Introduction
The Chinese rice-field eel (Monopterus albus), a member of the genus Monopterus Volta and family Synbranchidae, is a significant species in freshwater aquaculture in China, highly valued by consumers for its fast growth rate, delicious taste, and high nutritional value.1 In 2023, the artificial breeding production of rice-field eel in Hubei Province reached 169,752 tons, representing 47.79% of the total national output of rice-field eel and securing its position as the leading producer in China.2 However, as the scale of rice-field eel artificial breeding continues to expand, various disease problems have become increasingly prominent, causing serious economic losses to the rice-field farming.3–5 Gram-negative, facultatively anaerobic, rod-shaped A. hydrophila bacteria can be found in all water bodies across the planet. Numerous nations have reported outbreaks of A. hydrophila infectious illnesses, which were previously well-characterized as the pathology that causes cloacal bleeding, gastroenteritic hemorrhage, ascites, and septicemia with an open cutaneous ulcer.6,7 However, the symptoms and pathological abnormalities displayed by infected fish differ based on the particular bacterial isolates or strains. Elastase (ahyB), DNases (exu), Aerolysin (aer), lipase (lip), cholesterol acyltransferase (gcaT), type III secretion system (ascV), serine protease (ser), cytotonic enterotoxins (act, alt, ast), and polar flagella (fla) are among the virulence factors that are largely responsible for this bacterium’s capacity to cause illness.8–10 According to reports, aerolysin, cytotoxic enterotoxin, and extracellular serine protease are the three most crucial genes for identifying potentially dangerous strains of A. hydrophila.11,12 The information that is now available suggests that A. hydrophila’s pathogenicity is probably multifactorial. stemming from the combined effects of multiple virulence genes rather than a single gene.13,14
In May 2023, in Jianli, Hubei Province, a deadly disease outbreak affecting rice-field eels occurred. The diseased rice-field eels exhibited a darkened body color, varying degrees of hemorrhaging on their surface, and presented with red and swollen anuses. Additionally, they were observed to be floating slowly and weakly on the water’s surface. The mortality rate within one cage exceeded 80%, resulting in significant economic losses for local farmers. In this research, a bacterium was isolated from the diseased rice-field eel, and the pathogen responsible for the disease was identified as A. hydrophila through bacterial identification, Analysis of 16s rRNA and gyrB sequences, along with identification of physiological and biochemical traits, pathogenicity assessment, virulence gene examination, and growth characteristics of the A. hydrophila isolate. Additionally, artificial reproduction experiments were conducted. To help with efficient illness prevention and control, additional analysis was done on the histopathological changes caused by these bacteria as well as antibiotic sensitivity.
Materials and Methods
2.1. Experimental Monopterus albus
The diseased rice-field eel was taken from a rice-field eel farm in Jianli city, Hubei Province, and measured an average of 23 ± 1 cm in length and 10 ± 1 g in weight. Healthy rice-field eel (average body length of 9 ± 1 cm and a weight of 3 ± 1g) was purchased from a rice-field eel breeding factory in Tianmen city, Hubei Province, which did not report any cases of the illness. Before going to the experimental infection, Polymerase Chain Reaction (PCR) was used to test rice-field eels to ensure their health. The healthy rice-field eel was domesticated in our laboratory and fed twice daily.
2.2. Isolation of pathogenic bacteria
Five diseased rice-field eels were randomly selected from the rice-field eel farm where a disease outbreak occurred. The experimental fish were first inspected under a light microscope to check for fungi or parasites on their gills and body surface. The rice-field eels were then dissected in an aseptic setting. The liver, spleen, and kidneys of the rice-field eel were cut into a complete plane and lined on the Brain Heart Infusion (BHI, HopeBio, China) agar plates and cultured on BHI agar plates, which were then incubated at 28 ℃ for 24 hours. The resultant colonies’ morphological characteristics were examined. For purifying purposes, colonies with the biggest area on the plate and those with comparable color and form were considered dominant bacteria. To guarantee the acquisition of a pure culture, a single colony was chosen and cultivated on BHI agar plates at least three times. Furthermore, an individual colony was chosen for Gram staining and morphological analysis. This purified isolate was named YFI-C1 for subsequent analysis.
2.3. Physiological and biochemical characterization
YFI-C1 pure cultures were added to the BHI medium and incubated at 28 ℃ for the entire night. The physical qualities, including shape, size, and color, were then examined.
