1. Introduction
Global aquaculture has been accelerating since the 1980s. However, the outbreak of various infections has caused economic losses that cannot be ignored.1 In traditional aquaculture, chemical additives and veterinary drugs are mainly used to control bacterial and viral infections, especially antibiotics, which pose a significant risk to public health.2 Therefore, in the case of large-scale prohibition of antibiotics in the world, more and more attention has been paid to the use of environmental protection feed additives in the contemporary aquaculture industry, such as microbial supplements instead of antibiotics to improve the immune capacity of aquaculture-related species and solve the problem of production safety of the aquatic product.3
Probiotics are living microorganisms, which can improve the health and growth of the host by improving the balance of microbiota in the environment, with sufficient doses of probiotics supplemented in aquaculture waters or feed.2 Moreover, many functions of probiotics have been certificated, such as the improvement of the water environment, the increase of feed efficiency, the promotion of growth, reproductive performance, nutrition metabolism, and immune-related genes expression. Notably, gut microbiota is a potent regulator of host metabolism and immunity.4 Meanwhile, probiotics may ameliorate the composition of intestinal microbiota to promote the growth and immunity of many different aquaculture species, such as Sparus aurata,5 Dicentrarchus labrax,6 Ictalurus punctatus,7 Oreochromis niloticus,8 and Procambarus clarkii.9
Currently, there is a wide variety of commercial compound probiotics used in aquaculture for water or feed additives, with wide variations in efficiency and price. It is important to note that some products are often ineffective and may even harm fish with supernumerary economic losses.10,11 Inspired by these conditions, we urgently need to conduct a comprehensive evaluation of the efficiency of some well-known probiotic products. In the past two decades, commercial probiotics products, with mixed cultures of bacteria (Lactobacillus, Enterococcus, Clostridium, and Acetobacter) have been focused on the interest of researchers. It has been proved that these mixed cultures of probiotics could significantly increase the survival rate, feed conversion, and gonadosomatic index in several aquatic species.12 However, the effects of these mixed probiotics on intestinal health, intestinal histology and immune capacity of aquatic animals have been slightly reported, so further studies are needed.
The turbot (Scophthalmus maximus), originally native to Europe, was introduced to China in 1992. It is recognized as a crucial commercial fish species due to its rapid growth rate, low-temperature resistance, high feed conversion rate, and extensive industry chain, making it a cornerstone of marine industrial farming in China.13 However, turbot aquaculture is threatened seriously by bacterial diseases.14 Ma, Mb and Mc are three new commercial compound probiotics containing mainly Lactobacillus, Acetobacter pasteurianus and Enterococcus faecium strains. These strains have been shown to have a positive effect on growth performance and disease resistance in marine fish.15 The main objective of this study was to evaluate the effects of three commercial probiotics products on intestinal morphology, intestinal microbiota, and immunity of juvenile turbot as diet additives. Such information will aid in developing commercial probiotics products to support the aquaculture breeding industry and promote its sustainable development.
2. Materials and Methods
2.1. Experimental design
This experiment was conducted to study the effects of three commercial compound probiotics on the performance of juvenile turbots. According to the Lim method,16 the basic diet was mixed with the compound probiotic to make the three probiotic groups feed, and the probiotic was added at the ratio of 3% of the mass of the basic diet. The prepared feeds were naturally dried at room temperature and stored at 4 °C. To ensure the activity of the added strains, fresh diets should be prepared daily. The strain composition of probiotic complexes has been sequenced by Sangon Biotech Co., Ltd. (Shanghai, China). The four experimental groups were as follows:
C1: control group, fish was fed the basic diet (0.25-0.36 mm), with crude protein ≥ 55%, crude protein ≥ 8%, lysine ≥ 2.5%, crude fiber ≤ 3%, crude ash ≤ 16%, calcium ≤ 4%, and moisture ≤ 9%.
Ma: fish was fed the basic diet supplemented with 50 mL/kg Ma (viable bacteria ≥ 109 cfu/mL). The Ma water product mainly included Enterococcus faecium (38%), Lactobacillus acidophilus (15%), Lactobacillus casei (15%), Lactobacillus plantarum (10%), Pediococcus pentosaceus (8%), and Clostridium butyricum (7.5%).
