until death due to systemic failure.
Through the cross-sectional observation of the mantle, gills, and other tissues of oysters, it was found that the pathological symptoms of oysters infected with Polydora are similar to those of other parasites such as Perkinsus, including the disordered dissolution of connective tissue and muscle fibers in the mantle, increased dirt and secretion in the gills, the dullness of the soft parts, emaciation and ulceration, growth retardation, and the decline of reproductive ability. In the early stage of parasite infection, inflammation occurs only in the tissues around the pathogen, and secretion increases. In the later stage, most tissues will be infected to varying degrees. After oysters were infected with Perkinsus marinus, it was extremely emaciated, with loose meat, dark gray digestive glands, atrophy of mantle, blocked gonadal development, slow growth, and sometimes abscess.19 After P. marinus invades the oyster shell through the digestive tract epithelium, the primary lesion forms in the oyster shell, causing extensive abscess in the epithelial layer, and the epithelial cells and infiltrating blood cells are destroyed. After the worm body reaches the connective tissue, it reaches the whole body through the blood vessels, causing multiple abscesses in the connective tissue of various organs, especially in the mantle. Gao7 found that Polydora ciliata made oysters brittle, forcing shellfish to consume more energy to form shellfish to resist the invasion of polydora, rather than for their own growth and development, and at the same time the shellfish were more susceptible to infection and mortality increased due to the large number of microorganisms in the flocculent debris in the frostbite duct. The sick oysters showed weight loss, growth retardation or cessation, and decreased reproductive capacity. In 2021, the oyster parasite Thraustochytriu. caudivorum was first isolated from the gill tissue of crassostrea hongkongensis, and the flesh of infected oysters turned black, dissolved and even lead to death.20
Through the microscopic observation of oyster tissue, it was found that there were a large number of mucous cells in oyster tissue. Although mucous cells are common in molluscs, they have not been well studied and are still being explored. Mucous cells play a crucial role in fish immunity. AB-PAS staining showed the presence of various mucus cells on the marginal membrane cells of molluscs, and many RNA, acid polysaccharides, neutral polysaccharides, and high ACPase activity were found on mucus cells of molluscs such as Chlamys farreri, Nuttallia olivacea, and Meretrix meretrix.24 ACPase is an important hydrolase in animals and a marker enzyme for lysosomes, suggesting that the mantle mucus cells in molluscs, including oysters, have some immune protective functions. Abnormal enzyme activity is one of the features of histopathological changes. Diseased molluscs may experience decreased digestive function and metabolic disorders, leading to extensive damage to the tissues and organs. Oysters in the aquatic environment strongly rely on their innate immune system protection. The innate immune response includes cells (such as phagocytes) and body fluids (such as enzymes) to protect bivalve hosts from harmful microorganisms encountered in the natural environment.25 In general, immune cells (such as blood cells) are activated by pathogen associated molecular patterns (PAMP) stimulating receptors. This immune response includes signal cascades and the release of various molecules, such as hydrolases and antimicrobial peptides, which are part of the immune system against pathogens. This is supported by the presence of eosinophilia at the end of the mantle protrusion after oyster infection, as eosinophils are blood cells that mediate cellular immune responses in parasitic infections, indicating that infected oysters activate immune function by increasing cytokines such as blood cells.
Conclusions
Based on the research of this experiment, the following conclusions have been drawn. Through the observation of the mantle and gill infected by the Polydora, it was found that the Polydora caused significant damage to these two parts. The Polydora infected the shell and soft part, and then gradually affected the tissue cells of the whole oyster, causing damage or even death of the oyster’s soft tissue. The microstructure was mainly characterized by disordered secretion, abnormal rupture, and dissolution of muscle connective tissue. The frequency of abnormal or disappearing organelles was higher in the submicroscopic structure. In addition, the damage or death of infected oysters usually results from a combination of factors, including the parasitic behavior of Polydora and the secondary cross-infection caused by various pathogens, resulting in damage to the immune system.
Acknowledgments
This work was supported by a grant from the Professorial and Doctoral Scientific Research Foundation of Huizhou University (2020JB065); Team of Guangdong Provincial Department of Education: Marine shellfish ecological breeding and disease prevention and control innovation (2021KCXTDO57); Guangdong Provincial Rural Revitalization Strategy Special Funds for Seed Industry Revitalization Project (2024-SPY-02-003);Efficient Breeding and Industrialization of Marine Shellfish - Hemifusus tuba (2022CQ010010).
Authors’ Contribution
Conceptualization: Changyu Guo (Equal), Jiang-Yong Wang (Equal). Formal Analysis: Changyu Guo (Lead). Investigation: Changyu Guo (Lead). Writing – original draft: Changyu Guo (Lead). Writing – review & editing: Changyu Guo (Equal), Jiang-Yong Wang (Equal). Validation: Changyu Guo (Lead). Methodology: Jiang-Yong Wang (Lead). Funding acquisition: Jiang-Yong Wang (Lead). Resources: Jiang-Yong Wang (Lead). Supervision: Jiang-Yong Wang (Equal), Jing-Feng Sun (Equal), Zhao-Rui Wang (Equal). Software: Kai-Yuan (Equal), Jia-Hao Zhao (Equal).





__**.png)


__**.png)