1. Introduction
Reproductive success in marine fish aquaculture is highly dependent on the quality of gametes, particularly eggs, which are influenced by both intrinsic (e.g., maternal provisioning, hormonal status) and extrinsic (e.g., temperature, photoperiod, nutrition) factors. In aquaculture, controlling and enhancing gamete quality, particularly egg quality, plays an important role in ensuring reproductive success and improving larval survival. Egg quality is commonly defined as the ability of the egg to be fertilized and develop into a healthy, non-deformed embryo.1,2 However, egg quality is influenced by multiple factors, including broodstock nutrition, management practices, environmental conditions, and, notably, temporal factors specifically seasonal or monthly variations.3,4
In tropical marine fish such as the snubnose pompano (Trachinotus blochii), these temporal dynamics remain underexplored. The snubnose pompano (Trachinotus blochii) is a high-value marine fish species widely farmed in several Asian countries including Viet Nam. It is characterized by fast growth, adaptability to farming conditions, and high market demand.5 Nevertheless, under captive rearing conditions, egg production and larval quality from broodstock still show fluctuations, making it difficult to ensure a consistent supply of seeds throughout the year.6 Previous studies have indicated that reproductive indices such as gonadal maturation rate, fecundity, egg diameter, buoyancy, fertilization and hatching rates, as well as larval survival, can vary significantly over time, even on a monthly scale.7,8
Therefore, the present study was conducted to evaluate the monthly fluctuations in reproductive indices, egg quality, and larval survival of broodstock snubnose pompano (T. blochii) reared under captivity conditions. Identifying the periods with the highest egg quality and larval survival will contribute to optimizing seed production protocols and enhancing the overall efficiency of aquaculture practices for this species.
2. Materials and Methods
The study was conducted continuously over a 12-month period (from January to December 2024) at a commercial floating cage in Vietnam. Broodstock snubnose pompano (Trachinotus blochii) were reared in open sea floating cages (4x4x4 m) located in a sheltered coastal bay. Throughout the broodstock conditioning period, fish were fed a diet consisting mainly of trash fish at a feeding rate of 7% of body weight per day. To enhance reproductive performance, the diet was consistently supplemented twice weekly with shrimp, squid, and vitamins E and C. While the natural composition of trash fish may exhibit slight seasonal variation, the species used were similar in nutritional content and were sourced from the same local suppliers to ensure consistency. This feeding regime was maintained throughout the study to minimize dietary variability and its potential impact on broodstock fatty acid profiles. During each spawning cycle, broodstock exhibiting signs of gonadal maturity were hormonally induced to spawn using human chorionic gonadotropin (HCG). Female broodstock were administered HCG at a dosage of 1200 IU/kg body weight, while males received half this dose (600 IU/kg). The hormone was injected intramuscularly at the dorsal musculature in the morning time, and spawning was typically observed within 36–48 hours post-injection. This protocol was applied consistently across all months to standardize spawning induction.
Monthly monitoring of key water quality parameters in the broodstock cage farming area was conducted, including water temperature, salinity, dissolved oxygen, pH, and transparency. These environmental factors varied seasonally and are summarized in Table 1. Water temperatures ranged from 23.3°C in December to 29.9°C in September, salinity from 31 to 36 ppt, and pH levels remained relatively stable (8.10–8.30). Water transparency peaked in July–August (up to 11 m) and was lowest in December (2.6 m) (Table 1). These natural conditions provided a realistic backdrop for evaluating reproductive performance and egg quality over time.