Mb: fish was fed the basic diet supplemented with 50 mL/kg Mb (viable bacteria ≥ 109 cfu/mL). The Mb was composed of beneficial microorganisms, mainly including Acetobacter pasteurianus (44%), Klebsiella sp. A16-KP12 (29.5%), Lactobacillus buchneri (21%), and Pediococcus pentosaceus (1%).
Mc: fish was fed the basic diet supplemented with 50 mL/kg Mc (viable bacteria ≥ 109 cfu/mL). The Mc potent Lactobacillus product consisted of Lactobacillus plantarum (95%) and Enterococcus durans (3%).
2.2. Experimental fish and feeding trial
A total of 3600 juvenile turbots (30-day-old) were obtained from Weihai Jikun Aquatic Technology Co., Ltd. (Shandong Province, China) with an initial body weight of 0.15 ± 0.07 g/fish. After a 5-day acclimation period, healthy fish with uniform size were pooled and then randomly allocated into 12 tanks at a density of 300 fish per tank (three replicates per treatment). The water temperature, pH, and salinity were maintained at 17 ℃ ± 1 ℃, 8.0 ± 0.3, and 29.0 ± 0.5, respectively. All fish received the experimental diet twice daily (at 07:00 am and 19:00 pm) and were fed until full each time.
2.3. Sample collection
After a 36-day feeding experiment, the fish were feed-deprived for 24 h. 3 fish were randomly selected from each tank, and the hindgut was dissected and collected on ice and placed in sterile test tubes filled with 4% paraformaldehyde solution for intestinal morphometric analysis.17 An additional 20 fish were randomly selected from each tank for growth measurements and then dissected on ice to collect the intestine in sterile tubes for intestinal microbiota analysis.
2.4. Analytical methods
2.4.1. Analysis of intestinal morphology
The hindgut was quickly resected during sampling, immediately immobilized in a 4% paraformaldehyde solution, dehydrated with graded ethanol series and encased in paraffin wax,18 and finally stained with hematoxylin and eosin. The Image-Pro Plus 6.0 software was used to measure muscular thickness, villus length, and microvilli height under the 40 × microscope.
2.4.2. Analysis of intestinal microbiota
Total DNA was extracted from the intestinal tracts. The V3-V4 region of bacterial 16S rRNA in intestinal contents samples was amplified by PCR using universal primers 338F (5’-ACTCCTACGGGAGGCAGCA-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’). High-throughput sequencing was performed using Illumina Miseq sequencing by Sangon Biotech Co., Ltd. (Shanghai, China). The products were purified, quantified, and homogenized to form a sequencing library. The obtained high-quality data were analyzed in the following aspects: OTU analysis, species annotation and taxonomic analysis, diversity analysis, principal coordinate analysis (PCoA), association analysis, and network analyses.19 All analyses were conducted in R (version 3.5.1, R Development Core Team).
2.4.3. Challenge test of using* *Aeromonas salmonicida
The challenge test was carried out after the feeding test, the Aeromonas salmonicida (BHAS-1, CCTCC NO: M 2021264) used in this challenge test was provided by Yantai Aquatic Animal Disease Prevention and Control Center (Shandong, China). BHAS-1 strain, which was isolated and preserved, was activated in advance and cultured on the nutrition agar plate. The bacterial suspensions of about 1013 cfu/mL were prepared by washing the colonies with sterile physiological saline. In our previous study, we calculated 1.62×1013 CFU/mL as the semi-lethal concentration (LC50) of BHAS-1 by the karbers method.20 20 fish in each group were randomly selected, and 100 μL of BHAS-1 solution and 100 μL of saline were injected intraperitoneally into the probiotic and control groups, respectively, and recorded the death. The death number was recorded continuously for 14 days in the whole challenge test.
2.5. Statistical analysis
The results data were expressed as mean ± standard error (SE). The data were statistically evaluated using One-way analysis of variance (ANOVA) combined with Duncan’s multiple comparison test and polynomial regression (linear regression and quadratic regression), respectively. All statistics were performed using SPSS 19.0 (SPSS IBM, America), and Duncan’s multiple range test was applied. The significance was set at P < 0.05.
3. Results
3.1. Growth performance
On day 36, the growth performance of the fish was calculated. The probiotic groups exhibited significantly higher length and weight gain compared to the CK group (Table 1). Among them, the Ma group had the most significant facilitation effect.
