1. Introduction
The whiteleg shrimp (Litopenaeus vannamei) is one of the most commonly cultured aquatic species in the Mekong Delta region and many countries around the world. In Viet Nam, the total brackish water shrimp farming area remained relatively unchanged compared to 2022, estimated at approximately 737,000 hectares. Of this, the farming area for whiteleg shrimp accounted for 115,000 hectares, while black tiger shrimp (Penaeus monodon) farming occupied 622,000 hectares. Shrimp farming productivity was assessed to be relatively high compared to previous years, with a significant breakthrough in production, reaching 1,12 million tons, which is an increase of 5.5% compared to 2022. Specifically, whiteleg shrimp production reached 845,000 tons, and black tiger shrimp production reached 274,000 tons.1 In intensive shrimp ponds, the demand for feed is considerable, leading to substantial waste production from shrimp, which in turn increases nutrient levels in the pond water.2 Excessive nutrient accumulation in aquaculture ponds is a major factor promoting the proliferation of phytoplankton, triggering cyanobacterial blooms.3,4 Therefore, environmental management in shrimp ponds, especially algal control, is receiving increasing attention.
Cyanobacteria exhibit characteristics of both bacteria and phytoplankton. The genera commonly associated with bloom formation include Microcystis, Anabaena, Oscillatoria, and Phormidium.5,6 A study indicated that blue-green algae are also important as a potential resource for renewable energy and natural products.7 Microalgal growth and the rate of lipid buildup can be largely influenced by the cultivation conditions. Nutrient media composition, mode of cultivation, and physical parameters are key components to optimize and regulate cultivation conditions for high biomass and lipid production.8 In contrast, filamentous cyanobacteria such as Oscillatoria, Lyngbya, and Arthrospira are often dominant in ecological shrimp ponds in Ca Mau. These species offer little nutritional value and are considered detrimental to shrimp health. Due to the presence of intracellular gas vesicles, cyanobacteria, particularly in filamentous form, are indigestible to shrimp. Ingestion can lead to digestive disorders, including white feces disease in both whiteleg shrimp (L. vannamei) and black tiger shrimp (P. monodon). Additionally, these cyanobacteria may impart undesirable odors and flavors to shrimp. When entangled in gills, they secrete mucilage that clogs gill structure, potentially leading to shrimp mortality.9 Notably, Oscillatoria nigroviridis has been reported to produce both hepatotoxins and neurotoxins, posing severe risks to shrimp health.10 Members of the genus Phormidium are also capable of producing a range of cyanotoxins, and their proliferation in ponds can be particularly harmful to cultured shrimp.11,12 Environmental nutrient conditions play a crucial role in controlling cyanobacterial growth. In intensive whiteleg shrimp aquaculture systems, harmful cyanobacterial blooms significantly affect survival rate, growth performance, and overall yield. However, limited research has been conducted to quantify the density of harmful cyanobacteria in shrimp ponds. Therefore, this study aimed to evaluate the influence of different nutrient media on whiteleg shrimp growth and to determine the lethal concentration that causes 50% mortality (LC₅₀) in L. vannamei. The findings will provide a foundation for further studies on the impact of blue-green algae on the health and quality of whiteleg shrimp.
2. Materials and Methods
2.1. Research time and location
The study was conducted from August to December 2024 at the College of Aquaculture and Fisheries, Can Tho University.
2.2. Research materials
The nutrient medium used for algal cultivation included Walne,13 F/2,14 AGP (Algae growth promoter) and NPK (30:10:10).
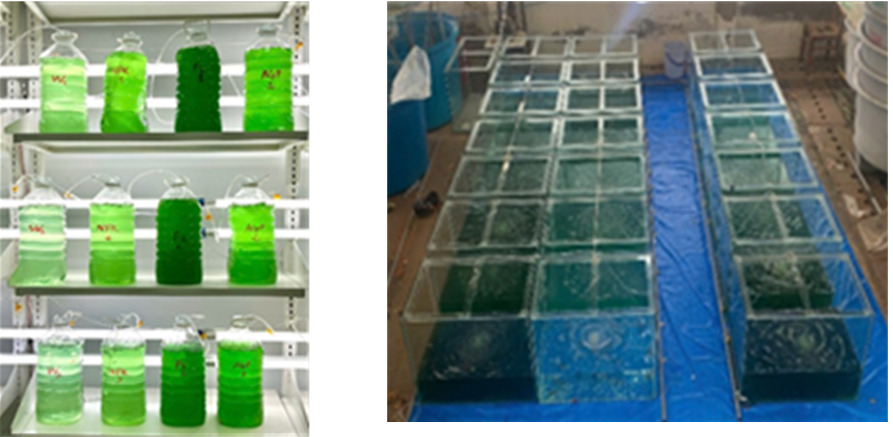
The algal strain P. chlorinum (Figure 1) was isolated from a whiteleg shrimp (L. vannamei) farming ponds in Bac Lieu Province. After collection, the algal samples were stored at temperatures of 4oC and transported to the laboratory for isolation and mass cultivation. Once sufficient biomass was obtained, experimental setups were conducted.
