1. Introduction
Babylonia areolata (commonly known as the Spotted Babylon Snail) is an economically valuable marine benthic gastropod belonging to the phylum Mollusca, class Gastropoda, order Neogastropoda, family Buccinidae. This species is predominantly distributed across Southeast Asia, Japan, and the coastal regions of southeastern China.1,2 Characterized by distinctive square-shaped patterns on its shell, B. areolata has gained significant consumer preference due to its tender texture and delicate flavor profile, establishing it as one of the most promising aquaculture species in the 21st century.3 Recent years have witnessed substantial expansion in its commercial cultivation, driven by technological advancements in aquaculture practices and growing market demands.4
Substrate selection constitutes a critical determinant in maintaining optimal benthic habitats for aquatic organisms in aquaculture systems 5. Appropriate substrate composition not only mitigates environmental stress but also enhances survival rates and overall aquaculture productivity.6 Previous investigations have demonstrated superior growth performance in certain bivalve species (e.g., Mysella vitrea, Telline deltoidalis, and Timoclea scabra) when cultivated in fine-sand substrates.7,8 V. O’Niam et al.9 reported that clay substrates in Portunus pelagicus (blue swimming crab) culture exhibited elevated organic matter content, pH levels, and ammonia concentrations compared to sandy loam or pure sand substrates. As an infaunal species, B. areolata exhibits characteristic sediment-burrowing behavior, spending >90% of its lifecycle buried in substrates except during feeding activities.10 This biological imperative renders substrate quality particularly crucial for its survival and growth.11 Comparative studies have confirmed significant survival advantages in concrete tank-cultured B. areolata populations when maintained in sand-layered environments versus bare-bottom conditions.12 Current intensive aquaculture of B. areolata predominantly utilizes fine sand substrates to accommodate their burrowing behavior.13 However, under prolonged farming cycles (>6 months) and high stocking densities (>1200 ind/m²), the substrate environment undergoes substantial deterioration: studies indicate that organic matter, total nitrogen (TN), and total phosphorus (TP) in the substrate accumulate to 7.5×, 30.3×, and 15.7× initial levels after six months of continuous cultivation,14 directly jeopardizing survival and growth.15 Nevertheless, inherent limitations of conventional fine sand substrates exacerbate systemic risks: (1) Frequent substrate cleaning or replacement may trigger abrupt water quality fluctuations, inducing physiological stress, mortality, and pathogen transmission risks during operations; (2) Fine sand particles exhibit smooth surfaces and lack porous structures, resulting in insufficient microbial colonization sites and unstable nitrifying/denitrifying functional microbial communities.16 This deficiency compromises substrate self-purification capacity, leading to persistent accumulation of toxic compounds (e.g., ammonia, nitrite) and subsequent benthic ecological imbalance. Consequently, there is an urgent demand to develop novel substrate materials that integrate physical compatibility with microbial regulation capabilities. By enhancing microbial colonization efficiency and metabolic activity, such materials could achieve in situ degradation of accumulated pollutants, thereby overcoming environmental constraints in high-density aquaculture systems.
Ceramsite, a porous sintered clay aggregate, demonstrates particular promise due to its unique physicochemical properties - including low density, high hydraulic conductivity, and adsorptive capacity - which have been successfully leveraged in ecological remediation applications.17,18 Mechanistic studies reveal that the porous structure of ceramsite supports nitrifying bacterial growth, enhancing pollutant breakdown via biofilms.19–21 The structure-function coupling mechanism of ceramsite manifests in particle-size-dependent effects: Micro-scale particles optimize contaminant adsorption via enhanced specific surface area, significantly improving water quality parameters; macro-scale particles enhance hydrodynamic flow and gas exchange, critical for sustaining benthic respiration.22 The structure-function coupling mechanism inherent to ceramsite substrates demonstrates considerable potential in aquatic stabilization through NH₃/NH₄⁺ adsorption equilibria, microbial community modulation via surface biofilm succession, and biological productivity enhancement, collectively establishing an innovative technological paradigm for benthic habitat engineering in sustainable aquaculture systems.23
Digestive and immune-related enzymatic activities serve as critical physiological biomarkers in aquatic benthic organisms, providing quantitative insights into their digestive efficiency, nutrient assimilation capacity, and immune response competence.24 In aquaculture systems, key digestive enzymes (lipase, amylase, and trypsin) are routinely monitored to evaluate metabolic performance, while immune defense mechanisms are assessed through antioxidant enzyme profiles, including glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and catalase (CAT).25,26 Mechanistically, substrate materials may exert indirect regulatory effects on enteric microbiota through multiple pathways: (1) modulating aqueous-phase nutrient dynamics, (2) maintaining substrate redox potential, and (3) shaping microbial community structure, thereby influencing enzymatic homeostasis in digestive and immune systems.27 Therefore, appropriate sediment improves water quality and enhances the growth and health of aquatic organisms by promoting digestion and boosting immunity.
This study conducted a systematic comparative aquaculture experiment utilizing five distinct substrate types: river sand, small ceramsite, medium ceramsite, large ceramsite, and quartz sand, to evaluate their impacts on B. areolata juveniles. The experimental design specifically targeted the elucidation of substrate-dependent effects on four critical parameters: water quality dynamics, growth performance metrics, digestive enzyme profiles, amylase, trypsin, and immune response biomarkers (SOD, CAT, GPx enzymatic capacities). Through this multidimensional evaluation framework, we aim to establish quantitative correlations between substrate characteristics and molluscan physiological responses, ultimately developing an optimized substrate selection matrix to enhance survival rates, growth efficiency, and disease resistance in commercial B. areolata cultivation systems.
2. Materials and Methods
2.1. Experimental design
The juvenile Babylonia areolata used in this experiment were provided by the Tropical Aquaculture Research and Development Center of the South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. The experiment was conducted at the Wenchang Fengjiawan Modern Fishery Industrial Park. Healthy juveniles with uniform size, normal burrowing behavior, and good vitality were selected as experimental subjects, exhibiting average shell height, shell width, and body weight of (10.40 ± 0.39) mm, (6.90 ± 0.27) mm, and (0.231 ± 0.020) g, respectively. Initial water quality parameters were maintained at salinity 33.00 ± 0.80 ppt, temperature 26.00 ± 1.00°C, ammonia nitrogen concentration <0.01 mg/L, nitrite concentration <0.04 mg/L, and dissolved oxygen (DO) >6.50 mg/L to ensure experimental consistency.