A total of 60 broodfish (30 females and 30 males) of uniform size (from 2.2-3.7 kg) were maintained and examined monthly. In the middle of each month, five randomly selected females were anesthetized using clove oil (40 ppm), and their gonads were assessed by gently pressing the abdomen to collect eggs. The harvested eggs were used to determine reproductive parameters, as described below:
2.1. Maturation rate (%)
The maturation rate was determined by counting the number of females that could be manually stripped for eggs (i.e., ovulated females) out of the total number of females examined each month, using the formula:
\[\small{Maturation\ rate\ (\%) = 100 \times \frac{Number\ of\ ovulated\ females}{Total\ number\ of\ females\ examined}}\]
2.2. Fecundity (eggs/kg female)
The total egg mass (g) collected from each female was weighed using a digital scale accurate to 0.01 g. Then, the number of eggs in 1 g of eggs was counted under a stereomicroscope to determine the egg density per gram. Fecundity was standardized as the number of eggs per kilogram of female body weight using the following formula:
\[\small{Fecundity\ (eggs/kg) = \frac{Estimated\ total\ number\ of\ eggs}{Weight\ of\ female\ (kg)}}\]
2.3. Egg diameter (mm)
From each female, 30 randomly selected eggs were measured using a microscope equipped with a digital camera and ImageJ software. The egg diameter, including the vitelline membrane, was measured at the widest point.
2.4. Egg buoyancy rate (%)
Approximately 200 eggs were placed in a 500 mL settling beaker filled with seawater (30–32‰). After 30 minutes, floating and sinking eggs were separated and counted using a petri dish, and the buoyancy rate was calculated as follows:
\[\small{Egg\ buoyancy\ rate\ (\%) = 100 \times \frac{Number\ of\ floating\ eggs}{Total\ number\ of\ eggs}}\]
2.5. Sample collection and fatty acid analysis
Egg samples were collected monthly from Trachinotus blochii broodstock between January and December. The collected eggs were analyzed to determine their fatty acid composition, expressed as a percentage of dry matter. The analysis focused on both individual fatty acids and summed groups of fatty acids, including saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), and highly unsaturated fatty acids (HUFA). Fatty acid profiles were analyzed from three pooled replicate egg samples per month, each comprising eggs from different females. Fatty acids were identified and quantified using gas chromatography, following lipid extraction from the dry matter of the egg samples. The data collected for each month were expressed as a percentage of total lipids. Lipid content was also recorded as a percentage of dry matter for each sample, and the total fatty acid content was calculated accordingly.
2.6. Fertilization rate (%)
Eggs were fertilized using sperm from one male per female and incubated in still seawater for about 30 minutes. Fertilized eggs were then observed under a microscope to check for cell cleavage. The fertilization rate was calculated as the percentage of eggs showing embryonic development.
2.7. Hatching rate (%)
Fertilized eggs were incubated under standard conditions (temperature: 28 ± 1°C; salinity: 32‰). Hatching rates were determined after 18–22 hours (depending on temperature) by counting the number of hatched larvae relative to the initial number of fertilized eggs:
\[\small{Hatching\ rate\ (\%) = 100 \times \frac{Number\ of\ hatched\ larvae}{Total\ number\ of\ fertilized\ eggs}}\]
2.8. Larval survival rate after 3 Days (%)
Newly hatched larvae were reared in 500 L plastic tanks at a density of 30 larvae/L, using Brachionus spp. rotifers enriched with DHA as feed. On day 3 post-hatching, the number of surviving larvae was counted and the survival rate was calculated as a percentage of the initial number of hatched larvae.
2.9. Statistical analysis
All data are presented as mean ± standard deviation. One-way analysis of variance (ANOVA) was used to evaluate monthly differences, followed by Tukey’s post hoc test for multiple comparisons, with statistical significance set at P < 0.05.
3. Results
3.1. Variations in maturation rate and fecundity
The maturity rate of broodstock varied throughout the year (Figure 1), ranging from 51.98% to 73.11%. The highest maturity rates were observed in May (72.61%), August (73.11%), and December (71.64%). Statistically significant differences (P < 0.05) were observed in the maturity rates across the months, as indicated by the different letters accompanying each column in the graph. The lowest maturity rate occurred in January (51.98%), with a slight increase in February (53.57%) and a peak in May. The maturity rate remained relatively high from May through August and declined slightly in September and October, before rising again in December.