The seawater used for algal inoculation had a salinity of 10‰ and was pre-treated with chlorine (20 mg L-1). Once chlorine residues were eliminated, alkalinity was adjusted to an optimal range of 80-120 mg CaCO3 L-1, and the water was filtered through a 1 µm microfiltration membrane prior to use. Whiteleg shrimp used in the experiment had an average body weight ranging from 7 to 12 grams.
2.3. Research method
2.3.1. Isolation and mass cultivation of algae
Algal sampling and isolation: Algal samples were collected from L. vannamei ponds and filtered sequentially through plankton nets with mesh sizes of 60 µm and 15 µm to remove debris and impurities. Samples were examined under a microscope to identify and select P. chlorinum as the dominant species for isolation. Morphological characteristics of P. chlorinum were observed, and species identification was identified using 16S rRNA gene sequencing. Amplification of the 16S rRNA gene obtained from blue-green algae DNA was approximately 1,500 bp. After DNA analysis, the results from BLAST-N alignment indicated that the DNA sequence of the algae isolated from whiteleg shrimp ponds revealed a high identity (98.84%) with the P. chlorinum reference species (accession number GQ859648.1).
Isolation by serial dilution method: A total of 10 test tubes were prepared, each containing 9 mL of water with 10‰ salinity, the same with original pond water conditions and supplemented with F/2 medium as the nutrient source. 1 mL of concentrated algal culture, previously enriched to favor the dominance of P. chlorinum was added to the first test tube and mixed thoroughly. Then, 1 mL was transferred from the first tube to the second, and this serial dilution process was continued through to the tenth tube. All tubes were incubated on a test tube rack under controlled laboratory conditions with continuous light exposure (24h light:0h dark photoperiod), at a temperature of 30°C, and light intensity of 3,000 Lux. Algal growth in each tube was monitored daily to assess the purity of P. chlorinum. Transfers were made to new media as needed until a pure culture of P. chlorinum was obtained. Once a test tube with a monoculture was confirmed, it was selected for mass cultivation and used for experimental setup.
2.3.2. Experimental setup
Experiment 1: Effects of different nutrient media on the growth of P. chlorinum.
The experiment was conducted at a sheltered experimental station. P. chlorinum was cultured in 6.0-liter plastic containers containing a culture algae volume of 5 L (Figure 2). The initial algal density was set at 200 ind mL⁻¹. Light was provided by white LED lights at an intensity of 3,000 Lux, with continuous light exposure (24 hours) and constant aeration. The experiment was finished when algal densities in the treatments declined for two consecutive days. Four types of nutrient media were used: Walne, F/2, AGP (Algae Growth Promoter), and NPK (30:10:10) to cultivate P. chlorinum. The experiment was randomly designed with four treatments corresponding to the four-nutrient media, each with three replicates. The application rate was 2 mL of nutrient solution per liter of algal culture. Nutrient supplementation was only done once at the beginning. The experimental duration was 8 days.
Experiment 2: Determination of P. chlorinum density causing 50% mortality (LC50) in whiteleg shrimp
Based on the results from Experiment 1, F/2 medium was selected for mass cultivation of P. chlorinum due to its superior algal growth performance. In Experiment 2, seven treatments were established, each with three replicates. Whiteleg shrimp (L. vannamei) with a body weight of 7–10 g was stocked at a density of 20 shrimp per tank, with a water volume of 80 liters and salinity of 10‰. Continuous aeration was provided throughout the experiment to maintain dissolved oxygen levels. From the results of algae sampling in shrimp ponds, the highest density of P. chlorinum algae was about 30,000 ind. mL-1. Based on this result, experiments were designed with different algae densities from 5,000 to 30,000 ind. mL-1 to find out the algae density that causes shrimp mortality at a rate of 50%. Shrimp were acclimated for one day prior to the addition of cyanobacteria at different densities: the control treatment without cyanobacteria (Control); 5,000 ind mL⁻¹ (T5); 10,000 ind mL⁻¹ (T10); 15,000 ind mL⁻¹ (T15); 20,000 ind mL⁻¹ (T20); 25,000 ind mL⁻¹ (T25) and 30,000 ind mL⁻¹ (T30). Following the addition of P. chlorinum, shrimp mortality was monitored at 30-minute intervals. Dead shrimp were immediately recorded and removed to prevent deterioration of water quality. Shrimp were fed to satiation four times a day at 7:00 PM, 11:00 AM, 16:00 PM, and 21:00 PM. The shrimp tanks were not water-changed during the experiment. Algal concentration was measured daily using a sedgewick-rafter counting chamber to ensure that the algal density was maintained across the experimental treatments. The experimental period lasted 14 days. The LC₅₀ value was calculated based on shrimp mortality rates across the different treatments.