The experiment was conducted in a recirculating aquaculture system using culture tanks (1m×0.8m×0.5m). Four experimental groups with different substrates were established: small ceramsite (2mm), medium ceramsite (5mm), large ceramsite (8mm), and quartz sand (1-2mm), along with a control group using river sand (1-2mm). Each group contained three replicates to minimize experimental error. All substrates were uniformly layered at the tank bottom with 2.5cm thickness.
The culture system maintained continuous oxygenation and water flow (20cm water depth) using pre-settled and sand-filtered seawater. A daily water exchange rate of 300% was implemented. Fresh oyster meat was administered once daily (8:00 AM) at 5-10% of body weight. Post-feeding observations at 30 and 60 minutes monitored residual feed and recorded burrowing/wall-climbing behaviors. At the 30-min monitoring interval, residual bait was volumetrically evaluated; if remaining bait fell below 30% of the initial provision, supplementary bait was administered with precise documentation of supplementation quantities. This protocol ensured maintenance of trace residual bait upon feeding termination, thereby facilitating accurate quantification of satiation-level consumption. At the 60-min terminal phase, residual bait was collected and gravimetrically analyzed to determine total consumption. During the 2-month trial period, water parameters remained stable: temperature 28.7 ± 0.8°C, salinity 1.045 ± 0.02‰, pH 8.3 ± 0.2, and DO 5.5 ± 0.5 mg/L. Regular monitoring of water quality indicators including ammonia nitrogen, nitrite nitrogen, TN, and chemical oxygen demand (COD) was conducted throughout the experimental period.
2.2. Sample Collection and Processing
During the experimental period, 10 randomly selected B. areolata from each test group were weighed using an electronic balance. one B. areolata was taken from each of the three parallel groups, the soft tissues (foot, mantle, and hepatopancreas) were dissected on ice, collected into pre-labeled cryotubes, and homogenized with 0.2 mg/L physiological saline at a 1:2 (w/v) ratio to ensure complete tissue disruption. The homogenate was centrifuged at 5,000 × g for 10 min at 4°C to separate the supernatant from cellular debris. The resulting supernatant was aliquoted into sterile Eppendorf tubes and stored at -80°C for subsequent biochemical analyses. Enzyme activities, including peroxidase (POD), GSH-Px, lipase, CAT, and lysozyme, were quantified using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, China). All procedures strictly adhered to the manufacturer’s protocols to ensure methodological consistency and data reliability.
2.3. Statistical Analysis
Use a fixed-area sampling box to sample at three different points in each tank, and then calculate the number and the survival of snails. The daily growth rate, specific growth rate(SGR), bait coefficient and bait efficiency were all carried out according to the methods of relevant literature.28
Survival (%) = [Average number of snails in the sampling box /(Area sampling box × Density)] ×100;
SGR (%)=100×(ln Final mean body weight − ln Initial mean body weight) / No. of days;
Yield per unit area (g/m2) = Survival × Density × mean body weight / Culture area;
Feed Conversion Ratio = Total weight of bait / (Final mean body weight − Initial mean body weight);
Feeding rate (%)= (Final mean body weight − Initial mean body weight) / Total weight of bait ×100 %;
Feed conversion efficiency (%)= (ln Final mean body weight − ln Initial mean body weight) / Total weight of bait × 100 %.
Experimental data were expressed as mean ± standard deviation (Mean ± SD) and analyzed using SPSS 21.0 (IBM Corp., USA). One-way analysis of variance (ANOVA) was first performed to assess significant differences among treatments. When statistical significance (p < 0.05) was detected, Duncan’s multiple range test was applied as a post hoc analysis for pairwise comparisons.
3. Results
3.1. Effects of Substrate Types on Water Quality Parameters in B. areolata Culture
Analysis of water quality parameters under different substrate conditions (Table 1) revealed significant variations in nitrogenous waste accumulation and organic load. Ammonia nitrogen (NH4+-N) concentrations were highest in the river sand control group, while all experimental substrates—quartz sand, small ceramsite, medium ceramsite, and large ceramsite—exhibited significantly lower NH4+-N levels compared to the control (p < 0.05). For nitrite nitrogen (NO2−-N), all substrate treatments showed statistically reduced concentrations relative to the control (p < 0.05), with the small ceramsite particle group demonstrating the lowest mean value (0.24 ± 0.04 mg/L). Similar trends were observed for TN, where all experimental substrates significantly outperformed the control (p < 0.05), again with small ceramsite yielding the lowest TN concentration (0.42 ± 0.04 mg/L). COD measurements further confirmed the superiority of engineered substrates. COD levels across all experimental groups were significantly lower than those in the control (p < 0.05), with the small ceramsite particle substrate achieving the minimal COD value (5.67 ± 0.40 mg/L), indicative of enhanced organic matter degradation efficiency.
3.2. Effects of Substrate Types on Growth Performance and Survival Rate of B. areolata
Analysis of growth parameters and survival rates across substrate treatments revealed significant substrate-dependent variations after a 60-day culture period (Table 2). B. areolata reared in small ceramsite and quartz sand exhibited significantly higher mean body weight (3.02 ± 0.08 g/individual) compared to the river sand control group (p < 0.05), with the small ceramsite group achieving maximal growth. No significant differences (p > 0.05) were observed between medium/large ceramsite groups (5 mm and 8 mm, respectively) and the control.
Survival rates followed a distinct pattern: the small ceramsite group demonstrated the highest survival (92.6 ± 3.29%), significantly exceeding the control (p < 0.05). While medium ceramsite and quartz sand showed comparable survival to the control (p > 0.05), large ceramsite yielded the lowest survival rate, significantly lower relative to all other groups (p < 0.05).
Production metrics displayed contrasting trends. Despite superior individual growth, the small ceramsite substrate showed significantly lower yield per unit area and specific growth rate (SGR) than the control (p < 0.05). Medium ceramsite and quartz and maintained parity with the control in these parameters (p > 0.05), whereas large ceramsite exhibited the poorest performance (p < 0.05).