The fecundity of broodstock varied significantly throughout the year (Figure 2), with the highest fecundity recorded in July (107,454 eggs/kg of female), followed by August (91,640 eggs/kg of female) and September (94,237 eggs/kg of female). Statistically significant differences (P < 0.05) were observed in fecundity across the months, as indicated by different letters accompanying each column in the graph. The lowest fecundity occurred in April (53,096 eggs/kg of female), while fecundity values in January, February, March, May, June, October, November, and December ranged from 70,989 to 89,357 eggs/kg of female. These months did not show statistically significant differences from one another, suggesting relatively stable fecundity during these periods, except for the dip in April and the peak during the summer months.
3.2. Egg Quality: Diameter, buoyancy, fertilization, and hatching rates
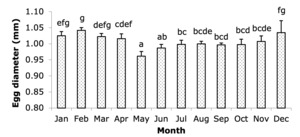
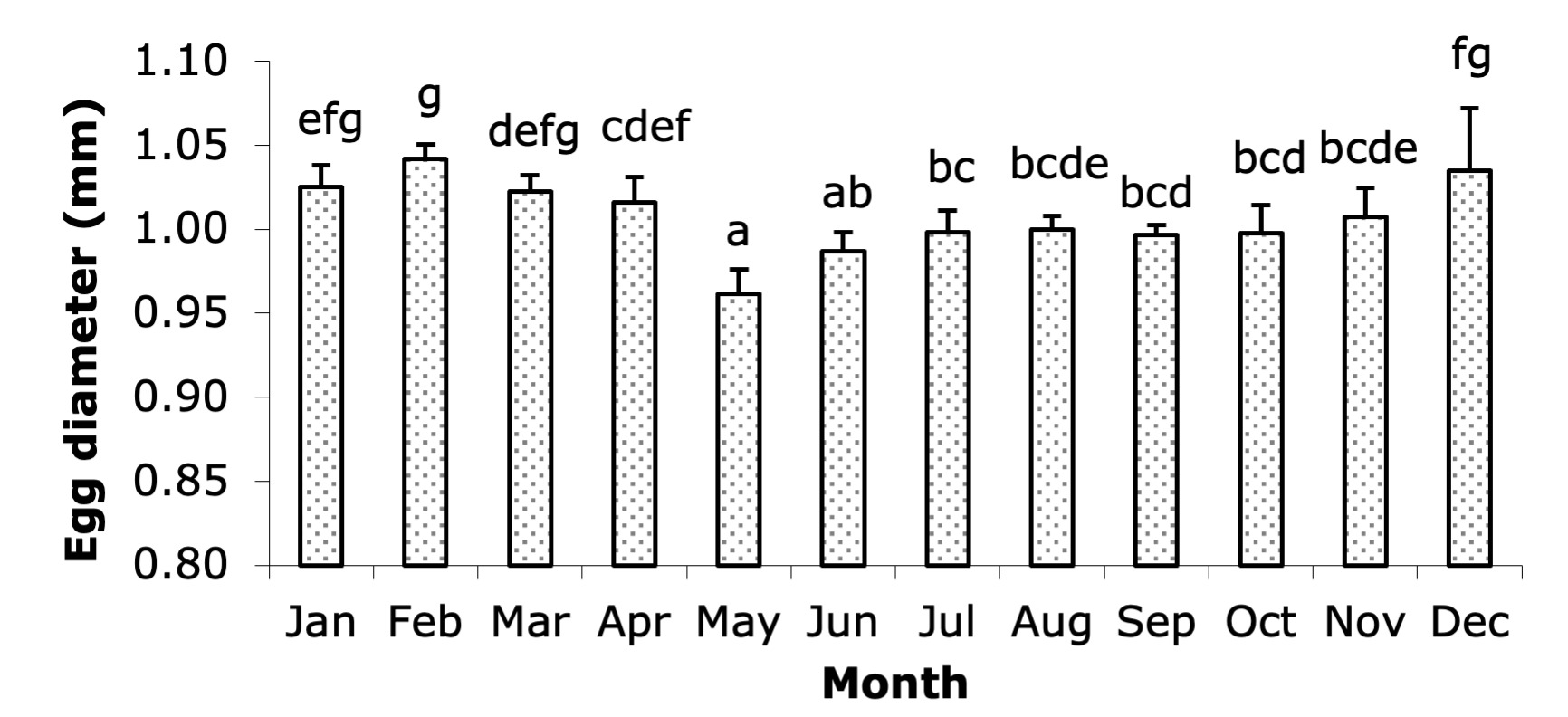
The egg diameter of broodstock varied significantly throughout the year (Figure 3), with statistically significant differences (P < 0.05) observed between the months, as indicated by the different letters accompanying each column in the graph. The largest egg diameters were recorded in February (1.04 mm) and December (1.03 mm). From January to April, the egg diameter remained relatively large, ranging from 1.01 to 1.04 mm. However, the egg diameter decreased significantly from May to September, with the smallest size observed in May (0.96 mm) and gradually increasing in the following months. From May to September, egg diameters ranged between 0.96 mm and 1.00 mm. In October and November, the egg diameter stabilized around 1.00 mm before increasing again in December. This seasonal variation suggests that egg quality, as indicated by egg size, tends to be higher during the cooler months of the year.