2.3.3. Monitoring parameters
Temperature and pH were measured twice daily at 08:00 and 14:00 using a multi-parameter water quality meter. Nutrient parameters including total ammonia nitrogen (TAN), nitrate (NO3-), phosphate (PO43-), chlorophyll-a, dry weight, and alkalinity were sampled every three days. The sampling and analytical methods for water quality parameters are detailed in Table 1.
Algal density was determined daily by collecting a 10 mL water sample, which was stored in a glass bottle and fixed with formalin (concentration of 4%). A 1 mL sample was placed in a Sedgewick-Rafter counting chamber, and the number of filaments was counted under a microscope, with each filament considered as one individual. Algal density was calculated using the method described by Boyd & Tucker.16
Algal density (ind mL-1) =
Where: T is the number of individuals counted, Vs is the volume of sample collected, Vc is the volume of the concentrated sample, N is the total number of counting squares, A is the area of one counting squares (1 mm2).
Dry weight: On day 5, a 50 mL algal sample (corresponding to a determined density) was filtered through a 0.22 µm Whatman filter paper that had been pre-dried at 60°C for 2 hours. The sample was then oven-dried at 60°C for 24 hours. A digital analytical balance (precision: 0.0001 g) was used to determine algal dry weight as follows:
Dry weight = IW (before drying)- FW (after drying)
Where: IW: algal dry weight before drying, FW: algal dry weight after dried.
Chlorophyll-a concentration: Chlorophyll-a concentration was measured every 3 days. A 50 mL sample was extracted with acetone and analyzed using a spectrophotometric colorimetric method.15 Absorbance was measured at 630, 647, 664, and 750 nm. The chlorophyll-a concentration (µg/L) was calculated using the following formula:
Chlorophyll-a=[11,85(E664-E750)-1,54(E647-E750)- 0,08(E630E750)]x[(V1x1000)]/V2(µg/ L)
Where: V1 is the volume of Acetone used (50 mL), V2 is the volume of water sample filtered
Algal size measurement: Algal size was measured on 100 randomly selected individuals using a microscope equipped with a digital camera (Eclipse Ci-L plus + DS-Ri + Nis Element AR). Measurements were taken at the start of the experiment and at the end of the exponential growth phase (day 5).
2.3.4. Data analysis
Data were analyzed for maximum, minimum, mean, and standard deviation using Microsoft Excel. Differences among treatment means were tested for statistical significance at p*<0.05* using SPSS software version 22.0. The LC₅₀ value was estimated using the Probit analysis method.17
3. Results
3.1. Effects of different nutrient media on the growth of P. chlorinum
3.1.1. Environmental parameters
3.1.1.1. Temperature and pH
Water temperature across treatments ranged from 28.3 to 28.4°C in the morning and from 31.7 to 31.8°C in the afternoon. Results in Table 2 show that the average temperature among treatments was not significantly different (p>0.05). Morning pH values across treatments ranged from 7.9 to 8.8, while afternoon values ranged from 8.3 to 8.9. The AGP treatment recorded significantly higher pH values in the morning compared to the Walne treatment (p<0.05), but no statistically significant differences were observed when compared with the F/2 and NPK treatments (Table 2).