3.2. Structure of intestinal microbiota
3.2.1. Alpha diversity analysis
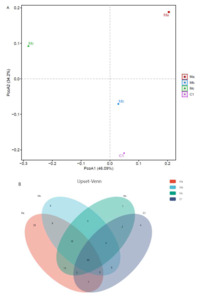
The OTUs richness and diversity index (ACE, Chao, Shannon, and Simpson) of intestinal microbial populations of turbots in different experimental groups are listed in Table 2. Among all groups, Ma had the highest OTUs richness (ACE and Chao) and Shannon indices and C1 had the highest Simpson index according to Venn diagram analysis.
3.2.2. Community composition and dominant species analysis
At the phylum level, the core microbial communities (top 10) of intestinal microbiota showed that Proteobacteria became the most dominant phylum in all groups. It is worth noting that probiotic groups can significantly reduce the relative abundance of Proteobacteria and increase the abundance of Firmicutes and Bacteroidetes compared with the C1 group (Figure 1A). In all groups, the effect of Ma was the most pronounced. At the genus level, the composition of the top 10 microbial changes significantly among different groups (Figure 1B). Compared to the CK group, the probiotic groups increased the relative abundance of Klebsiella, Brochothrix, Phenylobacterium, Lactobacillus, Acidovorax, and Ralstonia, and reduced the relative abundance of Pseudomonas and Shewanella (Figure 1B).
3.2.3. Beta diversity and OTU analysis
A PCoA was then conducted to visualize the differences in taxon composition among these groups (Figure 2A). Beta-diversity analysis showed that significant divergence occurred between probiotic groups and control groups. Compared to Mb and Mc, the Ma group showed a more significant separation. Next, we investigated differences in OTU amounts among different groups by Upset analysis (Figure 2B). A total of 55 OTUs were shared across all groups. In addition, 4 OTUs were unique in the C1 group, 25 OTUs were unique in the Ma group, 9 OTUs were unique in the Mb group, and 7 OTUs were unique in the Mc group.
3.2.4. Network analysis