3.3. Effects of Substrate Types on Digestive Enzyme Activities in B. areolata
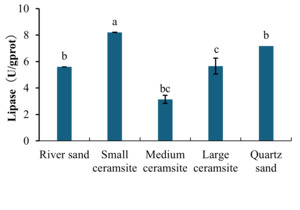
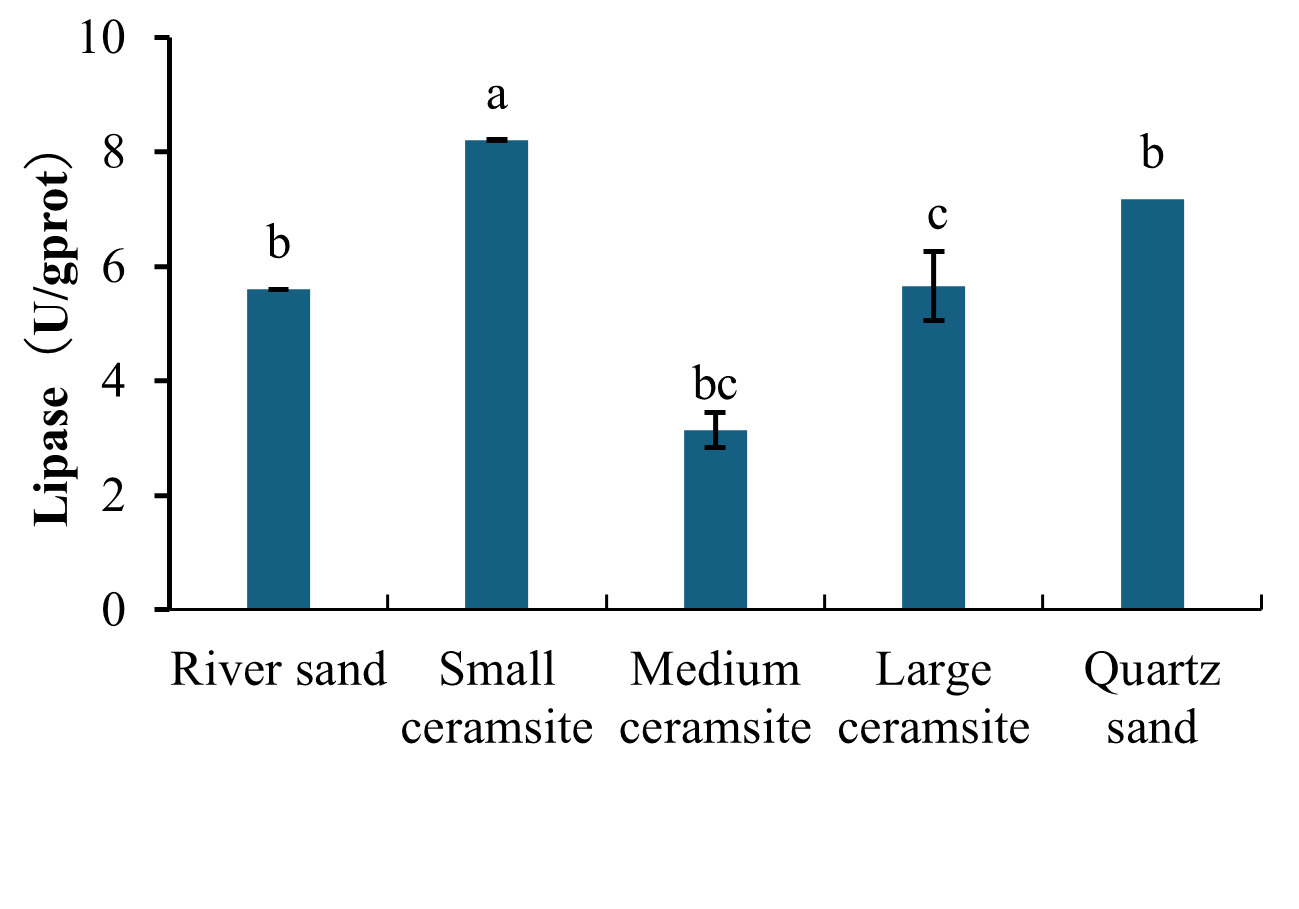
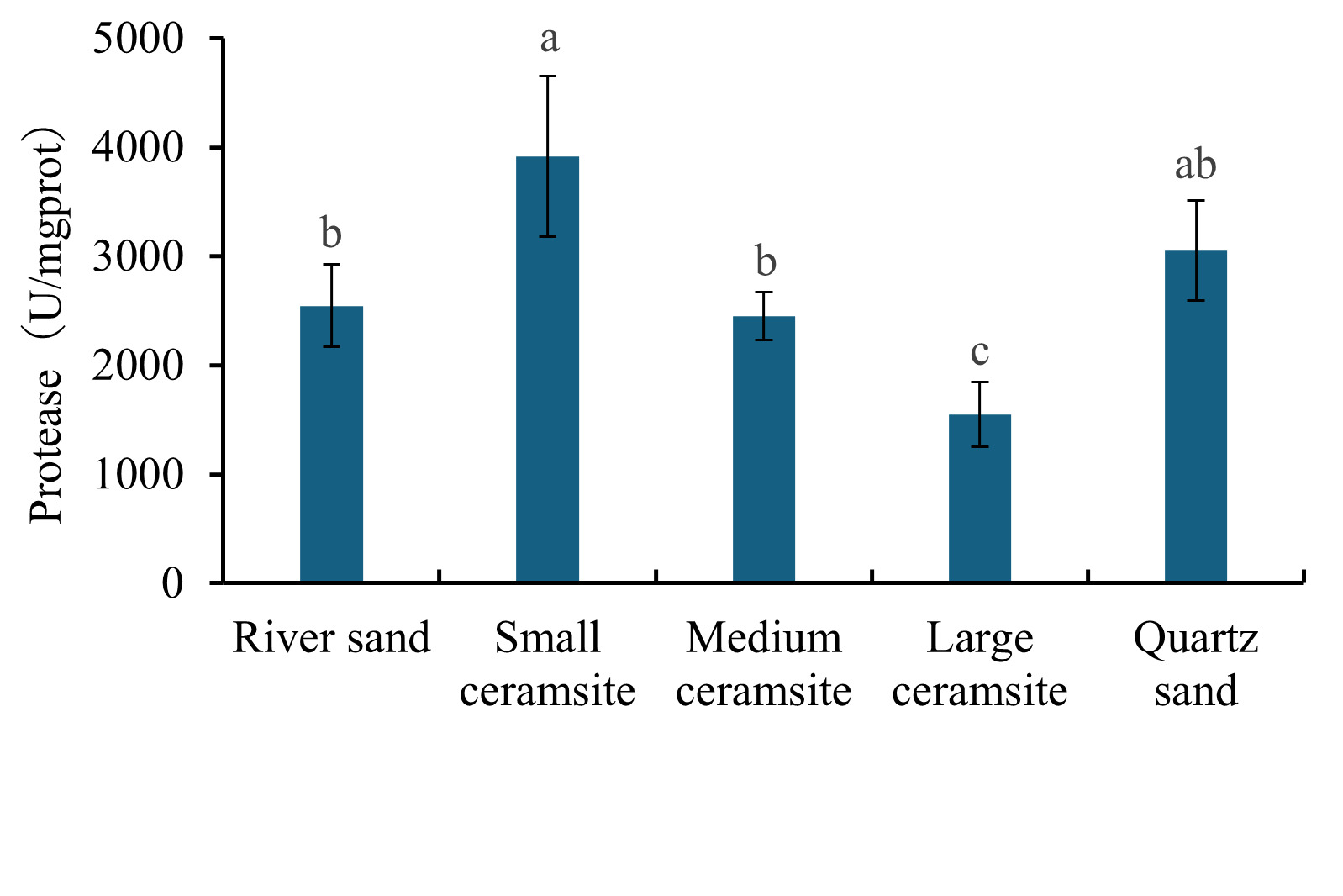
Analysis of hepatopancreatic enzyme activities revealed distinct substrate-dependent responses (Figures 1 and 2). For lipase activity (Figure 1), B. areolata cultured in small ceramsite exhibited significantly elevated enzymatic levels compared to the river sand control group (p < 0.05). No statistically significant differences (p > 0.05) were observed between the control and either medium ceramsite or quartz sand. Conversely, large ceramsite yielded the lowest lipase activity, with values significantly below the control (p < 0.05). A parallel trend was observed for trypsin (protease) activity (Figure 2). The small ceramsite group demonstrated peak trypsin activity, significantly surpassing the control (p < 0.05). Medium ceramsite and quartz sand showed enzymatic levels comparable to the control (p > 0.05), while large ceramsite again registered the lowest activity, significantly underperforming relative to all other groups (p < 0.05).
3.4. Effects of Substrate Types on Immune Enzyme Activities in B. areolata
3.4.1. Effects of Substrate Types on GSH-Px Activity
Analysis of GSH-Px activity across foot, mantle, and hepatopancreatic tissues (Figure 3) revealed substrate-specific modulation. In foot tissues, B. areolata cultured in small ceramsite exhibited significantly higher GSH-Px activity compared to the river sand control group (p < 0.05). Conversely, medium and large ceramsite substrates induced significantly reduced foot tissue GSH-Px activity relative to the control (p < 0.05), while quartz sand showed no statistical difference (p > 0.05). For mantle tissues, the control group (river sand) demonstrated the highest GSH-Px activity. All engineered substrates—small, medium, and large ceramsite, as well as quartz