1. Introduction
In China, the total aquaculture area has increased from 2.86 million hectares in 1978 to 7.11 million hectares in 2022. Meanwhile, total aquaculture production reached from 1.23 to 55.65 million tonnes, contributing 60%-70% of the world’s total aquaculture production.1,2 The rapid expansion and intensification of aquaculture farming in recent years has led to a rise in disease-related challenges and environmental degradation. These issues pose significant constraints on the sustainable development of the aquaculture industry.3 In intensive fish farming, chemical additives and antibiotics have been widely used to control diseases,4 however, the abuse of antibiotics causes series of problems, such as the emergence of drug-resistant bacterial strains, the alteration of microbiota in aqueous environment, and antibiotic residues in aquatic products, and finally harm aquatic products safety and consumer health.5 Therefore, there is an urgent need to find alternative, safe, and effective strategies for antibiotics in aquaculture to maintain good health and high production efficiency of animals. Yeast and yeast extracts have been reported to have excellent nutritive and probiotic properties for aquatic animals and could be used as protein-rich ingredients and probiotics in aquafeeds.6,7
In marine environments, red yeasts are ubiquitous and form part of the normal microflora of seawater.8 MRY, a type of yeast and aquatic eukaryote, is widely distributed across diverse aquatic environments, including oceans, deep seas, and lakes. It has been isolated from sources such as marine sediments and the digestive tracts of aquatic animals.9–11 Marine yeasts have the ability to grow in extreme environments (i.e., low light, low nutrition, high temperature, and high salinity) compared with terrestrial yeasts and to produce valuable enzymes and secondary metabolites.12–14 It has high nutritional value and is rich in protein, amino acids, unsaturated fatty acids, vitamins, carotenoids, especially powerful antioxidant astaxanthin, and other nutrients.10,15 MRY could secrete a variety of active metabolites, such as carotenoids, β-glucans, and mannan, which have high nutritional value and biological functions in animals.7,16 Therefore, MRY has the potential to be used as an important probiotic for aquaculture applications. It has been reported that the addition of MRY to feeds could be effective in promoting growth, shell and muscle pigmentation, antioxidant competence, immunoenhancement activity, intestinal microbiota balance, and disease resistance in aquatic animals.12,15,17–19
White-spotted conger (Conger myriaster) is a valuable farmed marine fish; it is naturally distributed from the East China Sea to the waters of Korea and Japan.20 This species presents great potential for fish farming because of its delicious meat, high nutritional value, and market value. Up to now, studies for the feeding habits and ecology, biological characteristics, early life history characteristics, genetic homogeneity and culture technique of this species have been reported.21 However, studies on the nutritional requirements of Conger myriaster are limited, and there are no commercially available feeds specifically formulated for Conger myriaster. Marine fish feed and iced-fresh fish are generally used when this fish is reared under farm conditions, which limits the development of this species reared in aquaculture. Therefore, the aim of this study was to evaluate the effect of dietary MRY supplementation on growth, digestive enzyme activities, antioxidant capacity, immunological indices and expression of genes in Conger myriaster.
2. Materials and methods
2.1. Preparation of MRY
The MRY strain used in the present study was presented by the Key Laboratory of Marine Bio-active Substances, SOA, China, and maintained in the Microbiology Laboratory of the Department of Marine Technology, Rizhao Polytechnic, China. In preparing the diets, 10% pure clones of MRY were inoculated in a 100-ml Erlenmeyer flask with YPD broth in a shaken incubator (140 rpm) at 30°C for 96 h. The bacteria were harvested by centrifugation (5,000 rpm for 15 min at 4°C) which was later washed twice with phosphate-buffered saline at pH 5. Cell pellets were resuspended, and the optical density (OD) was measured at 560 nm. The nutritional composition of MRY was crude protein (48.1%), crude lipid (23.58%), crude ash (14.89%), and carotenoid (84.5 μg/g).