Then we combined Ma, Mb, and Mc into the probiotic group, C1 into the control group, and further explored the bacterial co-occurrence patterns between the probiotics and control group using network analysis. In these two networks, the number of positive correlations was much higher than that of negative correlations (Figure 3 and Table 3). The values of graph density, avgCC, avgK, average weighted degree, and modularity in these empirical networks were significantly different between these groups, suggesting that the bacterial community compositions were significantly different between the probiotic group and the control group. Among the dominant bacterial communities in both groups, Proteobacteria, Firmicutes, Bacteroidota, Thermus, Tenericutes, Chloroflexi, Fibrobacteres, and Actinobacteriota tended to co-occur more than other phylum.
3.2.5. Key species and functional prediction
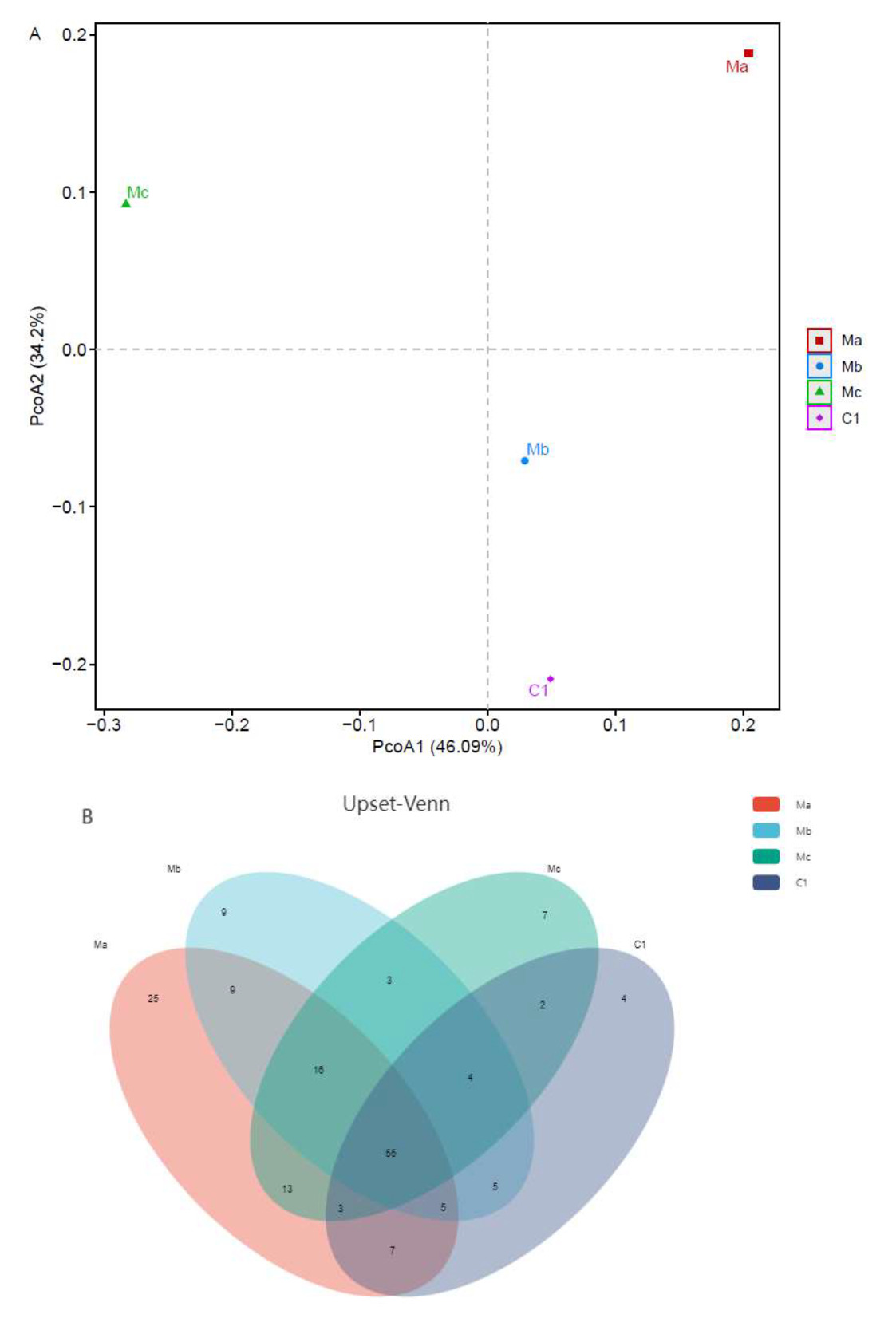
Due to the comparison of the potential effects, the distribution characteristics of key species among Ma, Mb, and Mc were further surveyed (Figure 4A). Ternary plots of key species across three probiotic groups showed that unclassified_Acidovorax, Propioniferax_sp._P7, Paracoccus_rhizosphaerae, unclassified_Ralstonia, Camellia_oleifera, and Arthrobacter_sp._GWS−BW−H45M contributed and enriched Ma, Pseudomonas_psychrophila and unclassified_PMMR1 contributed and enriched Mc, Shewanella_baltica, Shewanella_glacialipiscicola and Pseudomonas_sp._X−a10 contributed and enriched Mb.
The functional prediction analysis based on KEGG database is reported in Table 4. The analysis of functional classification prediction based on KEGG (level 2) showed that environmental information processing-membrane transport was the most abundant pathway in all experimental groups. Of these, metabolism-related functions were enriched in Ma compared with other groups, and they mainly belonged to the transcription factors, arginine, and proline metabolism (Figure 4B).
3.3. Intestinal morphology
The muscular thickness, intestinal villus length, and intestinal villus height were analyzed based on the intestinal morphology section (Figure 5). The muscular thickness did not show a significant difference in all experimental groups (P > 0.05) (Table 5). However, the intestinal villus length in the Ma, Mb, and Mc group was significantly higher than in the C1 group (P < 0.05). By comparing with C1 and Mb groups, the intestinal villus height in the Ma and Mc groups was significantly lower (P < 0.05).