3.1.1.2. TAN, NO3- , PO43- concentrations
The concentrations of water quality parameters such as total ammonia nitrogen (TAN), nitrate (NO3-), and phosphate (PO43-) showed a decreasing trend from day 1 to the end of the experiment (Figure 3). The average TAN concentration among treatments ranged from 0.037±0.001 to 6.540±0.009 mg L⁻¹. The highest TAN concentration was recorded in the NPK treatment throughout the sampling periods. In general, TAN levels declined by the end of the experiment across all treatments. Statistical analysis revealed significant differences in TAN concentrations among treatments during the sampling periods (p<0.05). Similarly, PO₄³⁻ concentrations ranged from 0.259±0.006 to 1.689±0.017 mg L⁻¹. Significant differences in PO43- concentrations were observed among treatments on day 1 and day 7. On day 1, the NPK treatment had the highest PO₄³⁻ level, while the Walne treatment showed the highest value on day 7. During the mid-phase of the experiment (day 4), PO43- concentrations in the Walne and F/2 treatments were significantly higher (p<0.05) compared to the NPK and AGP treatments. Additionally, NO3- concentrations during the experiment ranged from 0.073±0.001 to 4.300±0.020 mg L⁻¹. There were statistically significant differences (p<0.05) in NO₃⁻ levels among treatments throughout the sampling periods, with the Walne treatment consistently exhibiting higher NO3- concentrations compared to other treatments (Figure 3).
3.1.2. P. chlorinum growth under the influence of different nutrient medium
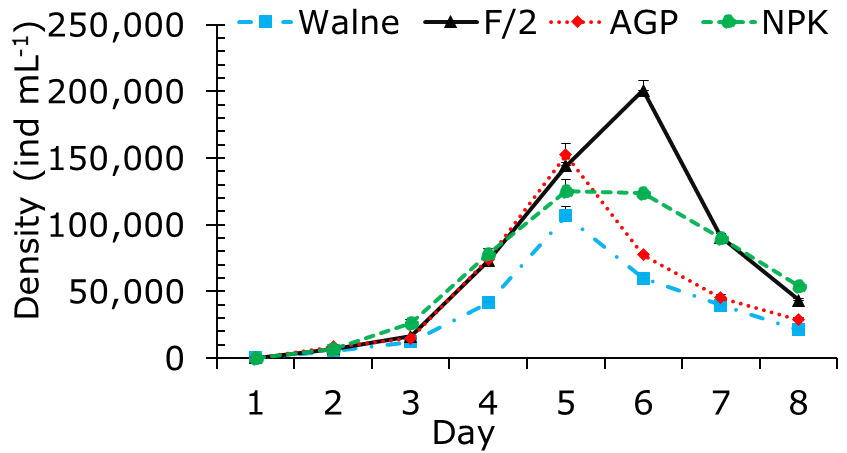
The algal density in all treatments began to increase significantly from day 3 to day 5. The densities of P. chlorinum in the Walne, AGP, and NPK treatments on day 5 were 106,611±6,993ind. mL⁻¹, 152,444±8,507 ind. mL⁻¹, and 125,500±8,023 ind. mL⁻¹, respectively. Thereafter, algal density continued to rise, reaching its peak at 200,741±7,389 ind. mL⁻¹ in the F/2 treatment on day 6. In contrast, algal densities in the other treatments showed a decreasing trend toward the end of the experiment. The results also indicated that the mean algal densities from day 4 until the end of the experiment were significantly different among treatments (p<0.05) (Figure 4).
The initial cell length of P. chlorinum across all treatments was 722.4±129.8 µm. After 5 days of culture, the sizes increased and reached values of 864.0±245.5 µm, 1,018.0±244.5 µm, 964.9±230.6 µm, and 903.2±190.9 µm in the Walne, F/2, AGP, and NPK treatments, respectively (Table 3). Results showed that on day 5, the F/2 treatment exhibited the highest size of P. chlorinum (1,018.0±244.5 µm), which was significantly different (p<0.05) from the Walne, AGP, and NPK treatments.
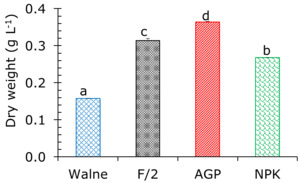
The dry weight of P. chlorinum ranged from 0.157 to 0.364 g L-1, with the highest value recorded in the AGP treatment (0.364±0.002 g L-1), followed by F/2 (0.314±0.005 g L-1), NPK (0.268±0.016 g L-1), and the lowest in the Walne treatment (0.157±0.001 g L-1). The dry weight of the algae differed significantly among treatments during the experimental period (p<0.05) (Figure 5). This result showed that when the algal density increased, the dry weight proliferated, and vice versa.