2.2. Experimental Diets
In this trial, the control diet was designed with white fish meal, fermented fish processing wastes, yeast powder, wheat meal, fish oil, vitamin premix, and mineral premix. Four isonitrogenous (crude protein, 45%) and is lipidic (crude lipid, 10%) experimental diets were formulated to contain 0%, 0.5%, 1.0%, and 1.5% MRY, which were coded as MRY0, MRY0.5, MRY1, and MRY1.5, respectively. All the ingredients were ground into fine powder through a 320-μm mesh. All the ingredients for each experimental diet were fully mixed with oil. Then, all diets were stored at −20°C until used. The composition and chemical analysis of the experimental diets are shown in Table 1.
2.3. Fish and feeding trial
The present study was approved by the Animal Care and Use Committee of Rizhao Polytechnic. All animal procedures were performed according to the Guideline for the Care and Use of Laboratory Animals in China.
Juveniles Conger myriaster were purchased from Shandong Rongsense Group (Rizhao, China), and reared in Rizhao Yuhai Hongqi Ocean Engineering Co., LTD (Rizhao, Shandong, China). Before the feeding trial, fish were raised in cement pools (25 m3) and fed the basal diet for 2 weeks to acclimate to the experimental conditions. A total of 180 fish (average initial body weight, 46.27 g) were randomly distributed into 12 net cages (1 m × 0.7 m × 1 m). Each diet was randomly assigned to triplicate cages, and each cage had 15 fish. Before feeding, water was added to diet (v/w =1:1) to produce dough, then the fish were hand-fed to apparent satiation twice times daily (7:00 and 17:00). During the feeding trial, the flow rate of water was maintained at 3.2 L min-1, temperature was 25°C~26.6°C, salinity was 28‰~29‰, pH was 7.14~7.54, dissolved oxygen level > 6 mg/L, nitrite-N was 0.11~0.15 mg/L, ammonia-N was 0.29~0.35 mg/L, the light intensity was poor light. The feeding duration was 56 days.
2.3. Sample collection
At the end of the feeding trial, fish were starved for 24 h before sampling. Firstly, fish in each cage were captured and anesthetized with tricaine methanesulfonate (200 mg L−1), and then the weight and survival of fish in each cage were measured and recorded. Five fish in each cage were randomly collected for the collection of the serum, intestine, liver and muscle samples. Blood was collected from caudal vein, and clot at 4°C for 4 h, then centrifuged at 4000 rpm at 4°C for 15 min, serum was extracted and kept at −80°C until used for analysis. Intestine, liver, and muscle samples were dissected and frozen at −80°C until further analysis.
2.4. Sample analysis
2.4.1. Digestive enzyme activity
Intestines of fish (n = 3) in each cage were used for digestive enzyme activity. The intestines were homogenized in ice-cold aseptic normal saline (0.86%, w/v =1/9) using a tissue homogenizer. Then, the homogenates were centrifuged at 3500 rpm for 15 min at 4°C, the supernatants were stored at −80°C for further analysis. The activities of trypsin, lipase, and amylase were analyzed using an assay kit (A080-2-2, A080-2-2, C016-1-2; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instruction.
2.4.2. Serum parameters assays
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities, total protein (TP), glucose (Glu), cholesterol (TC) and triglyceride (TG) content were determined using an automated biochemistry analyzer (BS-400, Mindray Bio Medical Co., Ltd., China). Serum acid phosphatase (ACP), alkaline phosphatase (ALP), nitric oxide synthase (NOS) activities, and lysozyme (LZM) content were analyzed by assay kits (A060-2-1, A059-2-2, A001-3-2, A050-1-1; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions.
2.4.3. Antioxidant enzymes analysis
The liver was homogenized in ice-cold sterile saline solution (0.86%, w/v =1/9). After that, the homogenates were centrifuged at 3000 rpm for 10 min at 4°C, then the supernatants were kept at −80°C for antioxidant enzymes analysis. Total antioxidant capacity (T-AOC), Catalase (CAT), total superoxide dismutase (T-SOD) and glutathione peroxidase (GSH-Px) activities, malondialdehyde (MDA) content were analyzed using assay kits (A015-2-1, A007-2-1, A001-3-2, A005-1-2, A003-1-2; Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and the detection methods were performed according to the instructions.
