Introduction
Cherax quadricarinatus, the Australian freshwater crayfish, is a significant freshwater commercial species, widely cultured in Southeast Asian countries such as Thailand, China, and Vietnam.1 The regulation of ovarian development remains a central topic in the study of economically important crustaceans. Previous studies have established that ovarian development in crustaceans is governed by steroid hormones, neuropeptides, neurotransmitters, and exogenous environmental factors.2,3 The ubiquitin-proteasome pathway (UPP),4 The cAMP-dependent protein kinase (PKA) pathway and the ecdysteroid receptor are also implicated in crustacean ovarian differentiation and development.5,6 Compared to other prawns, C. quadricarinatus produces fewer offspring, and the male-to-female in large-scale breeding is approximately 3:1, highlighting the need for a greater proportion of females for successful breeding. Monosex culture could optimize breeding by ensuring a higher number of female C. quadricarinatus. Thus, a deeper understanding of ovarian development is essential for further enhancing breeding practices.
Foxl2, a member of the FOX (forkhead box) family, plays a vital role in ovarian differentiation, development, and maintenance.7 In mammals, Foxl2 regulated ovarian development by inhibiting the expression of Sox9. Foxl2 is the first autosomal gene linked to premature ovarian failure in humans, with mutations in Foxl2 potentially leading to ovarian granulosa cell tumors. Knockout of Foxl2 in the ovaries of mature mice caused ovarian degeneration, which ultimately progressed to spermaries.7 Studies in fish have demonstrated significant sexual dimorphism in the expression levels of Foxl2 in glands.8 Foxl2 in Rana rugosa functioned similarly to mammalian Foxl2 during early ovarian differentiation.9 The expression levels of Foxl2 exhibited a marked rise in stage II female oyster Crassostrea gigasrelatives compared to males at the same stage or earlier stage.10 As expected, the expression of Foxl2 peaked in proliferative-stage ovaries of scallop Chlamys farreri, exhibiting a 62-fold elevation compared to testes.11
Recently, Foxl2 has been cloned in several crustacean species, and the functional study has received extensive attention as well. For instance, Foxl2 was first identified in the androgenic glands transcriptome of Macrobrachium nipponense,12 and tissue distributions showed that testes had significantly higher expression levels of Mn-Foxl2 mRNA than other tissues.13,14 High levels of FoxL2 expression were also observed in the testes of Fenneropenaeus merguiensis, and knockdown of the FoxL2 gene significantly increased vitellogenin expression, indicating its involvement in regulating reproduction.15 In contrast, Foxl2 in Procambarus clarkii is predominantly expressed in the ovaries, with minimal expression in the spermaries.16 Additionally, Foxl2 in Eriocheir sinensis was highly expressed in both ovaries and thoracic ganglia, indicating its role in regulating ovarian development.17 In summary, all the above reports suggested that Foxl2 might promote both male and female sexual development in crustaceans.
As known to us, understanding the sex-determination mechanism is the basis for sex-controlled breeding of aquatic species.18 Despite the fact that the crucial role of Foxl2 gene plays in regulating gonad development, the report of Cq-Foxl2 in C. quadricarinatus is still limited. In this study, the full-length cDNA sequence of Cq-Foxl2 was identified from the redclaw crayfish, and its structure characteristics were analyzed. Then, the mRNA expression patterans of Cq-Foxl2 were investigated in various tissues and at different developmental stages of the juvenile and ovary. Furthermore, the RNAi experiment was applied to explore the role of the Foxl2 gene in the early sex differentiation of C. quadricarinatus. The results will help to understand the molecular mechanisms of sex differentiation and provide a strong theoretical basis for the development of unisexual breeding techniques.