For ultrastructural analysis, the isolated and purified strains were fixed using a 2.5% glutaraldehyde fixative solution at 4 ℃ for over six hours. In the subsequent phase, the samples were cleaned using phosphate-buffered saline (PBS), dehydrated stepwise in an alcohol concentration gradient solution, dealcoholized gradually in a tert-butanol concentration gradient solution, and subsequently freeze-dried. The pathogen’s structural characteristics were examined with scanning electron microscopy.
Following the incubation of the isolated strains in a constant temperature incubator maintained at 28 ℃ for a period of 24 hours, individual colonies were selected and inoculated into Biolog inoculum IF-A (Biolog, USA). Each well of the GEN III plate received 100 μL of YFI-C1, which was then put in Bio’s fully automated microbial identification system to evaluate the isolated strains’ physiological and biochemical characteristics.15
2.4. 16S rRNA and gyrB gene sequence analysis and Concatenation of phylogenetic evolutionary trees
The genomic deoxyribonucleic acid (DNA) of isolate YFI-C1 was successfully extracted in compliance with the guidelines stipulated by the distributor of the Bacterial Genomic DNA Kit (Tiangen, Beijing, China). Subsequently, the collected DNA served as the PCR amplification template. Primers are designed according to Table 1, under the subsequent cycling parameters: 95 ℃ for 10 min, then 35 cycles of 94 ℃ for 45 s, 56 ℃ for 1 min, and 72 ℃ for 1 min, concluding with a final extension at 72 ℃ for 10 min. The PCR product was sequenced by Tianyi Huayu Biotechnology at Tianyi, China, Following the process of sequencing, a comparison was made between the 16S rRNA and gyrB sequences of the isolate clones and those stored within the NCBI database. This was achieved by means of the BLAST programme, which was used to analyse the sequence homology. Using the neighbor-joining approach in the MEGA 11.0 software, phylogenetic trees were created.16 Then, the two sequences obtained by sequencing were imported into SeqMan for splicing, and a new DNA characteristic sequence was obtained. The NCBI-published and whole-genome sequenced Aeromonas strains were to be downloaded, after which the 16S rRNA sequences and gyrB gene sequences of the strains were to be searched for using the standard sequences of the genes. The neighbor-joining method was utilized to construct a phylogenetic tree.17
2.5 Screening of Virulence-Related Genes
Using PCR, the existence of 12 virulence genes was examined. These genes include the nuclease (nuc), serine proteinase (ser), lipase (lip), haemolysin-aerolysin (aeraA), hemolysin (hlyA), cytolytic enterotoxin (alt), temperature-sensitive protease (eprCAI), a quorum sensing-controlled virulence factor (LuxS), type III secretion system (TTSS) (ascv), serine protease (ahp), flagellin (fla), and cytotoxic enterotoxin (act). The primers employed for amplifying these genes are detailed in Table 1. The PCR reaction mixture was prepared with a total volume of 25 µL, consisting of 12.5 µL of 2x PCR Master Mix (Takara, Japan), 1 µL from each primer, 1 µL of template DNA, and 9.5 µL of nuclease-free water. Following this, the products of the PCR were analyzed through electrophoresis using a 1% agarose gel, then, using the BLAST software, the isolate clones’ virulence-related gene sequences were examined for sequence homology in the NCBI database.
2.6 Experimental infections
Healthy rice-field eels were raised in tanks for a week in order to get used them to the conditions and facilities of the experiment. They were then split up into five groups, each group consists of 3 replicates, with each replicate containing 30 rice-field eels. Sterile PBS was used to wash the cultured colonies in order to create a bacterial suspension, which was diluted to 1 × 104, 1 × 105, 1 × 106 and 1 × 107 CFU/mL for the challenge test. Next, four different bacterial cell concentrations were administered intraperitoneally to rice-field eels in four groups, each receiving 100 µL of the bacterial suspension. The control group received 100 µL PBS. All rice-field eels were reared for a duration of 7 days at a temperature of 28 ℃ to observe and record any symptoms. Morbidity rates and rates of illness symptoms were noted. The isolation and re-identification of the pathogenic bacteria from the affected fish samples was carried out in accordance with standard procedure. The median lethal dose (LD50) for the YFI-C1 strain affecting rice-field eel was later assessed using the Reed-Muench method.27 In order to validate the conclusions, three repeated experiments were performed.