2.4.4. Gene expression
The mRNA levels of related genes were determined by qPCR. The extraction of total RNA from the liver and intestine was carried out using MiniBEST Universal RNA Extraction Kit (TaKaRa, Japan) according to the product manual, and the RNA concentration and quality were measured by 1.2% denaturing agarose gel (OD260/280=1.8-2.0). Subsequently, cDNA was synthesized using PrimeSciptTM 1st Strand cDNA Synthesis Kit (TaKaRa, Japan), then kept at −20°C for qPCR analysis. The primers are shown in Table 2.
The qPCR analysis of genes (HSP70, HSP90) was done with SYBR Prime Script TM RT-PCR Kit (TaKaRa, Japan) using a CFX96 real-time PCR detection system thermocycler (Bio-Rad) following the product manual. In this study, β-actin was used as internal references for normalization. The total volume of the PCR reactions was 20μL and consisted of: 10μL SYBR Green Premix Ex TaqII (2 ×), 1μL primer of each, 2μL cDNA, and 6μL distilled/deionized H2O. The RT-PCR initiated with a denaturation step at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, 58°C for 10 s. The target gene expression results were calculated using the 2−ΔΔCT method as described by Schmittgen and Livak.23
2.5. Statistical analysis
All data were subjected to one-way ANOVA after homogeneity in variance was tested using SPSS Statistics 23 (IBM, SPSS Inc., USA). If a significant difference was found (P<0.05), Duncan’s multiple range test was used to compare the mean means between individual treatment. The significant differences were found between individual treatment at P<0.05. The results were presented as means ± S.D.
3. Results
3.1. Growth performance and somatic indices
The growth performance, feed utilization and somatic indices of fish are shown in Table 3. No significant differences were found in the survival, weight gain (WG), specific growth rate (SGR), final body weight (FBW), hepatosomatic index (HSI), and viscerosomatic index (VSI) among fish-fed diets with different MRY levels (P>0.05). The fish fed diet MRY1 had significant higher condition factor (CF) than that fed diet MRY1.5, and no significant differences were found in the MRY0, MRY0.5 and MRY1 groups (P> 0.05).
3.2. Digestive enzyme activity
As shown in Table 4, no statistical differences were detected for trypsin, lipase and α-amylase activities among treatments (P>0.05).
3.3. Serum biochemical parameters
The serum biochemical parameters are shown in Table 5. Fish fed diets MRY1 and MRY1.5 had significantly lower ALT and AST activities than those fed diets MRY0 and MRY0.5 (P<0.05). Glu content showed an increased trend with increasing MRY level, and fish fed diet MRY1.5 had significant higher value than those fed diets MRY0 and MRY0.5 (P<0.05). TC and TG content exhibited a decreased trend with increasing MRY levels and fish fed diet MRY1 had the lowest value (P< 0.05).
As shown in Table 5, fish fed diets MRY1 and MRY1.5 had significantly higher serum NOS activity and TP content than fish fed diet MRY0 (P<0.05), and no significant difference in the MRY0.5, MRY1 and MRY1.5 groups (P> 0.05). Fish fed diets MRY1 and MRY1.5 had higher LZM values than that fed diets MRY0 and MRY0.5 (P<0.05), and there were no statistical differences between MRY1 and MRY1.5 groups, MRY0 and MRY0.5 groups (P>0.05). Fish fed diet MRY1 had higher ALP activity than fish fed diet MRY0 (P<0.05), and fish fed diet MRY1.5 had significantly higher ACP activity than fish fed diet MRY0 (P< 0.05), and no significant differences among the MRY0.5, MRY1 and MRY1.5 groups (P>0.05).
3.4. Antioxidant enzymes activity
The hepatic antioxidant enzyme activities are shown in Table 6. There were no significant differences in hepatic T-AOC, CAT activities and MDA content of fish fed different groups (P>0.05). Fish fed diet MRY0.5 had significantly higher GSH-Px activity than that fed diets MRY0 and MRY1.5 (P<0.05), but similar to that fed diet MRY1(P>0.05). T-SOD activity increased with increasing dietary MRY level, and fish fed diet MRY1.5 had significantly higher T-SOD activity than that fed diet MRY0 (P<0.05), and no significant differences in the MRY0.5, MRY1 and MRY1.5 groups (P> 0.05).