Materials and Methods
Experimental animals and sample collection
C. quadricarinatus (60.0 ± 0.5g, 14.5 ± 0.2cm) used in this study were sourced from a breeding farm at Zhejiang Institute of Freshwater Fisheries (Zhejiang province, China). All experimental individuals were maintained in circulating tanks at 28.1 ± 0.5 °C for temporary breeding. Ovaries, testes, hepatopancreas, muscles, intestines, and gills were separately dissected from three males and three females. Additionally, juveniles that cultured in the pool of a greenhouse were collected at different body length stages. Finally, ovaries at different developmental periods (I, undeveloped stage; II, developing stage with yellow follicle cell; III, developing stage with green follicle cell; IV, nearly ripe stage; V, ripe stage; VI, spent stage) were collected according to previously published criteria.19 All samples were immediately flash-frozen in liquid nitrogen and stored at - 80 °C.
cDNA sequence cloning of Cq-Foxl2
Using the conserved region of Foxl2 from GenBank as a query, we searched for Foxl2 homologous genes in the transcriptome database of C. quadricarinatus, and identified an Expressed Sequence Tag (EST) containing the FOX protein domain. Two pairs of primers, 5’RACE-1 and 5’RACE-2, 3’RACE-1 and 3’RACE-2 (Table 1), were designed based on transcriptome data from the sexual glands of C. quadricarinatus. 3’-RACE and 5’-RACE PCRs were performed using the SMART™ RACE cDNA Amplification Kit (Clontech, USA) following the manufacturer’s instructions. The PCR program: 5 cycles of 30 s, 94 °C; 2 min, 72 °C. 5 cycles of 30 s, 94 °C; 30 s, 68 °C; 2 min, 72 °C. 25 cycles of 30 s, 94 °C; 30 s, 63 °C; 3 min, 72 °C. 7 min, 72 °C. PCR products were detected by 1.5% agarose gel electrophoresis and purified using the Gel Extraction Kit (QIAGEN, Venlo). The target bands were cloned into the pMD18-T vector and sequenced. The PCR program was under the following conditions: 5 cycles at 94 °C for 30 s, 72 °C for 2 min; 5 cycles at 94 °C for 30 s, 68 °C for 30 s, 72 °C for 2 min; and 25 cycles at 94 °C for 30 s, 63 °C for 30 s, 72 °C for 3 min, followed by an extension at 72 °C for 7 min. PCR products were analyzed by 1.5% agarose gel electrophoresis, purified using the Gel Extraction Kit, cloned into the pMD18-T vector, and sequenced.
Sequence analysis
The open reading frame (ORF) and amino acid sequence were predicted using online software (http://www.ncbi.nlm.nih.gov/gorf/orfig.cgi), and secondary structure analysis was conducted using GOR4 (http://www.expasy.org/). BLAST searches (http://blast.ncbi.nlm.nih.gov/Blast.cgi) were performed to identify homologous genes from different species, including P. clarkii (KR135174.1), Eriocheir sinensis (KF806733.1), Macrobrachium nipponense (KM821275.1), Hyalella azteca (XM_018169860.1), Danio rerio (NM_001045252.2), Larimichthys crocea (XM_010731397.3), Scophthalmus maximus (XP_035502153.1), Clupea harengus (XP_031416120.1), Salmo salar (XP_014018845.1), Fundulus heteroclitus (XP_012718687.2) Oncorhynchus mykiss (NP_001117957.1) and Lepisosteus oculatus (XP_006637658.1).
Phylogenetic analysis
A multiple sequence alignment was conducted using the ClustalW algorithm in MEGA5.05 software, and a phylogenetic tree was constructed using the Neighbor-Joining method. All sequences were retrieved from NCBI (https://www.ncbi.nlm.nih.gov/).
RNA extraction and reverse transcription
Total RNA from all samples were extracted using TRIzol Reagent (Invitrogen, USA) according to the manufacturer’s instructions. To eliminate genomic DNA contamination, all RNA samples were treated with RNase-free DNase I. RNA concentration was quantified using a Nanodrop 2000 (Thermo Fisher Scientific, USA), and RNA integrity was assessed via 1% agarose gel electrophoresis. After dilution to 100 ng/μL, first-strand cDNA was synthesized using the PrimeScriptTM RT Master Mix reagent Kit (Takara Bio, Japan). The cDNA was stored at - 20 °C for future use.
Expression pattern of Cq-Foxl2 mRNA
Quantitative Real-time PCR (qRT-PCR) was carried out to measure the expression levels of Cq-Foxl2 in a LightCycler® 480 System (Roche, Switzerland) using SYBR PreMix Ex TaqTMⅡ (Takara, Japan). The Cq-18S rRNA (Table 1) was used as an internal control gene following the program described below: 98 °C for 3 min; 40 cycles of 95 °C for 10 s, and 60 °C for 20 s. Three replicates were performed for each sample, and the average of the three replicates was calculated using the -2-ΔΔCt method.