sand—resulted in significantly lower mantle GSH-Px activity than the control (p < 0.05). In hepatopancreatic tissues, small ceramsite enhanced GSH-Px activity significantly above control levels (p < 0.05). By contrast, medium ceramsite and quartz sand suppressed hepatopancreatic GSH-Px activity below control values (p < 0.05).
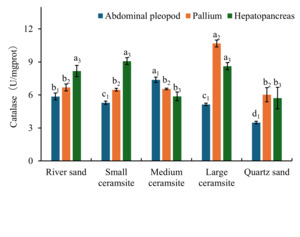
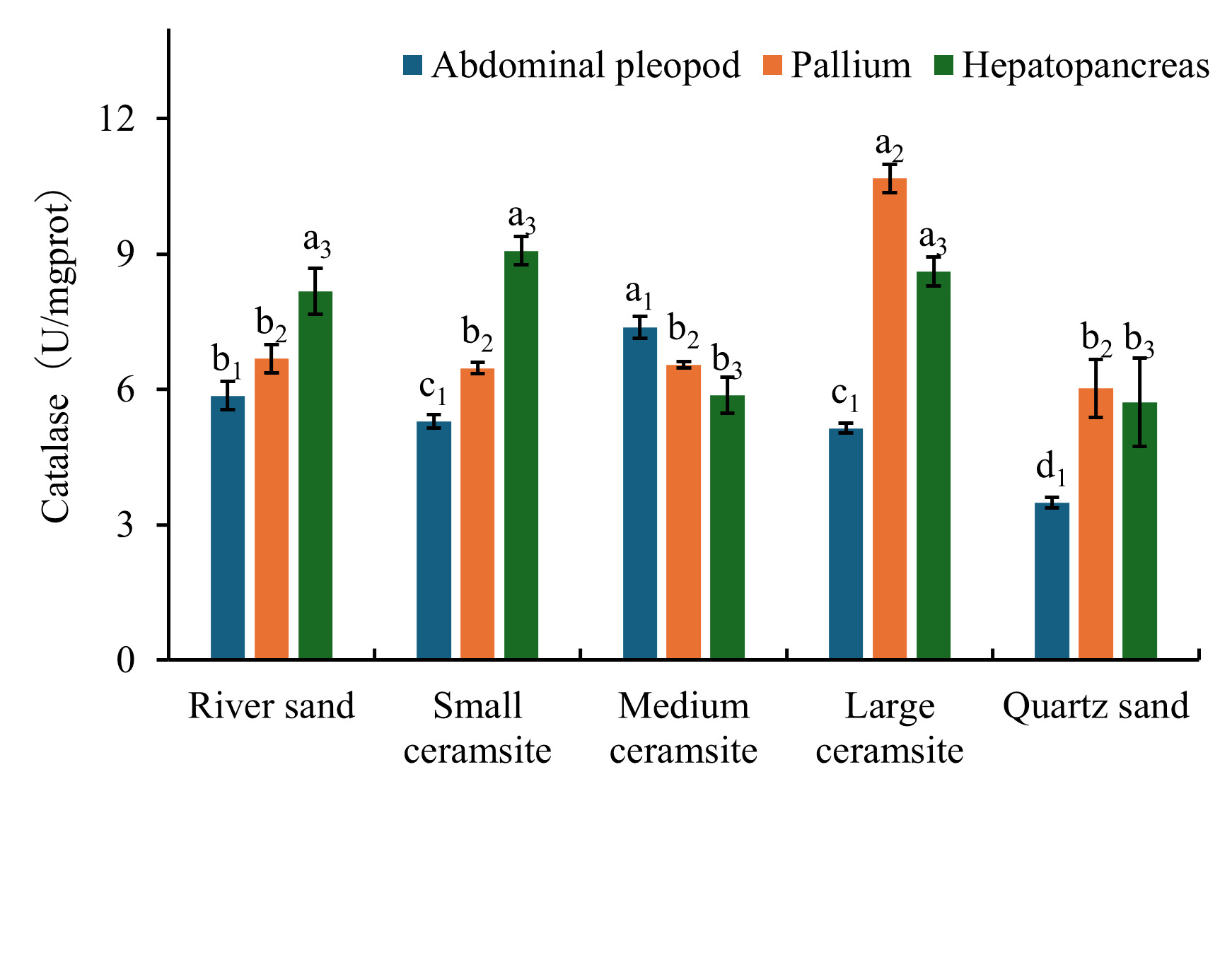
3.4.2. Effects of Substrate Types on CAT Activity
Analysis of CAT activity across tissues under different substrate conditions revealed tissue- and substrate-specific modulation (Figure 4). In foot tissues, CAT activity was significantly reduced in small ceramsite, large ceramsite, and quartz sand compared to the river sand control group (p < 0.05). Conversely, medium ceramsite induced significantly elevated foot CAT activity relative to the control (p < 0.05). For mantle tissues, large ceramsite elicited the highest CAT activity, significantly exceeding control levels (p < 0.05). No significant differences (p > 0.05) were observed between the control and small/medium ceramsite or quartz sand. In hepatopancreatic tissues, CAT activity was significantly suppressed in medium ceramsite and quartz sand compared to the control (p < 0.05), while small and large ceramsite maintained activity levels comparable to the control (p > 0.05). Tissue-specific hierarchy further demonstrated: Under river sand and small ceramsite, hepatopancreatic CAT activity surpassed levels in both foot and mantle tissues. Medium ceramsite prioritized CAT activity as: foot > mantle > hepatopancreas. Large ceramsite exhibited maximal mantle CAT activity, significantly higher than foot and hepatopancreatic levels (p < 0.05). Quartz sand induced the lowest foot CAT activity, significantly below mantle and hepatopancreatic values (p < 0.05).
3.4.3. Effects of Substrate Types on POD Activity