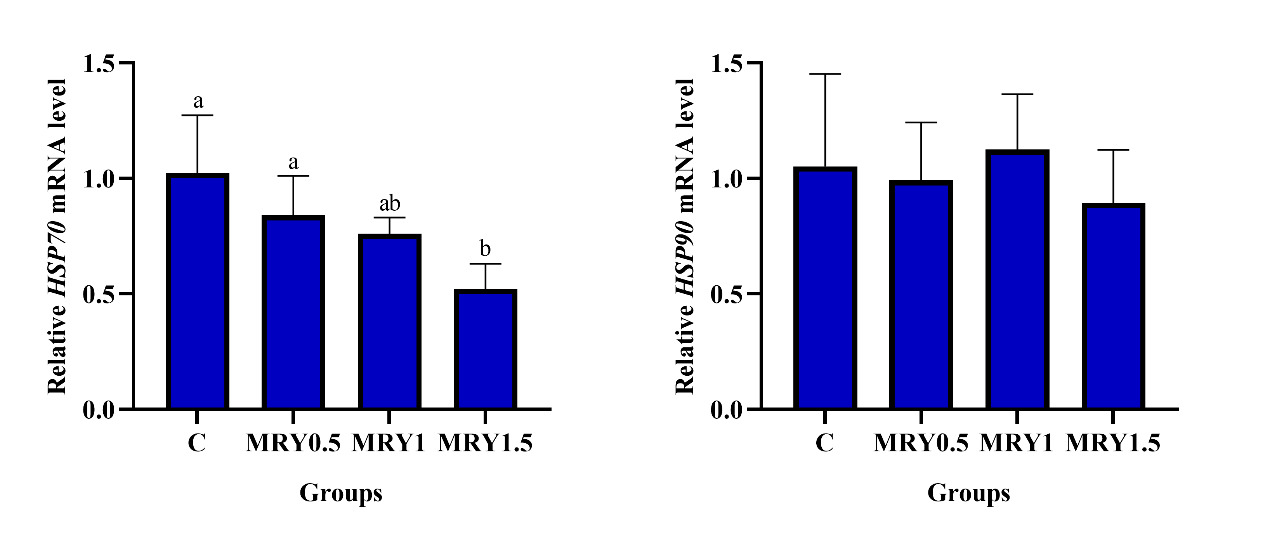
3.5. Gene expression levels of HSP70 and HSP90
As shown in Fig. 1, the gene HSP70 expression level in the liver of Conger myriaster showed a decreased trend following the supplementation with MRY. Fish fed diet MRY1.5 exhibited significantly lower relative expression of HSP70 in the liver than fish fed diets MRY0 and MRY0.5 (P<0.05), there was no significant difference was observed among MRY0, MRY0.5, and MRY1 groups (P>0.05) (Fig. 1). No significant difference was found in the relative expression of HSP90 in liver among all groups (P>0.05) (Fig. 1).