All data were calculated as means±S.D. and evaluated by one-way ANOVA. Significance analysis was performed using SPSS 19.0 software (*P<0.05, ** P<0.01, ***P< 0.001).
Target-silencing Cq-Foxl2 gene by RNA interference
DNA fragments of the Cq-Foxl2 (350 bp) and enhanced green fluorescent protein gene (eGFP, 359 bp) were first cloned into the vector pUC57. Then, the recombinant plasmids were extracted and used as templates for dsRNA (double-stranded RNA) synthesis with a pair of specific primers containing T7 promoters (Table 1). Cq-Foxl2-dsRNA and EGFP-dsRNA (used as control) were synthesized using a TranscriptAid T7 High Yield Transcription kit (Novozan, Nanjing). The reaction system was carried out in a final volume of 20 µL, containing 2 μL T7 Reaction Buffer, 2 μL ATP Solution, 2 μL CTP Solution, 2 μL GTP Solution, 2 μL UTP Solution, 2 μL T7 Enzyme Mix, and 1 μg of DNA templates. The reaction was incubated at 37 °C for 16 h. The integrity, purity, and concentration of the resulting dsRNA were evaluated by agarose gel electrophoresis and Nanodrop2000. For a short silencing experiment, undifferentiated juvenile crayfish (an average weight of 2 g and average body length of 2 cm) were selected and classified into two groups: Cq-Foxl2 dsRNA-injected (n=15) and EGFP dsRNA-injected (n=15). Each individual was intramuscularly injected with 5 µg/g of either Cq-Foxl2-dsRNA or Cq-eGFP-dsRNA. For checking the RNAi effect, cephalothoraxes of dsRNA-injected individuals were collected two weeks after injection, and qRT-PCR was performed to investigate the expression level of Cq-Foxl2 and Cq-Dsx.
Results
cDNA sequence analysis of Cq-Foxl2
Cq-Foxl2 cDNA sequence was assembled using 5’-RACE, 3’-RACE and RT-PCR techniques. The nucleotide and deduced amino acid sequences are presented in Figure 1a. The full-length cDNA sequence of Cq-Foxl2 comprised 2,325 nucleotides, including a 630 bp 5’-untranslated region (UTR), a 1,515 bp 3’-UTR, and an open reading frame of 1,695 bp encoding a protein of 564 amino acids (Figure 1). Secondary structure prediction revealed a typical forkhead box domain located between resideues 250-345 (Figure 1b).
Multiple sequence alignment and phylogenetic analysis of Cq-Foxl2
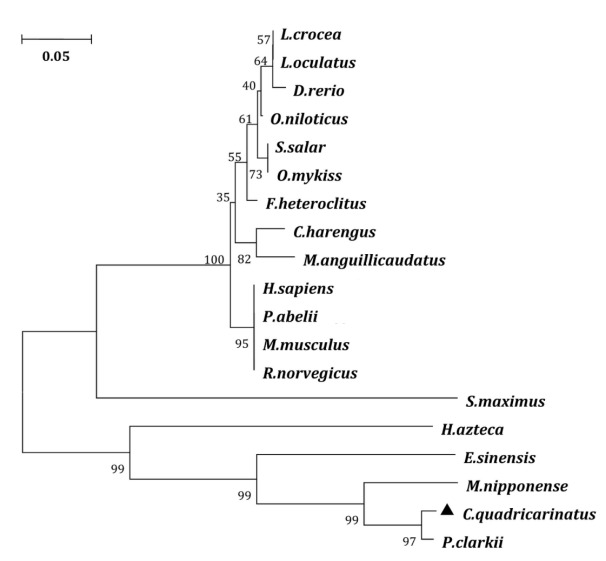
Foxl2 amino acid sequences from 18 species, including fishes, mammals, and crustaceans, were retrieved from the NCBI database for multiple sequence alignment. These alignments demonstrated species-specific variations in the Foxl2 amino acid sequence length, with fish Foxl2 being shorter than that of crustaceans and mammals (Figure 2). Despite this variation, all 19 species displayed the characteristic conserved forkhead box domain. The results of the multiple amino acid comparison analysis showed that the Cq-Foxl2 protein had a high similarity to that of the following species: Procambarus clarkii (KR135174.1, 70.12%), E. sinensis (KF806733.1, 41.17%), Clupea harengus (XP_031416120.1, 23.81%) and Larimichthys crocea (XM_010731397.3, 20.52%). The most conserved region of Foxl2 across fish, crustaceans, and mammals was within the forkhead box domain. A phylogenetic tree of Foxl2 was constructed using MEGA 5.05 software based on the multiple sequence alignment. The result revealed that the phylogenetic tree was divided into two branches, Foxl2 proteins from crustaceans were clustered into one clade, and others were clustered into another (Figure 3).