The floating egg rate of broodstock varied throughout the year (Figure 4), with statistically significant differences (P < 0.05) between the months, as indicated by different letters accompanying each column in the graph. The highest floating egg rate was observed in June (84.77%), followed by February (81.71%) and September (80.71%). These months showed the best buoyancy, indicating higher egg quality during this period. Conversely, the lowest floating egg rates were recorded in April (66.02%), January (66.74%), and October (65.79%). The floating egg rate remained relatively stable in other months, ranging from 69.86% to 77.86%. This variation in floating egg rate throughout the year suggests that environmental conditions and broodstock management practices influence the buoyancy and overall quality of the eggs.
The fertilization rate of broodstock eggs varied significantly throughout the year (Figure 5), with statistically significant differences (P < 0.05) observed between the months, as indicated by the different letters accompanying each column in the graph. The highest fertilization rate was recorded in November (91.61%), indicating optimal spawning conditions during this month. High fertilization rates were also observed in September (85.58%), January (82.75%), February (82.37%), and October (82.27%). The lowest fertilization rates occurred in March (72.09%) and April (72.98%). The rates during other months ranged from 76.41% to 80.15%, showing moderate fertilization success. This variation in fertilization rates suggests that environmental factors and broodstock condition influence the reproductive success of the eggs throughout the year.
The hatching rate of eggs varied throughout the year (Figure 6), with statistically significant differences (P < 0.05) between the months, as indicated by the different letters accompanying each column in the graph. The highest hatching rates were observed in November (90.81%), October (88.89%), and July (88.38%). The lowest hatching rate occurred in April (71.27%). Hatching rates in other months ranged from 76.32% to 86.63%, with relatively stable but slightly lower rates in January, February, August, and December. These results indicate that the hatching success was highest during the cooler months of October and November, and lowest in April, suggesting seasonal influences on egg viability and developmental success.
3.3. Monthly variations in fatty acid composition in eggs
Analysis of the fatty acid composition in Trachinotus blochii eggs revealed significant monthly fluctuations, reflecting the influence of environmental conditions and dietary factors on egg quality (Table 2). The total n-3 PUFA content ranged from 1.58% (November) to 1.96% (February). Higher levels were recorded in February (1.96%), October (1.88%), and June (1.82%). In particular, DHA (C22:6n-3) a critical component for embryonic development and the nervous system peaked in May (1.15%) and February (1.12%). These findings suggest that transitional seasons and early-year periods may be associated with better nutrient accumulation in eggs. Total n-6 HUFA content varied from 0.45% (November) to 0.65% (September), with relatively high values observed in January, May, and December (all at 0.64%). This indicates a generally even distribution of n-6 HUFA throughout the year, though a notable decline was evident in November. Total lipid content (% dry matter) ranged from 10.87% (May) to 13.25% (April). However, the total fatty acid-to-lipid ratio an indicator of the efficiency of fatty acid accumulation in egg lipids was highest in June (51.84%) and October (48.79%), suggesting that eggs during these months had relatively higher fatty acid content relative to total lipids. Total HUFA content (including EPA, DHA, and other long-chain fatty acids) ranged from 1.39% (March) to 1.81% (February). Elevated HUFA levels in February, July, August, and October suggest that eggs collected during these periods may have higher nutritional potential, supporting better larval development and survival after hatching.