2.7 Histopathological observation
The liver, spleen, and kidney of the diseased rice-field eel were fixed in 4% paraformaldehyde, followed by dehydration using graded alcohol and embedding in paraffin. Sections of the tissue were subsequently cut into 5 μm slices by means of a Leica Biosystems (Olympus, Germany) RM2255 microtome. Thereafter, the resulting sections were subjected to staining with hematoxylin and eosin, a procedure carried out by Tianyi, a company based in China. Following this, the pathological tissue sections were observed under the light microscope. The liver, spleen, and kidney of healthy yellow eels from the control group underwent identical processing procedures.
2.8 Antibiotic susceptibility
The investigation of antimicrobial susceptibility was conducted using the Kirby-Bauer disk diffusion method.28 The antimicrobials tested included Gentamicin, Ciprofloxacin, Doxycycline, Compound Sulfamethoxazoles, Cefothiophene, Neomycin, Florfenicol, Enrofloxacin, Trimethoprim-sulfamethoxazole, and Amikacin. The isolated strains were prepared into bacterial suspensions with nutrient broth. A volume of 200 µL was transferred onto a BHI plate within an ultra-clean bench environment. The bacterial solution was evenly spread using a coating rod. Subsequently, ten different antimicrobial susceptibility disks were placed uniformly on the BHI plate. Subsequently, the plates were tilted and maintained at a constant temperature of 28 ℃ for a duration of 24 hours. Following this, the results were carefully observed and the mean diameters of the resulting growth inhibition zones were recorded.
Results
3.1. Clinical examination
The diseased fish from the rice-field eel farms exhibited slow movement, reduced responsiveness, and a tendency to swim near the water’s surface. Clinical examination of the diseased rice-field eel revealed hemorrhaging in various superficial areas (Figure 1A), with notable erythema and swelling around the anus (Figure 1B). The infected fish’s autopsy revealed septicemia, which is characterized by a blackened liver (Figure 1C), and an ascitic fluid buildup inside the abdominal cavity (Figure 1D).
3.2. Histopathological observations