4. Discussion
4.1. Growth performance
MRY has the ability to secrete various active metabolites, such as β-carotene, astaxanthin, polysaccharides, and essential amino acids, making it highly biologically functional and nutritionally valuable for animals, and could be used as an effective functional probiotic.16 The positive effect of dietary supplementation of MRY on growth of sea cucumber (Apostichopus japonicus),15 Pacific white shrimp (Litopenaeus vannamei),12,18 tilapia (Oreochromis niloticus),19 golden pompano (Trachinotus ovatus)24 and hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus ♂)25 have been reported in previous studies. This suggests that the yeasts are rich in protein, carbohydrates, unsaturated fatty acids, B-group vitamins, minerals and enzymes, and other bioactive components, which could be used as nutritional and functional supplements for animals.6 Studies on rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar) have shown that the using of yeast-derived β-glucan and mannan-oligosaccharides in feeds could positively influence growth.26,27 Meanwhile, marine yeast strains colonize the gut of aquatic animals, producing a variety of growth factors, amino acids, vitamins, and digestive enzymes, enhancing the digestion and absorption of diet.6 However, the growth of Conger myriaster was not affected by dietary MRY supplementation in the present study. This result was consistent with other studies through adding yeast culture,28 yeast hydrolysate29 to the diet of largemouth bass and astaxanthin rich red yeast rainbow trout.9 One reason is that exogenous bacteria might consume partial dietary protein/amino acids additional of the host.30 Additionally, this may be due to the short term of feeding, and further study should focus on whether the long-term feeding duration induces the growth-promoting effects of MRY for this fish. On the contrary, Liu et al.19 found that the excessive addition of MRY did not further improve the growth of juvenile tilapia. This could be attributed to the fact that the presence of β-glucan in excessive levels of red yeast could negatively impact the feed utilization and growth of fish.31,32 Moreover, the competition among the resident bacterial flora of the intestine and exogenous MRY may lead to low MRY colonization in the digestive tract of fish, leading to a decrease in extracellular enzyme secretion, poor digestibility, and growth. Additionally, the excessive addition of red yeast in feed may disrupt the dynamic balance of microbial species and quantities in the digestive tract of fish, leading to inhibition of the digestive enzyme activity of fish, thereby reducing their ability to effectively digest and absorb nutrients. Finally, this inhibition negatively impacted the growth.33 The differences in growth in these studies could be due to a variety of factors, such as marine yeast species, dose and forms, fish species, diet formula, intestinal microflora, physiology and architecture of gastrointestinal tract, temperature, salinity, and feeding duration.
4.2. Digestive enzyme activity
Digestive enzymes play a vital role in the digestive capacity of food, and feed digestibility could be improved by increasing the digestive capacity of animal.34 It was found that compared to the control group, dietary addition of R. benthica D30 at 105, 106 and 107 CFU/g feed significantly increased amylase activity, cellulase activity and alginase activity of sea cucumber.15 Similar findings were also observed in white shrimp,12 tilapia,19 hybrid grouper25 and red swamp crayfish (Procambarus clarkii).35 The increased digestive enzyme activities may be due to the capacity of MRY to successfully colonize in the intestine, secrete diverse digestive enzymes, and enhance enzymatic activity.15 Moreover, MRY could enhance the ecological equilibrium of intestinal flora, increase the population of beneficial bacteria, and suppress the growth of harmful bacteria, thus leading to facilitated growth and elevating digestive enzyme activities.36 Additionally, it could enhance the maturation of the digestive system in aquatic animals and stimulate intestinal metabolism to improve overall digestive functions, leading to an increase in the synthesis of digestive enzymes.37 However, in this study, trypsin, α-amylase, and lipase activities were not significantly affected by dietary MRY supplementation, which indicated that no changes in digestive function were observed when fish fed MRY. This was in accord with the finding of Dai30 who found that the supplementation of Rhodotorula glutinis in diet of Litopenaeus vannamei had no obvious effects on improving digestive enzyme activity. However, Chi et al. found significantly lower amylase and trypsin activities in shrimp hepatopancreas when fed with yeast hydrolyzate.38 The digestibility of yeast products in aquatic animals depends on the type of yeast and the processing technique. Such processing techniques aim to disrupt the yeast’s rigid cell wall by inducing autolysis using heat, pressure, or enzymes, thereby increasing digestibility.39,40