Substrate-driven modulation of POD activity across tissues is summarized in Figure 5. In foot tissues, POD activity was significantly suppressed in small and medium ceramsite compared to the river sand control (p < 0.05). Conversely, large ceramsite and quartz sand induced significantly elevated foot POD activity relative to the control (p < 0.05). For mantle tissues, all experimental substrates (small/medium/large ceramsite and quartz sand) resulted in significantly lower POD activity than the control (p < 0.05). In hepatopancreatic tissues, POD activity was significantly enhanced in small/large ceramsite and quartz sand compared to the control (p < 0.05). Tissue-specific hierarchy analysis further demonstrated that under river sand, mantle POD activity significantly exceeded levels in both foot and hepatopancreatic tissues (p < 0.05), with foot activity being the lowest. In all engineered substrates (small/medium/large ceramsite, quartz sand), hepatopancreatic POD activity was significantly higher than foot and mantle values (p < 0.05).
3.4.4. Effects of Substrate Types on Lysozyme Activity
Analysis of lysozyme activity across tissues under varying substrate conditions is presented in Figure 6. In foot tissues, B. areolata cultured in medium and large ceramsite exhibited significantly reduced lysozyme activity compared to the river sand control group (p < 0.05), while small ceramsite and quartz sand showed activity levels comparable to the control (p > 0.05). For mantle and hepatopancreatic tissues, lysozyme activity was similarly suppressed in medium and large ceramsite groups relative to the control (p < 0.05). However, small ceramsite and quartz sand maintained mantle/hepatopancreatic lysozyme activity at control-equivalent levels (p > 0.05). Hepatopancreatic lysozyme activity consistently ranked highest across all substrate treatments (river sand, small/medium/large ceramsite, quartz sand), significantly exceeding values in foot and mantle tissues (p < 0.05).