Chlorophyll-a concentration
Chlorophyll-a concentrations in all treatments increased from the second sampling day to day 7 and then declined on day 8 (the final day of the experiment). After the first day of cultivation, chlorophyll-a levels were the lowest across all treatments, with no statistically significant differences observed (p>0.05). After four days, chlorophyll-a concentrations increased in all treatments, ranging from 298.3±2.3 to 457.6±7.4 µg L-1. The highest value at this stage was recorded in the NPK treatment, and statistically significant (p<0.05) differences were found among treatments. On day 7, chlorophyll-a concentrations increased significantly, reaching a peak value of 3,305.7± 5.7 µg L-1 in the F/2 treatment, which was significantly different (p<0.05) from the other treatments (Table 4). Based on the results of this experiment, it can be concluded that the F/2 medium, when applied at a dosage of 2 mL L-1, was more effective in promoting biomass production of P. chlorinum compared to the Walne, AGP, and NPK media. The corresponding values of algal density, dry weight, and chlorophyll-a concentration achieved in the F/2 treatment were 200,741±7,389 ind mL-1, 0.364±0.002 g L-1, and 3,305.7±5.7 µg L-1, respectively.
3.2 Determination of P. chlorinum densities causes mortality 50% (LC50) on white leg shrimp
Water quality parameters during the experiment
Throughout the experimental period, water temperature remained relatively stable across treatments. Morning temperatures ranged from 26.72±0.06 to 26.84±0.03 °C, while afternoon temperatures ranged from 28.30±0.04 to 28.54±0.12 °C (Table 5). These temperatures were within the optimal range for the growth and development of whiteleg shrimp. The pH values during the experiment ranged from 6.9 to 8.1 across treatments. Morning pH values ranged from 7.35±0.11 to 7.55±0.01, and afternoon pH values ranged from 7.48±0.08 to 7.69±0.00. There were no statistically significant differences (p>0.05) in average pH values between treatments in either the morning or afternoon (Table 5).
Dissolved oxygen concentrations remained relatively stable throughout the experimental period. DO levels among the treatments in the morning ranged from 5.14±0.08 to 5.36± 0.02 mg L-1, and in the afternoon from 5.12±0.09 to 5.24±0.06 mg L-1 (Table 5). Due to the continuous aeration system installed in the shrimp culture tanks, DO concentrations were consistently maintained within a stable range, with no statistically significant differences (p>0.05) observed among treatments. The results indicate that DO concentrations in all treatments were within the optimal range for shrimp development.
Total alkalinity among treatments ranged from 149 to 158 mg CaCO3 L-1. Alkalinity levels tended to decrease toward the end of the experiment, with no statistically significant differences (p>0.05) among treatments across sampling periods (Figure 6A).
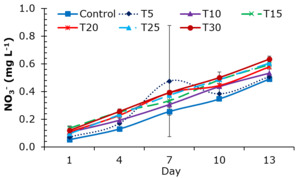
TAN concentrations in the treatments of experiment 2 ranged from 0.011 to 0.739 mg L-1. The highest mean TAN concentration recorded throughout the sampling periods was 0.27±0.02 mg L-1 in treatment T30. The remaining treatments showed TAN concentrations of 0.20±0.16 mg L-1 in treatment control; 0.20±0.06 mg L-1 in T5; 0.24±0.13 mg L-1 in T10; 0.20±0.10 mg L-1 in T15; 0.16±0.03 mg L-1 in T20; and 0.24±0.12 mg L-1 in T25 (Figure 6B). Nitrate (NO3-) concentrations exhibited an increasing trend from day 1 to the end of the experiment, with no statistically significant differences (p<0.05) observed among treatments (Figure 7). NO₃⁻ concentrations ranged from 0.046 to 0.940 mg L-1. The lowest mean NO3- concentration across sampling periods was observed in treatment control (0.25±0.17 mg L-1), while the highest was recorded in treatment T30 (0.38±0.20 mg L-1).
Phosphate (PO43-) concentrations in the water across all treatments during the experimental period ranged from 0.09 to 0.49 mg L-1. The average PO43- levels tended to increase toward the end of the experiment. The highest PO43- concentration was recorded in treatment control (0.26 mg L-1), while the lowest was observed in treatment T10 (0.15 mg L-1). In treatment control, PO43- levels increased on days 10 and 13 (Figure 8A). Overall, PO43- concentrations in all treatments exceeded the acceptable limits, indicating a high nutrient environment in the experimental shrimp culture tanks.
Chlorophyll-a concentrations varied significantly among treatments, ranging from 88.625 to 666.240 µg L-1. Chlorophyll-a levels tended to increase from day 1 to day 7 and subsequently declined by day 13 of the experiment (Figure 8B). Higher chlorophyll-a concentrations were observed in treatments with increased cyanobacterial biomass. Additionally, elevated concentrations of nutrients such as NO3- and PO43- promoted strong algal growth in the experimental whiteleg shrimp culture tanks.