3.4. Challenge experiment
The Aeromonas salmonicida challenge experiment in juvenile turbot after 36 days of feeding was conducted to examine the immunity performance. The cumulative mortality rate of the Ma group was 35% and 100% in the C1, Mb, and Mc groups. Moreover, in this challenging experiment, fish fed with the Ma diet began to die on the 8th day, but fish fed with other groups’ diets began to die on the 1st day. The deceased fish exhibited symptoms indicative of erythematous ulcers between the fins, an enlarged abdomen, and a red anus by morphological observation. The anatomical examination revealed the presence of ascites, congestion of the liver, swollen intestines, enlarged spleen, necrotic kidneys, and milky pus within the body.20 The symptoms exhibited by the fish are consistent with those reported in the aftermath of A. salmonicida infection. Consequently, it was possible to ascertain that the mortalities of the fish were due to A. salmonicida infections. Thus, feeding with Ma significantly improved the survival of A. salmonicid infected juvenile turbot than C1, Mb, and Mc.
4. Discussion
In recent years, the inclusion of multiple probiotics has gained interest. The advantage of using multiple-strain preparations is that they may further improve the overall beneficial effect of the probiotic formulation and are active against a wider range of conditions and species.21 However, fewer studies have been conducted on the use of compound probiotics in turbot applications. Therefore, this study was conducted to investigate the effect of different dietary compound probiotics on growth, intestinal microbiota, intestinal morphology, and immunity of juvenile turbots.
In this experiment, 30-day-old juvenile fish were chosen as the experiment subjects, and at this age, the fish were already able to feed on the basic diet. Studies have shown that the development of the digestive-absorptive system of turbot is basically complete by the age of 60-70 days, and the morphology and function of the digestive-absorptive system are close to those of adult fish.22 Thus, to ensure the effectiveness of the experiment, the experimental period was set at 36 days.
Our results showed that at the end of the 36-day feeding trial, the body weight and length of the treatment group receiving the probiotic complexes were increased compared to the control group, while there was a significant difference between the probiotic groups in terms of body weight and length, which was higher in Ma group than in other probiotic groups. Thus, our results not only showed that the probiotic complex had a positive effect on promoting the growth of turbot but also indicated that there were differences in the effects of different combinations of probiotics on the hosts.
The intestine plays a crucial role in digestion and defense.23 The Intestine microbiota has been identified as a significant regulator of host metabolism and immunity, making it an essential component of gut health.5 Previous studies had reported that the composition of the intestinal microbiota of aquatic organisms is mainly regulated by the environment and diet. The intestinal microbiota composition is closely related to the health of the host, which can improve host performance by promoting nutrient absorption, stimulating immune responses, and resisting disease.24
Thus, it is important to compare the potential effects of different probiotics on host intestinal microbiota, intestinal morphology, and immunity.
Significant divergence in intestinal microbiota was found in these groups. Ma group significantly improved the richness and diversity of the intestinal microbial community and promoted the stability of the intestinal microbial community, whereas Mb and Mc showed lower impact and efficiency. Likewise, rainbow trout diet supplemented with lactic acid bacteria could significantly improve the richness and diversity of the intestinal microbial community.25 In addition, compared with the C1 group, Ma could significantly improve the relative abundance of Firmicutes and Bacteroidota at the phylum level. Whereas the promotion effect of Mb and Mc on these bacteria was not ideal. There is evidence that the bacteria belonging to Firmicutes and Bacteroidota have been applied as probiotics due to their capacity for metabolism promotion.26 At the genus level, Ma dietary supplementation significantly reduced the relative abundance of pathogenic bacteria, such as Shewanellaceae and Pseudomonadaceae. In recent years, a large number of studies have been reported on the infection of marine-farmed animals by Shewanellaceae and Pseudomonadaceae27,28. Meanwhile, compared to the other group, the relative abundance of Lactobacillus was also higher in the Ma group. Studies have shown that Lactobacillus supplementation could improve the growth and disease resistance of fish and was recommended for commercial aquaculture production systems.29 Moreover, the key species of the Ma group also confirmed this view, such as unclassified_Acidovorax,30 Propioniferax_sp._P7,31 Camellia_oleifera,32 and Arthrobacter_sp.33 These findings further indicate that probiotic feeding can increase the abundance of potential probiotics in the intestinal of juvenile turbot to promote host health. Meanwhile, the count of potential probiotics enriched in Ma was more than Mb and Mc group. Network analysis provides a comprehensive understanding of the interactions, composition, and assemblage patterns of fish microbial communities, reflecting the ecological processes of fish microbial communities.34 Results showed that the networks between the probiotic and control group were significantly different and non-randomized, which is consistent with the topological features of a small-world and intrinsic modular architecture. It is worth noting that the strong interactions between intra- and inter-phylum taxa were found in the probiotic group which indicates the beneficial effect of the stability of the microbial community by probiotics feeding. Interestingly, according to the KEGG pathways abundance from high to low order in all the experimental groups, processing-membrane transport was the most abundant pathway, followed by the metabolism_amino acid metabolism pathways and the metabolism-carbohydrate metabolism pathways, which suggested that these pathways may be related to immunity and stress resistance of turbot, further study is needed in the future.