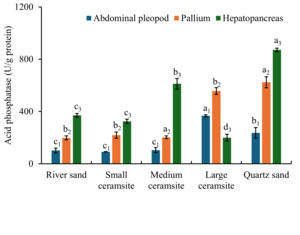
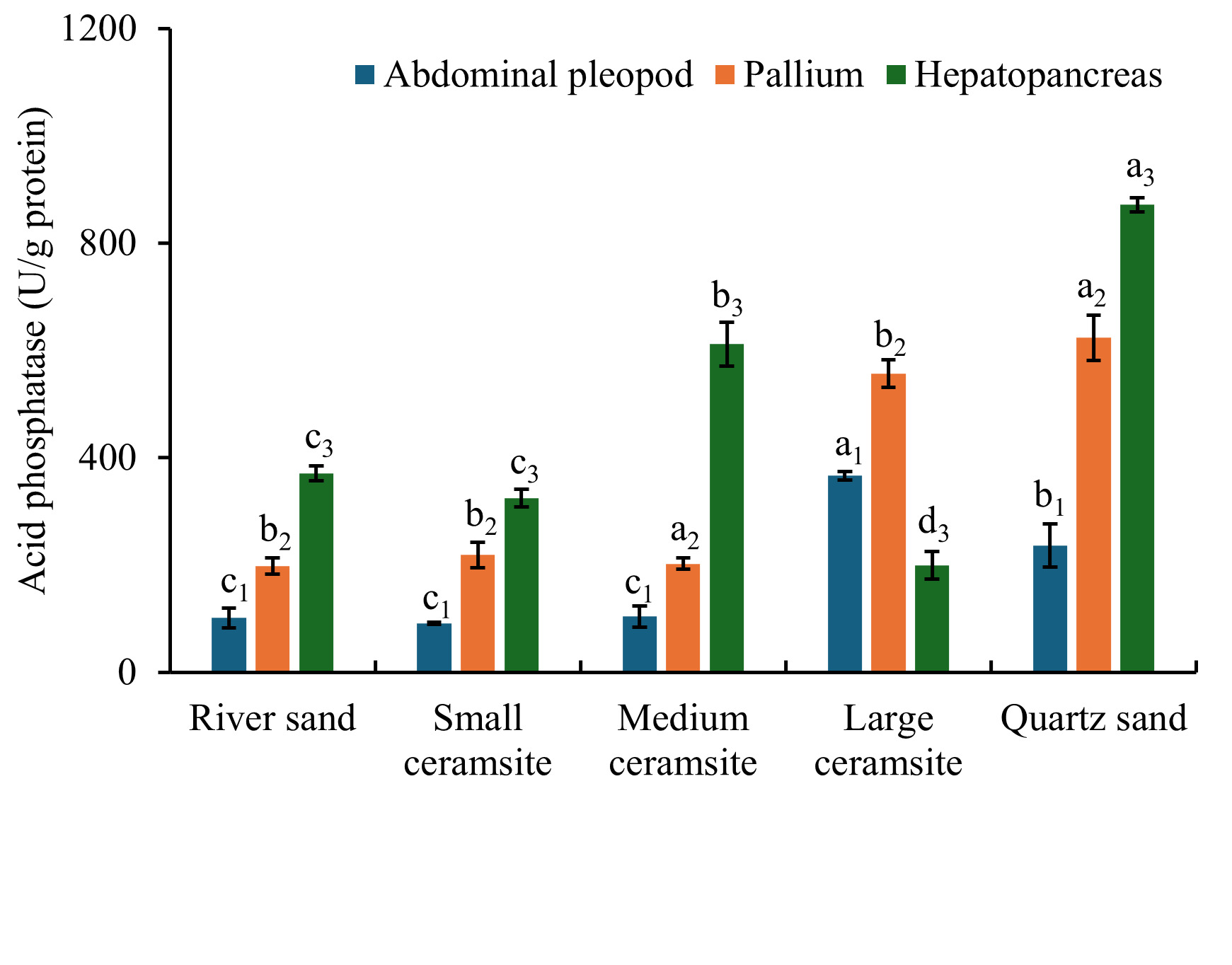
3.4.5. Effects of Substrate Types on Acid Phosphatase (ACP) Activity
Tissue-specific acid phosphatase activity under different substrates is illustrated in Figure 7. In foot tissues, ACP activity was significantly elevated in large ceramsite and quartz sand compared to the river sand control (p < 0.05), while small and medium ceramsite showed no statistical difference from the control (p > 0.05). For mantle tissues, medium ceramsite and quartz sand induced significantly higher ACP activity than the control (p < 0.05), whereas small and large ceramsite maintained control-equivalent activity levels (p > 0.05). In hepatopancreatic tissues, ACP activity was significantly enhanced in medium ceramsite and quartz sand (p < 0.05 vs. control) but suppressed in large ceramsite (p < 0.05). Cross-tissue comparisons revealed that under river sand, small/medium ceramsite, and quartz sand, hepatopancreatic ACP activity consistently exceeded levels in foot and mantle tissues (p < 0.05). Large ceramsite uniquely prioritized foot ACP activity, with values significantly surpassing mantle and hepatopancreatic levels (p < 0.05).
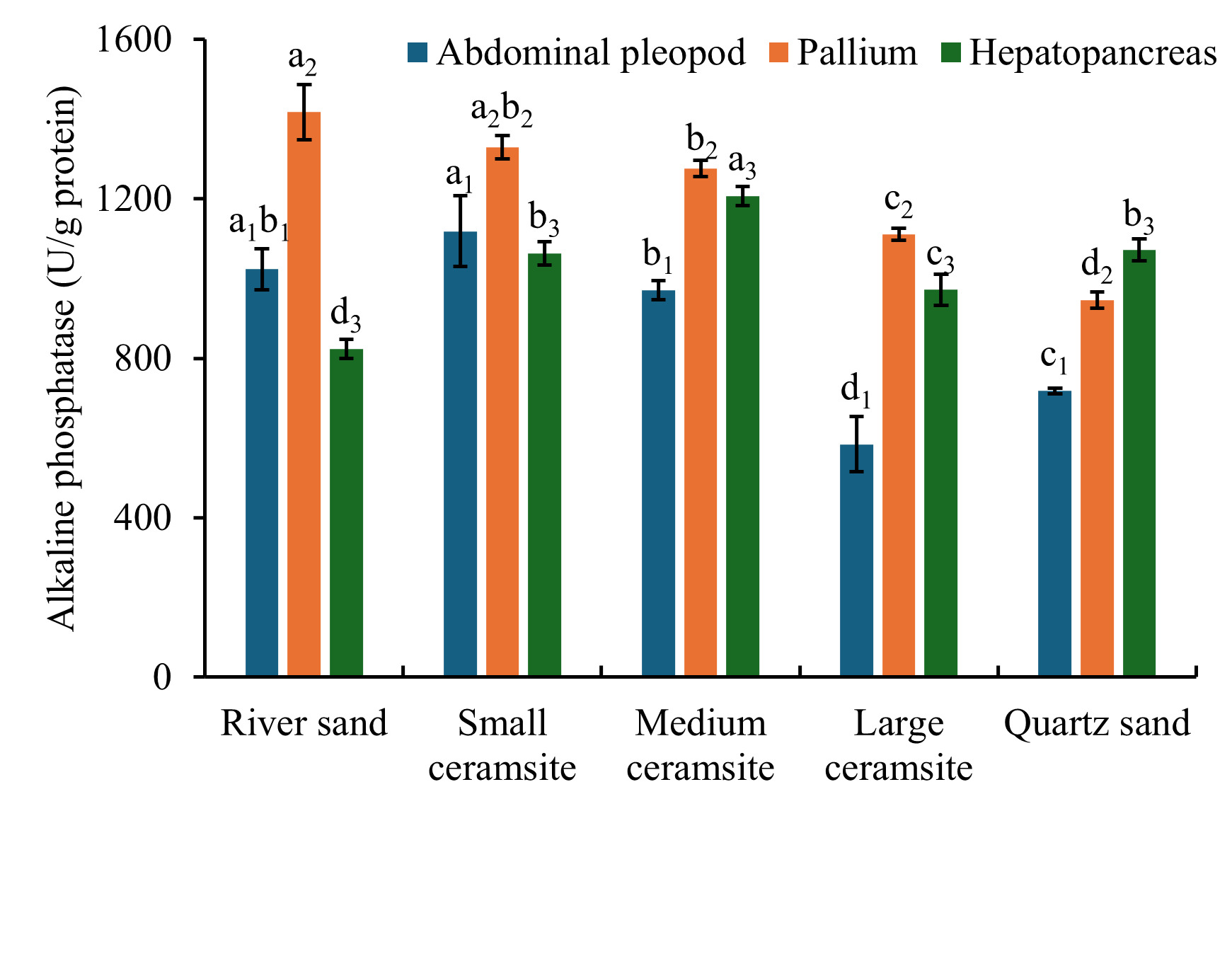
3.4.6. Effects of Substrate Types on Alkaline Phosphatase (AKP) Activity
Tissue-specific AKP activity under varying substrates is summarized in Figure 8. In foot tissues, AKP activity was significantly reduced in large ceramsite and quartz sand compared to the river sand control group (p < 0.05). A parallel suppression pattern was observed in mantle tissues, where medium and large ceramsite, along with quartz sand, exhibited significantly lower AKP activity than the control (p < 0.05). Conversely, hepatopancreatic tissues demonstrated a contrasting response: all experimental substrates (small/medium/large ceramsite, quartz sand) induced significantly higher AKP activity relative to the control (p < 0.05). Tissue prioritization analysis further revealed that under river sand, small/medium/large ceramsite, mantle AKP activity consistently surpassed levels in foot and hepatopancreatic tissues. In quartz sand, hepatopancreatic AKP activity dominated, significantly exceeding values in foot and mantle tissues.
4. Discussion
4.1. Impacts of Substrate Types on Water Quality, Growth Performance, and Survival Rate in Babylonia areolata Culture