The pathological changes observed in diseased rice-field eels revealed significant alterations. The liver exhibited severe cellular necrosis and structural disintegration, with margination of nuclear material, along with dilation of hepatic sinusoids and infiltration of inflammatory cells within the sinusoids (Figure 2B). In contrast, the hepatocytes of healthy rice-field eels show uniform staining, and the hepatic sinusoids are narrow. The spleen displayed extensive cellular necrosis and structural breakdown, accompanied by an increase in melanomacrophages and aggregation of lymphocytes (Figure 2D). Severe focal bleeding has been observed in the kidneys, accompanied by atrophy and distortion of the renal ductules, glomerular necrosis, vacuolation, and a substantial presence of inflammatory cells (Figure 2F).
3.3. Morphological Characterization of YFI-C1
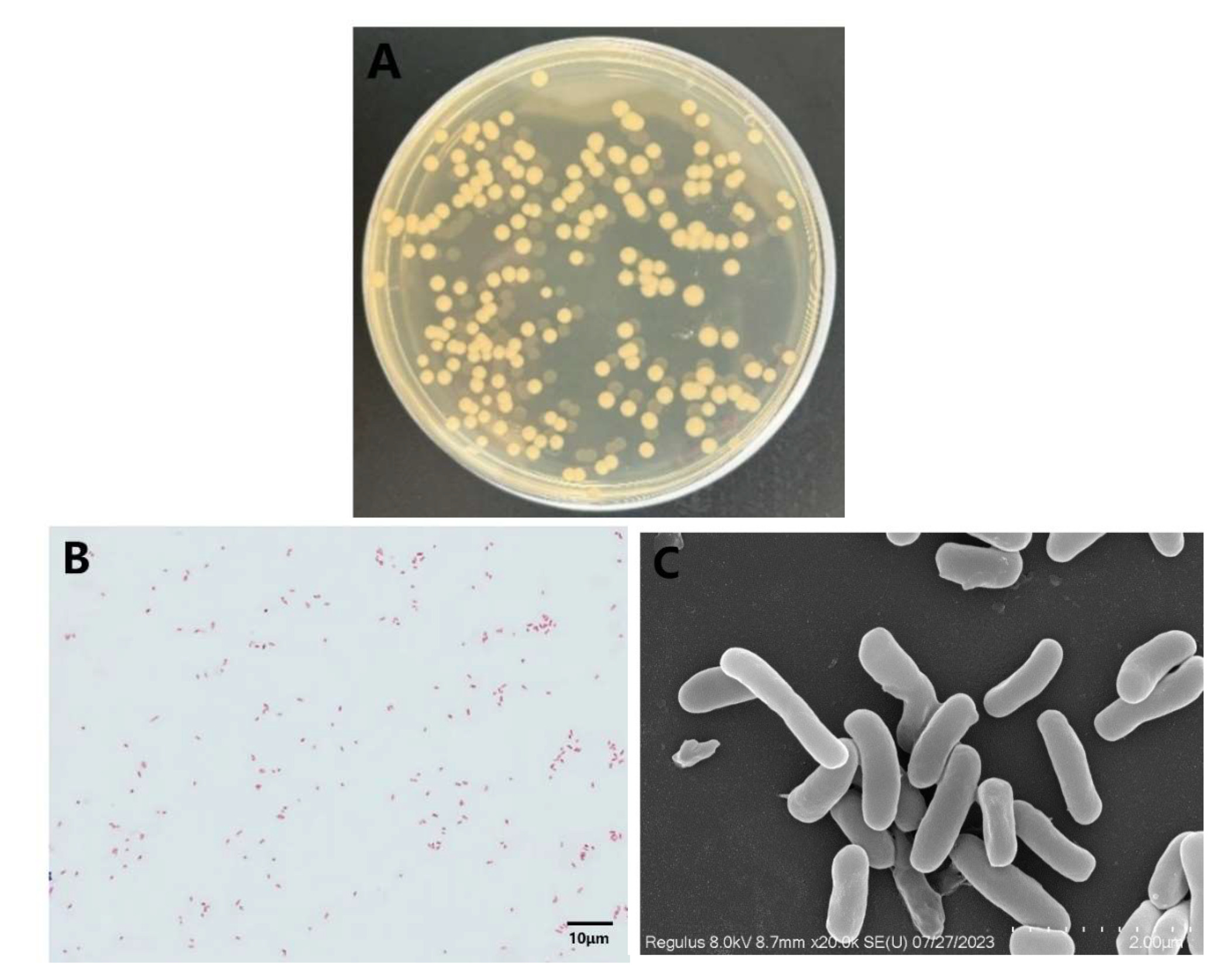
The liver, spleen, and kidney of the diseased rice-field eel were collected in an ultra-clean workbench for pathogen isolation and culture. The observed colonies manifested a round and convex form, accompanied by a smooth outer surface and edges; they exhibited a gray-white coloration with diameters ranging from 1.5 to 3.0 mm (Figure 3A). This strain has been tentatively designated as YFI-C1. Upon Gram staining, it displayed a red color (Figure 3B), indicating that it is a gram-negative bacterium. Scanning electron microscopy (SEM) analysis revealed that YFI-C1 is characterized by irregularly arranged rod-shaped morphology, measuring approximately 2 μm in length (Figure 3C).
3.4. Sequence analysis of the 16S rRNA and gyrB genes
The BLAST results indicated that isolate YFI-C1 shares 100% identity with other strains of A. hydrophila. The construction of phylogenetic trees was based on the analysis of 16S rRNA and gyrB gene sequences (Figure 4A and B), isolate YFI-C1 distinctly clustered with a group of known A. hydrophila strains. Subsequently, these two gene sequences were concatenated to create a novel DNA characteristic sequence, which facilitated the construction of phylogenetic evolutionary trees capable of effectively distinguishing different Aeromonas spp. strains. The results confirmed that YFI-C1 is identified as A. hydrophila (Figure 4C).
3.5. Virulence determinant genes
In the present study, twelve virulence-related genes (ascV, nuc, ser, lip, aeraA, hly, alt, eprCAI, act, LuxS, ahp and fla) were screened using PCR assays. The results obtained from the analysis of the amplified genes from YFI-C1 are displayed in Figure 5. Ten virulence-related genes (nuc, ser, lip, aeraA, hly, alt, eprCAI, act, LuxS, fla) were detected in strain YFI-C1; however, the remaining two genes (ascV, ahp) were not detected.