3.4. Larval survival
The survival rate of 3-day-old larvae varied significantly throughout the year (Figure 7), with statistically significant differences (P < 0.05) observed between the months, as indicated by the different letters accompanying each column in the graph. The highest survival rates were recorded in July (59.81%) and June (58.76%), followed closely by September (57.42%) and December (56.96%). The lowest survival rates occurred in May (42.58%) and April (43.42%). In other months, the survival rates ranged from 49.08% to 54.03%, showing moderate survival success. The results suggest that the survival of larvae tends to be higher during the mid-year months, particularly in June and July, and lower in the spring months of April and May. This seasonal variation highlights the influence of environmental conditions and broodstock management on larval survival.
4. Discussion
The reproductive performance of snubnose pompano (Trachinotus blochii) reared under captive conditions exhibited clear seasonal and monthly fluctuations. These variations were closely associated with environmental factors and nutritional status, similar to many marine fish species with cyclical reproductive patterns.3,4
Monthly changes in reproductive indices, egg quality, and larval survival of T. blochii broodstock were closely associated with natural environmental variations in the sea cage area. Higher reproductive performance was observed from May to September, when water temperature (28–30°C) and transparency (>6 m) peaked. These conditions are favorable for oocyte maturation and larval development. In contrast, lower temperatures (23–25°C) and reduced water clarity during winter months (December–February) corresponded with declines in spawning success and larval survival. Salinity and pH remained stable throughout the year, contributing to relatively consistent fertilization rates. However, slight fluctuations in dissolved oxygen may have affected embryonic development in some months. These findings highlight the influence of seasonal environmental conditions on broodstock performance and suggest that seed production should be scheduled during warmer months to optimize hatchery outcomes.
4.1. Variations in maturation rate and fecundity
The highest gonadal maturation rates were recorded in May, August, and December, coinciding with transitional periods between dry and rainy seasons. This suggests that snubnose pompano displays strong physiological responsiveness to environmental changes in temperature, salinity, and photoperiod factors known to influence reproductive cycles in fish.4 The highest fecundity, observed in July (107,454 eggs/kg female), reflects increased reproductive activity during the rainy season, when environmental conditions and natural food availability are generally more favorable. These findings are consistent with those of Yanes-Roca et al.9 in snook (Centropomus undecimalis).
4.2. Egg Quality: Diameter, buoyancy, fertilization, and hatching rates
Egg quality indicators such as diameter, buoyancy rate, fertilization rate, and hatching rate varied significantly over time. The largest egg sizes were recorded in February and December, indicating high maternal nutritional investment.2 The highest buoyancy rate in June (84.77%) serves as an early indicator of egg quality and embryonic development potential.10 The highest fertilization (91.61%) and hatching (90.81%) rates occurred in November, which may be linked to the stable environmental conditions of the dry season. Such conditions support sperm viability and promote synchronized fertilization and embryonic development.11
4.3. Larval survival and the role of egg biochemistry
Although fertilization and hatching rates peaked in November, the highest 3-day larval survival rate was observed in July. This suggests that egg quality particularly biochemical composition such as polyunsaturated fatty acids (n-3 HUFA) is a more decisive factor for post-hatch survival than reproductive indices alone.8,12 The months with higher larval survival coincided with elevated DHA and n-3 PUFA levels in eggs (July and November), supporting the idea that DHA plays a crucial role in the development of the central nervous system and cellular membrane function.1,9 Moreover, July and November, which showed the highest larval survival, also had favorable fatty acid profiles with DHA/EPA ratios >2.0 and n-3/n-6 ratios >5.0, consistent with previous studies indicating these thresholds are critical for proper embryonic development and membrane fluidity.13,14 This reinforces that absolute DHA content and the balance between key fatty acids are essential for early larval survival.