Substrate properties critically govern aquaculture water quality by modulating organic matter accumulation, nutrient cycling efficiency, and microbial community structure.29 Fine-grained substrates, characterized by higher specific surface area and superior hydraulic conductivity, enhance microbial biofilm formation and optimize nitrogenous waste degradation through improved nitrifier colonization.30,31 Our results demonstrate significant substrate-driven water quality improvements, with quartz sand and ceramsite substrates (small/medium/large) outperforming the river sand control in reducing NH4+-N, NO2−-N, TN, and COD. Notably, small ceramsite achieved the lowest concentrations of NH4+-N (0.33 mg/L), NO2−-N (0.24 mg/L), and TN (0.42 mg/g), suggesting their superior bioremediation capacity. This enhancement likely arises from their optimized surface area-to-volume ratio (promoting nitrifying bacterial attachment) and interstitial flow dynamics, which accelerate nitrogen transformation pathways. The minimal COD (5.67 mg/L) under small ceramsite further corroborates their efficacy in organic matter mineralization. Studies demonstrate that ceramsite can significantly increase the microbial population in water, particularly nitrogen-cycling bacteria, thereby enhancing the removal of ammonia nitrogen and TN.32 Additionally, ceramsite can further improve the removal efficiency of ammonia nitrogen, TN, and TP by immobilizing effective microbial communities.33 In this study, ceramsite was directly used as the substrate for the culture of B. areolata, providing a habitat while achieving in-situ purification of nitrogen, phosphorus, and other substances.
Substrate granularity also profoundly influences benthic organism behavior and survival strategies. For B. areolata, optimal substrates enhance nutrient accessibility while minimizing physiological stress.34,35 Consistent with reports of accelerated growth in fine-grained substrates,16,23 small ceramsite maximized juvenile B. areolata growth (3.02 g/individual, p < 0.05 vs. control), while quartz sand also supported favorable growth metrics. Medium/large ceramsite (5–8 mm) showed growth performance comparable to the control (p > 0.05), likely due to suboptimal microhabitat conditions. Survival rates exhibited granularity-dependent trends: small ceramsite achieved the highest survival (92.6%, p < 0.05), attributable to their dual role in stabilizing water quality (reducing NH3/NO2− toxicity) and facilitating burrowing behavior. Conversely, large ceramsite significantly reduced survival (p < 0.05), potentially due to their dense physical structure impeding juvenile substrate penetration and increasing energy expenditure during locomotion. This mechanical barrier effect, combined with limited microbial activity in coarse substrates, likely exacerbated metabolic stress and mortality. Additionally, the cost-effectiveness and superior aquacultural performance of sintered ceramsite substantiate their viability as a cultivation substrate for B. areolata from a profitability perspective.