3.6. Biochemical characterization of strain YFI-C1
YFI-C1 was subjected to biochemical tests using a biochemical analyzer (Table 2). The results indicated that YFI-C1 was positive for Dextrin, Acidic PH PH6, N-Acetyl-D-Glucosamine, D-Salicin, 1% NaCl, α-D-Glucose, β-Methyl-D-Glucoside, D-Mannose, L-Aspartic Acid, N-Acetyl-D-Galactosamine, D-Fuctose, D-Galactose, Niaproof 4, Gelatin, L-Serine, Guanidine HCl, L-Glutamic Acid, D-Galactonic Acid, Vancomycin, Methyl Pyruvate, D-Lactic Acid Methyl Ester, Lactic Acid, Nalidixic Acid, Tween 40 reaction, Tetrazolium Blue, L-Malic Acid, α-Hydroxy- Butyric-Acid, and Acetoacetic Acid, but was negative for D-Saccharic Acid, Citric Acid, Quinic Acid, Mucic Acid, D-Malic Acid, P-Hydroxy-Phenylacetic Acid, L-Pyroglutamic Acid, Fusidic Acid, 4% NaCl, 8% NaCl, Tetrazolium Violet, D-Glucuronic Acid, Potassium Tellurite, Formic Acid, Propionic Acid, and Sodium Bromate. Based on the morphological and biochemical analyses, isolate YFI-C1 has been tentatively identified as A. hydrophila.
3.7. Antibiotic susceptibility test
The results of the antibiotic susceptibility tests conducted on strain YFI-C1 against ten different antibiotics are presented in Table 3. Strain YFI-C1 demonstrated sensitivity to six antibiotics, namely Ciprofloxacin, Trimethoprim-sulfamethoxazole, Doxycycline, Enrofloxacin, Compound Sulfamethoxazoles, Florfenicol. Conversely, it exhibited resistance to Cefothiophene and displayed only intermediate resistance to Gentamicin, Neomycin, Amikacin.
3.8. Experimental infections and the isolated strain’s pathogenicity
An infection assay was conducted on healthy rice-field eel to confirm the pathogenicity of isolate YFI-C1. The fish exhibiting signs of challenge were exposed to a bacterial solution at a level of 1 × 107 CFU/mL, exhibited a mortality rate of 70% within seven days post-challenge. The calculated LD50 of A. hydrophila YFI-C1 was 1 × 106 CFU/mL (Figure 6). Affected fish had a red and enlarged anus, slowed swimming, and decreased food intake during the early stages of illness. The symptoms associated with A. hydrophila infections were consistent with those observed in cases of bacterial septicemia in naturally infected fish. Over time, the mortality rate among the fish increased significantly, and pronounced bleeding symptoms became evident on the surface of diseased individuals. Reisolating the bacteria from the sick fish proved successful; these isolates exhibited identical morphological and biochemical characteristics as those seen in YFI-C1. In contrast, the control group demonstrated normal feeding behavior and activity levels, with no signs of disease or mortality observed.
Discussion
A. hydrophila was first isolated from infected frogs by Sanarelli in 1891. This bacteria is the main pathogen affecting a variety of aquatic species and is extensively dispersed over different natural water bodies.29 In addition, it has the capacity to infect individuals who have both a strong immune system and those with compromised immune systems alike, this can lead to a variety of systemic illnesses and gastroenteritis. This poses a risk for workers and farmers who have direct contact with diseased fish.30,31 Consequently, it has become a prominent disease linked to comorbidity between humans, animals, and fish.32 A. hydrophila has been found in Nile tilapia in recent years.33, Siberian sturgeon (Acipenser baerii).34, Basa catfish (P. bocourti).35, and rainbow trout (Oncorhynchus mykiss).36 In our study, the diseased rice-field eels exhibited surface bleeding, skin ulcers, increased mucus production, and red, swollen anuses. Upon dissection of the rice-field eels, the presence of ascites within the abdominal cavity, in conjunction with hepatomegaly, was observed; in more severe cases, liver necrosis was noted, along with renal congestion and renal papillary dilatation. By using purifying culture techniques, biochemical characterization, and investigation of 16S rRNA and gyrB gene sequences, the causal agent was determined to be A. hydrophila.