Expression pattern of Cq-Foxl2 in C. quadricarinatus
qRT-PCR analysis of Cq-Foxl2 expression in various tissues revealed high expression in the ovary, minimal expression in the testes, and no detectable expression in muscle, gill, intestine, or hepatopancreas (Figure 4a). At different developmental stages of juvenile crayfish, we detected the mRNA expression abundance of Cq-Foxl2 in a body length of 2 cm to 10 cm, and its expression was invariably higher in the female than in the male (Figure 4b). Additionally, Foxl2 mRNA was widely expressed in all examined ovarian development stages, but displayed a sharp decrease to minimal levels at stage VI (Figure 4c).
Effect of Cq-Foxl2 dsRNA injection on the expression levels of Cq-Dsx
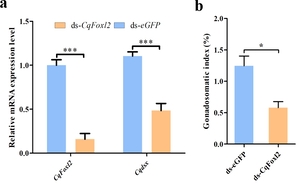
To further explore the function of Cq-Foxl2, RNA interference silencing was employed via dsRNA injection into undifferentiated crayfish. The results revealed that, compared to the Cq-dsRNA-eGFP injection group, both Cq-Dsx and Cq-Foxl2 expression levels were significantly suppressed following Cq-Foxl2-dsRNA injection (Figure 5a). Additionally, a comparison of gonadal indices between the two groups revealed a significant decrease in the gonadal index following Cq-Foxl2-dsRNA injection compared to the Cq-eGFP-dsRNA injection group (Figure 5b).
Discussion
Foxl2 functions have been extensively studied in vertebrates,7,20,21 but research in crustaceans remains limited. In this study, a 2,325 bp Cq-Foxl2 cDNA, with an ORF of 1,695 bp encoding 564 amino acids, was isolated and sequenced using rapid amplification of cDNA ends method. Cq-Foxl2 was found to contain a typical forkhead box of approximately 100 amino acids, similar to other forkhead box family members. While Foxl2 was conserved across many species, the amino acid sequence length varies among species. Multiple sequence alignments and phylogenetic analysis revealed that the Cq-Foxl2 protein was genetically closer to that of crustaceans than to mammals and fish. Notably, the Cq-Foxl2 sequence exhibited the highest identity with the orthologue from P. clarkii, which suggested evolutionary conservation of Foxl2 protein.
Foxl2 was first cloned and identified in mammals as a key factor in ovarian differentiation.20 Previous studies showed that Foxl2 was highly expressed in the ovary and minimally expressed in the testis of adult mice, indicating its critical role in ovarian development.7 Additionally, Foxl2 was highly expressed in the ovaries of most fish, similar to mammals, with a sexual dimorphismic expression pattern.22,23 These findings aligned with our results where Cq-Foxl2 expression was significantly higher in the ovary than in the testis, also exhibiting sexual dimorphism. Inversely, studies on F. merguiensis,15 M. rosenbergii,24 and M. nipponense14 reported higher expression of Foxl2 in the testis compared to the ovary. These results suggested that Foxl2 from crustaceans might produce the functional difference with mammalian and fish species due to a long evolutionary relationship, which played regulatory role in both males and females. Previous studies found that Foxl2 mRNA was widely expressed in all detected tissues,22,25 while in current study, the expression level of Cq-Foxl2 was restricted to the gonads, implying Foxl2 in C. quadricarinatus was a gonad-specific gene.