4.4. Practical implications for aquaculture
The findings on the monthly variations in reproductive indices and egg quality of broodstock snubnose pompano (Trachinotus blochii) provide critical insights for optimizing hatchery protocols in aquaculture. Identifying specific months with high gonadal maturation, fertilization, hatching, and larval survival rates enables hatchery operators to better plan breeding activities, thereby ensuring a consistent and high-quality seed supply in line with market demand. Specifically, the months of May, July, November, and December have been identified as peak reproductive periods, making them ideal for large-scale egg collection and intensified seed production.
Moreover, the study suggests that cyclic broodstock nutritional management can improve egg quality and larval survival, particularly during low-performance periods such as March–April. Supplementing essential fatty acids, especially n-3 HUFA such as DHA and EPA, in female diets before spawning has been shown to enhance egg quality in many marine fish species.3,9 This represents a simple yet effective nutritional strategy that can be readily integrated into current broodstock management programs.
In addition, the detailed monthly data on reproductive traits provide a foundation for future studies on spawning synchronization, the seasonal application of ovulation-inducing hormones, and improvements to incubation systems tailored to specific times of the year. This research also contributes to a better understanding of the reproductive mechanisms of T. blochii under captivity conditions an essential factor in the development of new cultured species and the sustainable upgrading of seed production systems, replacing the increasingly limited reliance on wild-caught juveniles.5,8
In summary, this study not only contributes academically to the understanding of seasonal reproductive patterns in snubnose pompano but also holds substantial practical value in formulating proactive, efficient, and sustainable seed production strategies for modern marine aquaculture.
5. Conclusion and recommendations
This study clarified the monthly variations in reproductive indices, egg quality, and larval survival of snubnose pompano (Trachinotus blochii) broodstock reared under captivity conditions. The results revealed that reproductive parameters including gonadal maturation rate, fecundity, egg diameter, buoyancy rate, fertilization, hatching, and larval survival fluctuated throughout the year. Among these, May, July, November, and December were identified as peak periods for reproductive performance and egg quality. Notably, the alignment of high maturation rates with stable environmental conditions such as temperature and salinity suggests that T. blochii may exhibit environmentally driven cyclic reproduction, similar to other economically important marine species.
Furthermore, egg quality indicated by buoyancy rate and nutrient content, particularly n-3 polyunsaturated fatty acids such as DHA plays a critical role in improving larval survival, emphasizing the importance of nutrition during the maturation and spawning phases in females.
Based on the findings, we recommend that snubnose pompano hatcheries prioritize egg collection and artificial spawning during the months with favorable reproductive indicators namely May, July, November, and December to optimize seed output and quality. At the same time, it is essential to enhance the broodstock diet with essential nutrients, especially n-3 HUFA, prior to and during the spawning season to improve egg quality and larval viability. Additionally, this study opens new avenues for regulating spawning periods through environmental control (e.g., temperature, photoperiod), combined with the application of hormonal stimulation, to ensure a stable year-round seed supply an essential requirement for the sustainable development of industrial marine aquaculture.1,5 Future research on photothermal manipulation may facilitate year-round seed production of T. blochii under hatchery conditions, aligning with practices used in other marine finfish.
Acknowledgments
This research was funded by Nha Trang University (NTU) under grant number TR2020-13-18. We are grateful to Mr. Nguyen Tan Khang for his assistance with the experiment and to Prof. Paul B. Brown for proofreading the manuscript.
Authors’ Contribution
Methodology: Manh V. Ngo (Equal), Hoang M. Le (Equal). Formal Analysis: Manh V. Ngo (Lead). Writing – original draft: Manh V. Ngo (Lead), Hoang M. Le (Equal). Funding acquisition: Manh V. Ngo (Lead). Conceptualization: Hoang M. Le (Lead). Writing – review & editing: Hoang M. Le (Lead). Supervision: Hoang M. Le (Lead).
Competing Interest – COPE
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Conduct Approval – IACUC
Following Vietnam’s National Regulations on the Use of Animals in Research (Decree 32/2006/ND-CP, 2006), snubnose pompano is not classified under either Group IB (endangered and critically endangered species) or Group IIB (threatened and rare species). As such, conducting this study did not necessitate obtaining a permit or ethical approval. Nonetheless, the researchers adhered to the highest standards of ethical practice in their treatment of animals throughout the study.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.