The 16S rRNA gene has been considered a reliable and precise indicator for the identification of bacterial genera for a considerable period.37 Nevertheless, the existence of microheterogeneities, in conjunction with the elevated sequence similarity observed among closely related species, renders this gene inadequate for the purpose of distinguishing between Aeromonas spp.38 In contrast, it has been shown that gyrB gene phylogenetic analysis is a useful method for identifying closely related species, making it more appropriate for differentiating species within the genus Aeromonas.39 In instances where 16S rRNA sequencing is employed to identify Aeromonas, re-sequencing of the gyrB gene can provide additional sequence information that complements the 16S rRNA data. This combined approach facilitates the construction of a phylogenetic tree that allows for more precise identification of Aeromonas at the species level.17 Consequently, in the present study, both the 16S rRNA and gyrB genes were utilized for the purpose of identification. Furthermore, both the pathogen and A. hydrophila clustered together, according to phylogenetic analysis. Based on morphological characteristics observed in conjunction with the 16S rRNA and gyrB gene sequence analysis, YFI-C1 was identified as A. hydrophila.
Our isolate’s pathogenicity toward healthy rice-field eels was validated by the outcomes of our experimental challenge test. Additionally, the pathogenicity assessment revealed that the LD50 was 1 × 106 CFU/mL per fish weight. In this work, rice-field eels infected with A. hydrophila isolate YFI-C1 showed severe bleeding in multiple body sections and internal organ destruction. Aeromonas spp. infections were also characterized by histological changes in the internal organs of rice-field eels. In the liver, the alterations were characterised by focal hepatocyte necrosis, the rise in the quantity of cells exhibiting inflammatory characteristics, and elevated levels of hemosiderin within the liver.40–43 Significant necrosis of the splenic surface was noted as well, corroborating findings from other authors.44 Necrosis affecting renal glomerular and tubular epithelia was also observed, along with inflammatory infiltration within the kidney parenchyma. The renal tubules exhibited atrophy and deformation, filled with a substantial number of inflammatory cells; many cells displayed signs of constriction and nuclear lysis. Some hepatocytes in diseased rice-field eels underwent disintegration, infiltration of inflammatory cells within the sinusoids. These signs and symptoms are typical of fish infected with A. hydrophila.
The fisheries and aquaculture sector is crucial in developing nations, as it bolsters local economies and offers nutritious food options. However, bacterial infectious diseases significantly diminish the economic value of fish.45 A. hydrophila stands out as the primary pathogen behind hemorrhagic disease in rice-field eel farming, causing significant setbacks in aquaculture due to elevated rates of illness and death among the rice-field eels.46–48 Therefore, Identifying the pathogen’s frequency and describing its efficacy and antibiotic resistance are crucial first stages in creating efficient management plans for fish raised for food.
Previous research indicates that the pathogenicity of Aeromonas is influenced by a multitude of factors that function synergistically. Numerous known virulence factors have been found, such as outer membrane proteins, quorum sensing systems, secretion systems, ironion acquisition systems, toxins, proteases, and motility-related factors, among others.49,50 As of now, studies have shown a link between the presence of particular virulence genes in Aeromonas strains and their ability to be pathogenic as well as hemolytic and cytotoxic.51,52 It is evident that these virulence factors play a pivotal role in the progression of diseases. In our study, YFI-C1 was tested for 12 virulence genes (ascV, nuc, ser, lip, aeraA, hly, alt, eprCAI, act, LuxS, ahp and fla) by the PCR assay. It had been found that the A. hydrophila isolate YFI-C1 had cytotoxic enterotoxin, lipase, serine proteinase, hae-molysin-aerolysin, hemolysin, temperature-sensitive protease, cytolytic enterotoxin, a quorum sensing-controlled virulence, flagellin and nuclease. Infections are greatly influenced by these virulence factors.53 The pathogenicity of Aeromonas isolates may be influenced by differences in the relative position of possible virulence genes.54 The study by Sha et al.12 revealed complex interactions between various enterotoxins in Aeromonas species. Their findings indicated that different combinations of these enterotoxins observed across bacterial isolates may modulate the expression levels of specific enterotoxin genes, thereby potentially influencing the clinical severity of diarrheal symptoms. Abrami et al.55 revealed that lip and ser, two virulence factors, work together to contribute to the overall pathogenicity of aeromonad infections.56 Furthermore, the concurrent presence of the ser gene alongside aeraA amplifies pathogenic potential due to aerolysin activation by a serine protease. In a word, the multiple virulence genes identified in isolate YFI-C1 suggest that synergistic interactions among these genetic components may play an essential role in its pathogenic capabilities.