Furthermore, examining the expression of Cq-Foxl2 at different developmental stages of juvenile crayfish revealed that Cq-Foxl2 was gradually expressed in a body length of 2 cm to 10 cm. These findings revealed that Cq-Foxl2 played an essential role in early gonadal differentiation in C. quadricarinatus, and a body length of 2 cm might be a sex-sensitive period. Previous studies on Coilia nasus demonstrated that Foxl2 expression began early in ovarian development, gradually increasing as the development of ovary, and then decreasing in the later stage of ovarian maturation.21 Research on Scatophagus argus found that Foxl2 expression in the testis peaked initially, followed by a decline to a low or undetectable level.26 Our results showed that Cq-Foxl2 expression increased progressively from stage I to stage V, reaching its peak at stage V, followed by a significant decrease at stage VI, which was consistent with these studies, showing temporal and spatial differences in Cq-Foxl2 expression during ovarian development. This expression pattern suggests that Cq-Foxl2 might play a role in promoting oocyte growth and maturation in the ovary.
Studies in mice showed that Foxl2 knockout resulted in the ovary transforming into the testicular-like structure.27 Similarly, the simultaneous deletion of Foxl2a and Foxl2b in zebrafish successfully induced the transformation of females into phenotypic males.8 At present, the explicit mechanisms underlying sex differentiation and determination of Foxl2 regulation remains unclear in crustaceans. It has been proven that ovarian development in crustaceans is characterized by the rapid production of egg yolk protein in a process called vitellogenesis.28 As already mentioned, Foxl2 negatively affected the vitellogenin synthesis at the mature stage of the ovary in E. sinensis.15 Besides, previous studies revealed that Cq-Foxl2 was involved in several signal pathways related to ovarian development, including ovarian steroid synthesis, estrogen signaling pathway, oocyte meiosis, and progestin-mediated oocyte maturation.29 Moreover, in our previous study, Cq-Dsx was found to play essential role in the female ovarian development/differentiation of the redclaw crayfish,30 which pointed us in the direction to further explore the interaction relationship between Cq-Foxl2 and Cq-Dsx. As expected, substantial change was observed in the expression level of down-regulated in Cq-Dsx after treatment with Cq-Foxl2 dsRNA injection, meanwhile resulting in a significant reduction in the ovarian gonadal index. These results strongly suggest that Cq-Foxl2 represented a positive effect on the maintenance of ovarian function and development by regulating Cq-Dsx expression in the redclaw crayfish.
In conclusion, this study reported the characterization of Cq-Foxl2 in the redclaw crayfish. Tissues distribution found that Cq-Foxl2 expression was restricted to the ovary and testis with a significant sexually dimorphic expression pattern. In addition, gene expression analysis at different stages of juveniles and ovarian development revealed its key role in early gonadal differentiation and ovarian functional maintenance. Moreover, RNAi assay further demonstrated that Cq-Foxl2 had a positive regulatory effect on ovarian development by regulating Cq-Dsx expression. Overall, these findings provided valuable insights for further investigation into the mechanism of crustacean ovarian development.
Acknowledgments
This study was supported by the Key Scientific and Technological Grant of Zhejiang for Breeding New Agricultural Varieties (2021C02069-4-5).
Authors’ Contribution
Conceptualization: Yucheng Zhang (Lead), Shun Cheng (Equal). Investigation: Yucheng Zhang (Equal), Jianbo Zheng (Equal), Yangda Zhou (Equal), Fei Li (Equal), Shili Liu (Equal). Writing – original draft: Yucheng Zhang (Equal). Supervision: Yucheng Zhang (Equal), Jianbo Zheng (Equal), Yangda Zhou (Equal), Fei Li (Equal), Shili Liu (Equal), Wenping Jiang (Equal), Meili Chi (Equal), Haiqi Zhang (Equal). Formal Analysis: Jianbo Zheng (Equal), Yangda Zhou (Equal), Fei Li (Equal), Shun Cheng (Equal).
Competing of Interest – COPE
No competing interests were disclosed. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Conduct Approval – IACUC
The sample collection and experiments in the study were in compliance with the Animal Ethics Committee of Zhejiang Institute of Freshwater Fisheries (Animal Ethics no. 1067, March 6, 2019).
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All data are available upon reasonable request.

_nucleotide_and_deduced_amino_acid_sequences_of_*cq-foxl2*._an_open_reading_frame_of_16.jpeg)


_the_expression_pattern.jpeg)
_nucleotide_and_deduced_amino_acid_sequences_of_*cq-foxl2*._an_open_reading_frame_of_16.jpeg)

_the_expression_pattern.jpeg)