3.2.2. Shrimp mortality rate (LC50)
When the cyanobacterium P. chlorinum was introduced into the experimental whiteleg shrimp culture tanks at different densities, the recorded shrimp mortality rates during a 7-day period were the highest in treatment T30 (45%), which differed significantly compared to the other treatments with death rates ranging from 0-10%. Algal density causing shrimp death with a rate of 50% was estimated to be approximately 34,672 ind. mL-1. Similarly, shrimp mortality rates in a 14-day period were 23.3±2.9%, 25.0±5.0%, 35.0±21.8%, 46.7±20.2%, 48.3±16.1%, and 76.7±7.6% for treatments T5, T10, T15, T20, T25, and T30, respectively. The highest death rate was observed in treatment T30, which was significantly different (p<0.05) from the other treatments (Figure 9). In the control group (without the addition of P. chlorinum), no shrimp mortality was observed, and the survival rate remained at 100%. The estimated algal density causing shrimp mortality at a rate of 50% over a 14-day period was nearly 26,432 ind.mL-1.
Discussion
Water environmental parameters such as temperature, pH, salinity, and nutrient content in water affect the growth of blue-green algae. Algal species of Phormidium ambiguum and P. foetida develop best at temperatures of 25°C to 33°C.18 Some genera of Oscillatoria, Anabaena, Phormidium, Nostoc, and Lyngbya thrive in waters with pH from 7.5 to > 9.0.19 In addition, water pH levels from 7.5 to 9.0 were suitable for Oscillatoria sp. and Microcystis sp. development, while a pH below or above these ranges caused a significant decrease in the growth of these blue-green algae.20 Filaments of P. chlorinum can be split up into smaller filaments when the pH of the water is out of the desired range. In general, the temperature and pH in experiment 1 were within the suitable range for the growth of blue-green algae P. chlorinum.
The nutrient contents in the water environment, including TAN, NO₃⁻, and PO₄³⁻, gradually decreased at the end of the experiment. This showed that the algae absorb nutrients over the culture time to grow, so these parameters tend to decrease at the end of the experiment. On day 4, the TAN content in the treatment control was greater than in other treatments and significantly different (p<0.05) from treatments T5, T20, and T25. The shrimp waste products had a high nutritional value but were not extensively absorbed, whereas the remaining treatments were taken in by the blue-green algae. However, on day 7, the decomposition of dead algae and organic matter increased the TAN level in treatments T10, T15, and T25 and was significantly different (p<0.05) compared to the control treatment and T20. During the study period the harmful nitrogenous waste was effectively removed by phytoplankton and microbial activity.21,22 With adequate amounts of water, air, sunlight, and nutrients like phosphorus and nitrogen, blue-green algae grow very rapidly and form a big bloom.23 In the experiment on the effect of different nutrition media on the growth of blue-green algae (Microcystis sp.), the levels of TAN, NO₃⁻, and PO₄³⁻ also decreased gradually through the sampling stages.24 Most algae use nitrogen in the form of NH₄⁺ and NO₃⁻; green algae and blue-green algae need nitrogen content from 0.1 to 1 mg L⁻¹. In water bodies, phosphorus content is often low; the suitable phosphorus content for algae growth is 0.018-0.098 mg L⁻¹; when phosphorus exceeds 18 mg L⁻¹, algae are completely inhibited.6 As Phormidium sp. algae has a strong capability to grow in nutrient-enriched or polluted water.25 A study agreed that high phosphate concentrations usually encouraged the growth of Cyanobacteria.26 Algae of Phormidium yuhuli were cultured with 10 mM glucose as a carbon source; the growth rate of the algae increased in the early days of the culture cycle with a density of 101,741 ind. mL-1.27 In this study, the highest density of blue-green algae was 200,741±7,389 ind. mL⁻¹ in the treatment F/2 after 6 days of culture. The medium F/2 is the best environment for the optimal growth of blue-green algae Oscillatoria agardhii when the pH is ranging from 7.5 to 9, salinity > 15‰, and the highest algae biomass was 5,983 mg L⁻¹ after 12 days of culture.28 In addition, the study using different nutrient media, including NPK, F/2, AGP, and Walne, to cultivate blue-green algae Planktothrix pseudagardhii, the results revealed that the maximum algae density was 216,722 ± 111 ind. mL⁻¹ in medium F/2 after 7 days of cultivation.29 This suggested that differing cultivation conditions also affect how long it takes for the algal density to peak. The size of P. chlorinum algae in the current study reached the highest at 1,018.0±244.5 µm in the treatment F/2. Similarly, the average size of Planktothrix pseudagardhii algae also increased the highest (1,065.0±412.6 µm) when cultivated in an environment F/2.29 Thus, it can be seen that algae size changes depending on the nutritional composition of the culture environment because each type of medium has different N and P contents.