4.2. Effects of Substrate Types on Digestive Enzyme Activities in Babylonia areolata
Substrates in aquaculture systems serve not only as habitats for benthic mollusks but also as critical reservoirs for nutrient deposition and organic matter accumulation.36 However, excessive sediment loading beyond ecological thresholds can release toxic compounds into the water column, compromising the physiological health of cultured organisms.37 Digestive enzymes such as lipase and trypsin are key indicators of nutrient assimilation efficiency, directly influencing growth performance and metabolic homeostasis in aquatic species.38 Lipase, a glycerol ester hydrolase, regulates fatty acid digestion and absorption by hydrolyzing triglyceride bonds,39 while trypsin catalyzes the proteolytic cleavage of macromolecular proteins into absorbable peptides and amino acids—a vital process for carnivorous mollusks.28 Previous studies have demonstrated that fine-grained substrates enhance pepsin activity in B. areolata compared to coarse-grained alternatives.40
Our results reveal significant substrate-dependent modulation of hepatopancreatic digestive enzyme activities. B. areolata cultured in small ceramsite exhibited markedly elevated lipase and trypsin activities compared to the river sand control group (p < 0.05). This enhancement likely stems from the hierarchical pore architecture of small ceramsite, which synergistically optimizes microbial and hydrological dynamics.40 Microbial facilitation, the multi-scale porosity provides extensive surface area for biofilm colonization, accelerating organic matter mineralization and nutrient solubilization. Interfacial exchange enhanced substrate-water interaction, promotes redox gradients and oxygen diffusion, stimulating enzymatic cascades. In contrast, large ceramsite showed the lowest enzymatic activities, attributable to their limited hydraulic conductivity and reduced microbial colonization. The restricted pore connectivity in coarse substrates impedes organic matter degradation, thereby suppressing enzyme secretion and activity through.23 Nutrient deprivation reduced bioavailability of organic substrates, limiting enzyme-substrate interactions. Hypoxia induces poor oxygen diffusion, inhibiting energy-dependent enzymatic processes. These findings demonstrate that substrate physicochemical properties regulate molluscan digestive physiology via a microbiome-substrate functionality-metabolic axis. The superior performance of small ceramsite underscores their bio-chemo-mechanical synergy in enhancing nutrient utilization—a critical advantage for optimizing benthic aquaculture productivity.
4.3. Effects of Substrate Types on Immune Enzyme Activities in Babylonia areolata
The antioxidant enzyme system in aquatic organisms serves as a critical defense mechanism against oxidative stress.41 CAT and POD are pivotal antioxidants that neutralize reactive oxygen species (ROS), while GSH-Px mitigates lipid peroxidation by decomposing hydrogen peroxide (H2O2) into water and oxygen, thereby preserving cellular membrane integrity.42 Antioxidant systems, comprising both enzymatic (e.g., CAT, POD, GSH-Px) and non-enzymatic components, function synergistically to scavenge excess ROS and prevent oxidative damage.43 Total antioxidant capacity (T-AOC), a comprehensive indicator of ROS-neutralizing potential, correlates positively with CAT and POD activities, reflecting enhanced stress resistance and free radical elimination efficiency.44 Concurrently, lysozyme strengthens innate immunity by hydrolyzing bacterial cell walls,45 while ACP and AKP act as metabolic biomarkers, reflecting nutritional status and detoxification capacity in hepatopancreatic tissues.45,46 Substrate granularity has been shown to inversely regulate antioxidant enzyme activities (e.g., SOD, CAT, AKP, T-AOC) in crustaceans, highlighting the profound impact of sediment physicochemical properties on oxidative stress responses.47
Extensive research has established that the enzymatic activities of CAT, SOD, POD, and GSH-Px are modulated by environmental variables, dietary regimes, and physiological states in marine invertebrates.28,48 In the gastropod B. areolata, which lacks adaptive immunity, immunological competence primarily relies on its antioxidant defense system. This study reveals that substrate types significantly modulated immune-antioxidant enzyme profiles in B. areolata. Small ceramsite elevated GSH-Px activity in foot and hepatopancreatic tissues (p < 0.05 vs. river sand control), whereas medium and large ceramsite suppressed GSH-Px levels (p < 0.05). Similar granularity-dependent trends were observed for CAT and POD activities, suggesting substrate-mediated regulation of oxidative stress intensity.42 The enhanced antioxidant capacity under small ceramsite likely stems from improved redox homeostasis via optimized microbial activity and interstitial oxygen diffusion. Lysozyme activity declined significantly in quartz sand and medium ceramsite (p < 0.05), potentially due to substrate-driven shifts in pathogenic microbial communities.49 Furthermore, ACP and AKP activities exhibited marked variations under large ceramsite and quartz sand, with coarse substrates impairing metabolic adaptation (p < 0.05). These findings underscore the dual role of substrate granularity in modulating immune-metabolic homeostasis, where fine-grained sediments enhance enzymatic defense mechanisms, while coarse substrates exacerbate oxidative and metabolic stress.
5. Conclusions
This study demonstrates that substrate composition critically modulates aquaculture performance, physiological resilience, and immune responses in juvenile Babylonia areolata. Small ceramsite significantly improved water quality by reducing ammonia nitrogen, nitrite nitrogen, and COD, while enhancing growth metrics and survival rates. Mechanistically, the hierarchical porosity of small ceramsite amplified hepatopancreatic lipase and trypsin activities, compared to river sand controls, indicating superior nutrient assimilation. Concurrently, small ceramsite elevated GSH-Px activity in foot and hepatopancreatic tissues, reinforcing antioxidant defenses against oxidative stress. In contrast, large ceramsite suppressed digestive enzyme activities and compromised immune function through reduced lysozyme and acid phosphatase (ACP) activity, likely due to restricted microbial colonization and impaired redox dynamics. While river sand and medium ceramsite supported moderate immune enzyme levels, small ceramsite uniquely synergized metabolic efficiency and pathogen resistance. These findings establish small ceramsite substrates as optimal for B. areolata culture, leveraging bio-chemo-physical interactions to enhance growth, oxidative stress resilience, and disease resistance.
Acknowledgements
This research was financially supported by Hainan Province Science and Technology Special Fund (ZDYF2022XDNY351), Research on breeding technology of candidate species for Guangdong modern marine ranching (2024-MRB-00-001), Central Public-interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (2023TD58), The earmarked fund for CARS (CARS-49), and Financial Fund of Ministry of Agriculture and Rural affairs of China (NHYYSWZZZYKZX2020).
Authors Contributions
Cheng ZHOU and Wang ZHAO conceived and designed the project, wrote the manuscript. Rui YANG, Junhua HUANG and Haipeng QIN measured and collected the data. Zhenhua MA, Xiaoyu Wang collected the samples and carried out analysis. All listed authors have read, edited, and approved the final manuscript.
Competing Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
The experiments were in compliance with the regulations and guidelines established by the Animal Care and Use Committee of South China Sea fisheries Research Institute, Chinese Academy of Fishery Sciences and permitted by this committee.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.