4.3. Serum biochemical indices
Serum biochemical parameters have proven to be an important indicator for assessing the nutritional, metabolic, and health status of aquatic animals.41 ALT and AST levels are used as common indexes for the diagnosis of liver function, and higher ALT and AST activities indicate tissue damage and dysfunction.9,42 In the present study, compared to the MRY0 group, fish-fed diets with MRY decreased serum AST and ALT activities. Higher serum ALT and AST activities and lipid peroxides (LPO) levels caused by fish fed oxidized oil were considerably decreased by the use of 10.87 g/kg red yeast in rainbow trout feed.9 Similar results have been reported in red swamp crayfish fed yeast hydrolysate,35 hybrid grouper, and yellow catfish (Pelteobagurs fulvidraco) fed yeast culture.25,43 This result indicated that yeasts have the ability to ameliorate the liver function of fish. Serum TG and TC levels are essential indices of cardiovascular disorder and potential treatment targets.44 In our study, serum TG and TC levels decreased with increasing MRY levels, and this can be interpreted as the beneficial effect of MRY. Similarly, rainbow trout fed diet with oxidized oil had significantly higher serum TG, TC, and phospholipids levels, and the dietary administration of red yeast could significantly decrease these parameters levels; the serum lipid content-lowering effect of red yeast is attributed to its antioxidative action, which normalizes the malfunction in the lipid metabolisms of the liver.9
The innate immune system is the first line of host defense in pathogenic organisms. Serum TP, ACP, ALP, lysozyme, NOS, and other immune parameters could prevent microbial adherence and colonization, resulting in decreased infection and diseases, these are important parameters of cell injury and are used to evaluate the non-specific immune capacity of fish.45,46 In this study, we found that fish fed diets MRY1 and MRY1.5 had significantly higher serum TP, ACP, ALP, lysozyme, and NOS level than fish fed diet MRY0, and this indicates that the use of MRY could be more effective in stimulating the antibacterial immune responses and better resistance against pathogens. Similarly, Wang et al.18 also found that ACP, ALP, LYZ, and T-NOS activities in the serum of shrimp were significantly higher in R. mucilaginosa JM-01 in different forms groups compared to the control group. Different MRY levels significantly increased the lysozyme and ALP activities, IgM and complement protein 3 contents in tilapia serum.19 Sarlin and Philip17 found that marine yeasts Debaryomyces hansenii and Candida tropicalis had immunostimulatory effects, provided better protection to Fenneropenaeus indicus against white spot syndrome virus, and performed better than the baker’s yeast. Similar results were reported in sea cucumber,15 golden pompano,24 Pacific red snapper (Lutjanus peru),13 L. vannamei,47 and hybrid grouper.25 The use of yeast has multifaceted benefits might be due in part to its chemical composition and biochemical functions, such as yeast wall polysaccharides.13,48,49 Fish with yeasts administration could enhance the immune system of the host by increasing the humoral immune system or by the interaction with immune cells.50
4.4 Antioxidant enzymes
Excessive production of reactive oxygen species (ROS) would induce cell malfunctions, cell membrane oxidation, DNA damage, and so on. The antioxidant system of fish is formed by antioxidant enzymes, such as GSH-Px, SOD, and CAT, and regulates the production and elimination of ROS.51 The antioxidant enzymes are used as the biomarkers of antioxidant capacity and oxidative stress of aquatic animals.51,52 SOD provides cellular defense against ROS by catalyzing the dismutation of O2− to O2 and H2O2. This reaction played a key role in protecting cells against the oxidative damage.53 In the present study, fish fed diet with 1% MRY had higher hepatic GSH-Px and T-SOD activities than those fed diet MRY0, and this indicated that the addition of MRY could boost the antioxidant capacity of Conger myriaster. Similarly, Liu et al. also observed that tilapia fed diets with 0.25%-1.0% MRY had higher serum and hepatic SOD, CAT, GSH-Px and T-AOC activities, lower MDA content.19 Fish fed diets with 0.2%-0.4% Rhodotorula mucilaginosa had significantly higher hepatic SOD activity and lower MDA content.24 Dietary addition of 105 and 106 CFU/g Rhodotorula benthica D30 increased the SOD activity in Apostichopus japonicus, while SOD decreased when the level enhanced to 107 CFU/g; this could be explained by higher amount of supplementation induced immune fatigue of animal.15 The antioxidant capacities were improved in the serum, hepatopancreases and muscle of Litopenaeus vannamei by the administration of 1% dry yeast or 108 live yeast cells g−1 diet12 and 1010 cfu/kg Rhodotorula mucilaginosa strain.18 These results indicated that the MRY might regulate the ROS intermediate system and activate the antioxidation system in fish to eliminate excess free radicals efficiently, resulting in reduced cell damage and improved antioxidant abilities.54 Carotenoids act as an antioxidant that protects the cell membrane phospholipid and other lipid components by removing singlet oxygen.16