The density of P. chlorinum algae in the treatment Walne was determined to be the lowest among the treatments, possibly due to the nutritional environment not being suitable for algae growth. Normally, the chlorophyll-a level is directly proportional to the algae density. In this study, the algae abundance and chlorophyll-a content were determined to be the lowest in the treatment Walne (106,611±6,993 ind L⁻¹ and 1,944.7±3.2 µg L⁻¹) and the highest in the treatment F/2 (200,741±7,389 ind L⁻¹ and 3,305.7±5.7 µg L⁻¹). Chlorophyll-a concentration has been widely used as a convenient correlation of biomass in estimations of phytoplankton growth.30 Maximization of chlorophyll-a in Phormidium sp. algae (9,300 µg L⁻¹) was induced by green light, while total carotenoids and β-carotene (3.05 and 0.89 µg mL-1, respectively) were induced by high white light,31 which were higher than the chlorophyll-a content recorded in this study. In addition, the highest chlorophyll-a content of 3,046±712 µg L-1 was achieved in the culture of Oscillatoria sp. at 10 ppt salinity after 10 days of experiment.20 The treatment F/2 exhibited the maximum algal density and chlorophyll-a content, but the treatment AGP had the highest measured dry weight of algae.
Algal growth and water quality characteristics are essential for whiteleg shrimp development. High nutrient content in water is favorable for algal growth, especially for species belonging to the cyanobacteria phylum. The variation in water quality parameters is closely related and indirectly affects shrimp growth rate and aquaculture productivity.32 The pH level affects all the chemical reactions of water and thus the physiological conditions of the shrimp.33 In culturing systems, the optimal pH range for whiteleg shrimp is reported to be between 7 and 9.34 The optimal DO and pH range for whiteleg shrimp farming is above 3 mg L⁻¹ and 7.5-8.5, with a pH fluctuation range of 0.5.35,36 The best temperature for the growth of whiteleg shrimp is from 25 to 32°C.37 In general, the temperature, pH, and DO parameters in the experiment were suitable for the growth and development of whiteleg shrimp. The alkalinity level in treatment T7 decreased to the lowest at the end of the experiment but was still within the appropriate range for shrimp growth. The alkalinity level should be maintained within an optimal range (75 to 150 mg L⁻¹); if alkalinity is found to be very low (<20 mg L⁻¹), that leads to poor phytoplankton growth.38 The change of temperature, pH, TAN, and PO₄³⁻ level in the pond affected the shrimp growth rate by approximately 16.6%, 55.7%, 1.8%, and 14.3%, respectively.39 In this study, the TAN content was highest on day 4 in treatment control, while at the end of the experiment, TAN was highest in treatments T25 and T30. In addition, the NO₃⁻ and PO₄³⁻ contents increased at the end of the experiment. Treatment control had the highest PO43- content increase at the end of the experiment. The shrimp tanks were not supplemented with blue-green algae, so PO43- was not significantly absorbed by algae, resulting in a high PO43- content. The decomposition of organic matter (dead algae, molted shrimp shells) increased the nutrient content in the water environment in treatments T5, T10, T15, T20, T25, and T30 at the end of the experiment. The optimal level of ammonia is below 0.05 to 0.01 mg L⁻¹, and nitrite and nitrate are below 0.01-0.1 mg L⁻¹ in the whiteleg shrimp farming ponds.38 In shrimp ponds, the ideal TAN concentration varies from 0.2 to 2 mg L⁻¹, while in aquaculture, the optimal PO₄³⁻ concentration falls from 0.005 to 0.2 mg L⁻¹ (Boyd, 1998). The chlorophyll a contents in black tiger shrimp ponds in Malaysia were found to range from 5.03±2.17 to 32.61±0.35 μg L⁻¹ throughout the cultivation period.40 The treatments with different densities of blue-green algae added to the shrimp tanks caused the chlorophyll-a concentration to increase very high and peaked on day 7. Of which, treatment T30 had the highest chlorophyll-a concentration. The highest shrimp mortality rate was also recorded in treatment T30. Blue-green algae blooms can cause severe water quality deterioration, including scum formation and toxin production.41 Species of the genus Phormidium can produce a variety of cyanotoxins, which are harmful to shrimp when grown in large quantities.11,12 Therefore, the