The aquaculture business and public health are becoming increasingly concerned about the emergence of antibiotic resistance in aquaculture. In this study, strain YFI-C1 exhibited sensitivity to six antibiotics, including Ciprofloxacin, Trimethoprim-sulfamethoxazole, Doxycycline, Enrofloxacin, Compound Sulfamethoxazoles, and Florfenicol. However, it demonstrated resistance to Cefothiophene and only intermediate resistance to Gentamicin, Neomycin, Amikacin. Similar findings have been reported in previously published research. For instance, It was discovered that the A. hydrophila isolated from the highly endangered Acipenser baerii was susceptible to Enrofloxacin, Florfenicol, Sulfamethoxazole, Norfloxacin, Gentamicin, Streptomycin, Nitrofurantoi34; A. hydrophila isolated from Basa (P. bocourti)35 was resistant to two antibiotics, Ampicillin and Penicillin, and susceptible to eight antibiotics, including Cefazolin, Sulfamethoxazole, Norfloxacin, Amikacin, Chloramphenicol, Ciprofloxacin, Gentamicin, and Erythromycin. A research study examined the occurrence and related death rates, as well as the patterns of antibiotic resistance of A. hydrophila isolates discovered in Nile tilapia from four different aquaculture farms located in different parts of Egypt. In addition, the isolates’ characteristics of antibiotic resistance were investigated. The study revealed that every isolate demonstrated resistance to Ampicillin and Amoxicillin. However, the lowest levels of resistance were recorded for Doxycycline and Gentamicin. Additionally, resistance was also identified for other antimicrobial agents, including Chloramphenicol, Streptomycin, Erythromycin, Colistin, and Trimethoprim-sulfamethoxazole, all the isolates that were subjected to testing were susceptible to ciprofloxacin, with a 100% response rate.57 Thus, it can be inferred that slight differences among studies may be attributed to variations in geographical or host environments. In this study, A. hydrophila YFI-C1 exhibited the highest sensitivity to Florfenicol, suggesting that Florfenicol may be the preferred therapeutic agent for managing this hemorrhagic disease in rice-field eels. Moreover, given that Aeromonas is also harmful to people and can cause diarrheal infections with a comparatively low infectious dose, future health risks could be significantly increased by the presence of pathogenicity factors and relative resistance to Cefothiophene.30 Consequently, more thorough epidemiological research on A. hydrophila is desperately needed.
Conclusion
In the current research, an isolate of A. hydrophila was identified from diseased rice-field eels and designated as YFI-C1. The characteristics of this isolate indicate a significant level of virulence, leading to acute infections in fish. Furthermore, the virulence of this strain was assessed through challenge tests, analysis of virulence-related genes, and histopathological examinations following injection. Additionally, potential treatments for infections brought on by A. hydrophila were provided by the findings of antibiotic susceptibility testing. Consequently, this study lays a foundational framework for the management A. hydrophila infections and serves as a valuable reference for future research.
Acknowledgments
This work was supported by the National Key R&D Program of China (grant number 2023YFD2400705), the Fourth Modern Agricultural Industry Technology System of Hubei Province (grant number 2023HBSTX4-05), the National Natural Science Foundation of China (grant number 32060824) and the Central Public-interest Scientific Institution Basal Research Fund, CAFS (grant number 2023TD46).
Authors’ Contribution
Conceptualization: Jingwen Jiang (Equal), Yong Zhou (Equal), Qiwang Zhong (Equal), Yuding Fan (Equal). Data curation: Jingwen Jiang (Equal), Yisha Liu (Equal), Mengmeng Li (Equal). Formal Analysis: Jingwen Jiang (Equal), Nan Jiang (Equal). Investigation: Jingwen Jiang (Equal). Writing – original draft: Jingwen Jiang (Lead). Software: Mingyang Xue (Equal), Wenzhi Liu (Equal). Funding acquisition: Yong Zhou (Equal), Qiwang Zhong (Equal), Yuding Fan (Equal). Writing – review & editing: Yong Zhou (Equal), Qiwang Zhong (Equal), Yuding Fan (Equal). Supervision: Yong Zhou (Equal), Qiwang Zhong (Equal), Yuding Fan (Equal).
Competing of Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
All animal experiments were approved by the Animal Experimental Ethical Inspection of Laboratory Animal Centre, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences (ID Number: YFI 2024-fanyuding-2207).
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.


.png)




.png)