The histological information of the intestinal tract can effectively evaluate the digestive capacity and potential health status of fish fed with different dietary ingredients (Yarahmadi et al. 2011). Among them, the length of the intestinal villus, the height of intestinal villus, and the thickness of muscular are important indicators for judging intestinal absorption capacity and health.23 In this experiment, both Ma, Mb, and Mc had a positive effect on the hindgut villus length of juvenile turbot, indicating that these three commercial probiotic products used in this experiment can improve intestinal absorption capacity and intestinal health by increasing the length of the intestinal villus. However, the hindgut villus height in the current study was significantly decreased in the Ma and Mb group, which was contrary to the trend of intestinal villus length. The main reason for this phenomenon may be that the change in intestinal villus height is related to the intestinal diameter, according to a previous study.23
A. salmonicida is one of the most harmful fish pathogens in aquaculture worldwide.35 Previous research showed that acute infection of turbot with A. salmonides usually led to fatal diseases, while chronic infection showed multifocal granulomatous dermatitis.36 Studies have demonstrated that probiotics can reduce the mortality of fish infected by pathogens. For example, higher survival rates were exhibited in the Carassius auratus immunized with recombinant Lactobacillus casei after challenge with A. veronii.37 Compared to the control group, Siniperca chuatsi fed the diet supplemented with Lactococcus lactis had lower mortality rates.38 Similarly, in the current study, after challenge test with A. salmonicida, the cumulative mortality rate in the Ma group was 35%, while in the C1, Mb, and Mc group, the cumulative mortality rate was 100%, and fish fed with Ma diet began to die on the 8th day, but fish fed with other groups’ diet began to die on the 1st day. This result was consistent with the result of intestinal microbiota. It showed that turbot-fed the Ma supplemented group can directly affect intestinal microorganisms and act on microbial products, host products, or food components so as to regulate the host immune system and reduce the cumulative mortality rate after infection, with better effects than the Mb and Mc group.
5. Conclusions
In summary, this experiment confirmed the importance of dietary probiotics supplementation in stimulating growth performance, intestinal morphology, intestinal microbiota, and immunity of juvenile turbot (S. maximus). Considering the effects of dietary probiotics’ types and levels on intestinal morphology, intestinal microbiota, and immunity. The findings indicate Ma was the better optimum product supplemented to the juvenile turbot diet through improving the abundance of potential probiotics in the intestinal and the survival rates under A. salmonicida infection. In the next step, the mechanism of action of the probiotic will be analyzed from the perspective of transcriptomics and metabolomics. Meanwhile, the effect on the probiotic effect of the strains was explored in terms of changing the frequency of feeding and the concentration of the additions.
Acknowledgments
The authors gratefully acknowledge financial support from Yantai Science and Technology Innovation Development Programme Project (grant number: 2024YD079). We would like to thank Dr. Prof. Yuzhe Han from Dalian Ocean University for helpful comments on the manuscript.
Authors’ Contribution
Writing – original draft: Hanzhi Xu (Lead). Investigation: Hanzhi Xu (Equal), Xiaowei Zhao (Equal), Kun Chen (Equal), Yanan Cao (Equal), Xiaodan Liu (Equal). Data curation: Xiaowei Zhao (Equal), Hua Huang (Equal). Visualization: Han Wang (Equal), Xiaodan Liu (Equal). Resources: He Wang (Equal), Zhanjun Li (Equal). Writing – review & editing: He Wang, Zhanjun Li (Equal).
Competing Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
This study was performed in accordance with the Guidelines for Care and Use of Laboratory Animals of the Chinese Association for Laboratory Animal Sciences (No. 2011-2).
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.