addition of P. chlorinum to shrimp tanks had a great impact on shrimp survival rates. Research results revealed that shrimp mortality rates increase when P. chlorinum was added to shrimp tanks at higher densities. The presence of filamentous blue-green algae (P. chlorinum) at high densities clogs shrimp gills, thereby affecting shrimp mobility and respiratory activity. In addition, blue-green algae may produce toxins that are lethal to the shrimp. Besides, algal blooms in ponds can cause local hypoxia at night, leading to hypoxia in the blood and mass mortality of shrimp.42 Shrimp health depends on environmental factors, and a stressful environment reduces the immunity of shrimp.43 The addition of blue-green algae at the end of the culture cycle did not significantly affect shrimp length and weight. However, the survival rate of shrimp decreased significantly when adding Planktothrix pseudagardhii algae to shrimp tanks at higher densities.44 Moreover, the cyanobacteria Limnothrix strain LmTK01 revealed a negative direct and indirect effect on the shrimp survival rate and production. It was found that shrimp died due to the molting of shrimp and the filament of algae attached to the appendages, gills, and digestive tract. Shrimp died in over 50% of all treatments were inoculated with Limnothrix strain LmTK01 (336.17, 703.70, and 1,390.63 µg chl-a L-1) in 96 hours.45 In addition to the above problems, some species of blue-green algae can produce odorous compounds, which can cause off-flavors in the cultivated shrimp.
Conclusions
For biomass cultivation of P. chlorinum algae, the F/2 medium was determined to be the most appropriate for its growth when compared to Walne, F/2, AGP, and NPK nutritional media. Peak values for density, dry weight, and chlorophyll-a concentration of blue-green algae were observed on day 5 of the cultivation cycle. Treatments supplemented with higher algal densities resulted in lower shrimp survival rates. The mortality rate of white leg shrimp in the treatment with P. chlorinum added at a density of 30,000 ind. mL⁻¹ was significantly higher than that in the other treatments. The predicted algal level causing 50% shrimp mortality in the 7-day period was 34,672 ind mL-1, while the concentration causing shrimp to die at the same rate in the 14-day period was 26,432 ind mL-1. The lethal cyanobacterial toxin content for whiteleg shrimp should be conducted in further studies.
Acknowledgments
The authors are thankful to the colleagues and students of the College of Aquaculture and Fisheries, Can Tho University, for their great support in completing the study. This study was funded by the Ministry of Education of Vietnam under a project of “Research on the species composition of harmful algae and its potential to cause aquatic organisms in freshwater and brackish-marine water environments”, Project code: B2023-TCT-12 (Project coordinator: Nguyen Thị Kim Lien).
Authors’ Contribution
Methodology: Nguyen Thi Kim Lien (Lead). Formal Analysis: Phan Thi Cam Tu (Equal), Vo Thanh Toan (Equal), Au Van Hoa (Equal). Investigation: Phan Thi Cam Tu (Equal), Vo Thanh Toan (Equal), Au Van Hoa (Equal). Writing – original draft: Nguyen Thi Kim Lien (Lead), Vu Ngoc Ut (Equal). Resources: Phan Thi Cam Tu (Equal), Vo Thanh Toan (Equal), Au Van Hoa (Equal). Writing – review & editing: Nguyen Thi Kim Lien (Lead), Vu Ngoc Ut (Equal). Conceptualization: Nguyen Thi Kim Lien (Lead). Funding acquisition: Nguyen Thi Kim Lien (Lead). Supervision: Nguyen Thi Kim Lien (Lead).
Competing Interest – COPE
No competing interests were disclosed
Ethical Conduct Approval – IACUC
The experiment was carried out in accordance with national guidelines on the protection of animals and experimental animal welfare in Vietnam (Law on Animal Health, 2015).
(Reference: Law on Animal Health, 2015. Vietnam National Assembly, No. 79/2015/QH13).
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
The data that has been used is confidential.

.png)



_and_tan_(b)_of_all_treatments_at_experiment_2.png)
_and_chlorophyll-a_(b)_concentrations_of_the_treatments_over_the_sampling_per.png)
_of_whiteleg_shrimp_in_7_day_and_14_day_periods.png)
.png)


_and_tan_(b)_of_all_treatments_at_experiment_2.png)

_and_chlorophyll-a_(b)_concentrations_of_the_treatments_over_the_sampling_per.png)
_of_whiteleg_shrimp_in_7_day_and_14_day_periods.png)