4.5. Genes expression
Heat-shock proteins (HSPs) are a family of highly conserved cellular that are produced in almost all organisms, and play an important role in protein folding, transport, and expression.55 HSPs could mitigate oxidative damage by scavenging reactive oxygen species, and induced very strong humoral and cellular immune responses.56 HSP70 is usually used as one of the biomarkers of stress response and has powerful immune regulatory effects.57 HSP90 activates natural killer cells and dendritic cells via Toll-like receptors, thus acting as a potent inducer of both the innate and adaptive immune responses.58 HSP70 and HSP90 function as important regulators in viral infection, including driving viral entry into cells, enhancing viral replication and gene expression, promoting the folding and assembly of viral proteins, as well as regulating apoptosis and host immunity.59 Generally, the HSP70 gene expression level was down-regulated in fish fed probiotics/prebiotics and increased fish welfare, indicating the increased anti-stress response.60–62 In this study, the expression levels of HSP70 mRNA in fish fed diet MRY1.5 was significantly lower than that of the MRY0 and MRY0.5 groups, and no significant difference was found in the relative expression of HSP90 in the liver among all groups. Similarly, Huyben et al.63 reported that no significant effects were found for the expression of HSP in the intestine of fish fed diet with 21.4% live yeast, but resulted in a decreased tendency in HSP90 expression, and indicates the beneficial effects of yeast as HSP90 is associated with nutrient transport and cellular repair.64 Tilapia fed diet with 0.1% live yeast significantly decreased the intestinal HSP70 expression under crowding conditions, while heat-inactivated yeast did not decrease HSP70 expression, suggesting that live yeast supplementation alleviated the cellular stress response in intestine.65 However, some results indicated that the marine yeast strains or extract may not affect66 or upregulate54,67 the expression of HSPs in fish. Liu et al.61 found that HSP70 expression in the intestine was significantly upregulated at days 10 and 35 but significantly downregulated at day 20. The changes in intestinal HSP70 expression were coincident with differences in the similarity coefficient of the intestinal autochthonous bacterial communities between dietary treatments and the initial control.61
5. Conclusion
In conclusion, dietary supplementation with MRY showed no positive influence on the growth of Conger myriaster under the experimental conditions, whereas it could increase the health status by elevating immune responses and antioxidant capacity. The present study indicates that 1%–1.5% dietary MRY could be a potential feed additive to enhance fish immunity. Further studies on the intestinal microbiota composition of Conger myriaster fed MRY should be elucidated to ascertain what led to the changes in immune responses.
Acknowledgments
This study was financially supported by the Foundation Program of Rizhao Excellent Youth Science (RZ2021ZR15) and Rizhao Youth Science (RZ2022ZR55). The authors extend their sincerest appreciation to Xiang Guo and Yingming Zhang for their assistance in this experiment.
Authorship contribution statement
Writing – review & editing: Yuyu Wang (Equal), Meiling An (Equal). Software: Yuyu Wang (Lead). Data curation: Yuyu Wang (Equal), Jianjun Guo (Equal), Fuhai Teng (Equal). Project administration: Meiling An (Equal), Yuanyuan Li (Equal), Junxia Wang (Equal), Ming Hu (Equal). Formal Analysis: Hengxia Shen (Equal), Tong Chen (Equal), Yanjun Dong (Equal). Resources: Yuanyuan Li (Equal), Fuhai Teng (Equal), Ming Hu (Equal). Supervision: Yuanyuan Li (Equal), Junxia Wang (Equal), Ming Hu (Equal). Investigation: Jianjun Guo (Equal), Fuhai Teng (Equal). Methodology: Junxia Wang (Equal).
Yuyu Wang: Writing-review & editing, Software, Data curation. Meiling An: Writing-review & editing, Project administration. Tong Chen, Hengxia Shen and Yanjun Dong: Analyzed the samples and data. Jianjun Guo: Investigation, Data curation. Fuhai Teng: Resources, Data Curation, Investigation. Yuanyuan Li and Ming Hu: Resources, Project administration, supervision. Junxia Wang: Methodology, Project Administration, Supervision.
Conflicts of interest
The authors have no conflict of interest to declare.
Data Availability Statement
All data included in this study are available upon request by contact with the corresponding author.