1. Introduction
The Pacific white shrimp, Litopenaeus vannamei (Boone, 1931), holds a dominant position in global aquaculture due to its high economic value, rapid growth, adaptability to diverse environments, and strong market demand.1 While some taxonomic ambiguity remains (often synonymized with Penaeus vannamei), recent taxonomic updates reaffirm the latter as the accurate classification,2 highlighting the dynamic nature of systematics in aquaculture. Global shrimp farming has expanded substantially, with production increasing significantly over the past decade.3,4 L. vannamei accounts for approximately 83% of total output, a trend driven by its biological advantages and economic appeal. However, this intensification has concurrently led to heightened disease risks, threatening production sustainability and causing significant financial losses.5 In response, recent attention has increasingly turned towards managing the gut microbiota as a strategic approach to bolster shrimp health and resilience, as the intestinal microbiome plays an essential role in modulating immune response, digestion, and nutrient utilization.6
Probiotic supplementation has been recognized as a promising and environmentally friendly strategy to enhance shrimp performance through gut microbiota modulation. Dietary inclusion of beneficial bacteria, such as Bacillus subtilis and Lactobacillus spp., has been widely investigated and shown to improve growth, feed efficiency, and immune responses in L. vannamei.7–9 For instance, specific dosages of Lactococcus lactis and B. subtilis have been identified to effectively promote growth, improve feed conversion, and enhance antioxidant activity and digestive enzyme function, contributing to better overall health and disease resistance.7 The shrimp intestine harbors a complex, metabolically active microbial ecosystem. Host-microbiota interactions, shaped by co-evolution, profoundly influence critical physiological processes such as immune modulation, nutrient absorption, and disease resistance.10 While mammalian studies have significantly advanced our understanding of gut microbiota functions, knowledge in aquaculture species, particularly shrimp, remains comparatively limited and often descriptive.11 Although studies on single strains of probiotics have provided useful insights, a more integrated understanding of how entire microbial communities respond to specific probiotic interventions, especially over extended culture periods and across different developmental stages, is still lacking.12,13
Recent advances in metagenomics and metabolomics provide new insights into these complex microbial interactions and their applications for aquaculture production. A balanced and diverse gut microbiota has been consistently associated with improved feed utilization, enhanced immune function, and increased survival in L. vannamei.14,15 Conversely, dysbiosis induced by environmental stress or pathogen exposure can severely impair shrimp performance.16 This research comprehensively investigated the long-term effects of dietary Bacillus subtilis, Lactobacillus acidophilus, and their combination on L. vannamei (PL10 to adult stages). We focused on key parameters including growth performance, survival, feed conversion ratio (FCR), and the temporal dynamics of the gut microbiota. Ultimately, this study aims to provide evidence-based strategies for optimizing feed efficiency and shrimp health, thereby supporting sustainable intensive aquaculture by enhancing our understanding of host-microbe interactions.
2. Materials and Methods
2.1. Experiment design
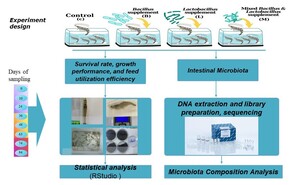
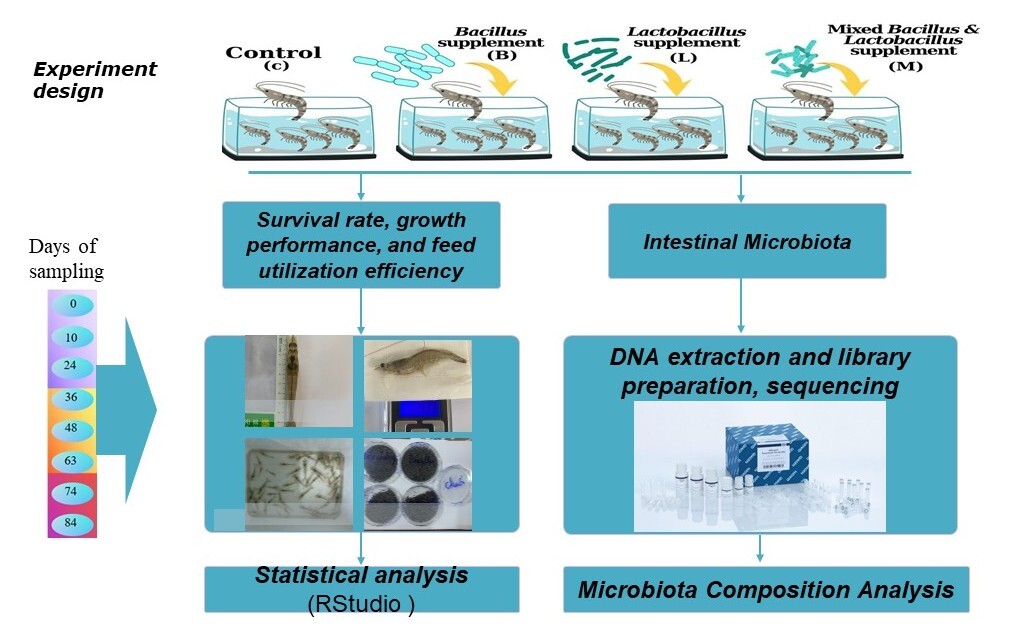
The study was conducted at the Cam Ranh Aquaculture Experiment Center (11.8247°N, 109.1255°E), Institute of Aquaculture, Nha Trang University. Pacific white shrimp (Litopenaeus vannamei) post-larvae at PL10 stage, sourced from Viet-Uc Company, underwent a seven-day acclimation period, during which they were fed a commercial (see Appendix 1. for nutrient content), probiotic-free diet. Following acclimation, shrimp with an average body length of 13–15 mm was randomly allocated to 20 experimental tanks (1 m³ volume each), with 5 replicate tanks per treatment. Each tank was stocked with 500 individuals.
Four dietary groups were established (Figure 1) consisting of commercial pellets in the following groups: (1) Control (C): a probiotic-free diet; (2) Lactobacillus (L): supplemented with 10⁷ CFU/g Lactobacillus acidophilus (ATCC 4356); (3) Bacillus (B): supplemented with 10⁷ CFU/g Bacillus subtilis (ATCC 6633); and (4) Mix (M): supplemented with a combination of 0.5x10⁷ CFU/g Lactobacillus and 0.5x10⁷ CFU/g Bacillus. Experimental diets were freshly prepared by thoroughly mixing the respective probiotic strains (except the control) with the commercial pellets daily, immediately before feeding.
The experiment lasted for 84 days. Note, the Baseline sample is the sample collected at day 0 (denoted as F0 as the starting point of the study) before they consumed the experimental diet. The Baseline sample serves as the basis for the initial bacterial communities. Throughout this period, environmental conditions in all tanks were meticulously maintained within optimal ranges to minimize variability and stress: temperature 28–31°C, salinity 30–32 PSU, pH 8.0–8.4, dissolved oxygen (DO) >5 ppm, alkalinity 140–160 ppm, and ammonia <0.1 ppm. Water quality management included twice-daily siphoning and a 10–30% water exchange (adjusted according to the shrimp’s culture stage), performed at 9:00 AM and 8:00 PM. To ensure that observed effects were attributable solely to the probiotic supplementation and to prevent confounding factors, no antibiotics were used at any point during the experimental duration.
2.2. Data Collection and Formulas
Shrimp survival, growth performance, and feed utilization were meticulously monitored every 10 ± 5 day throughout the experimental period. Various metrics were calculated to assess the effects of the different dietary treatments.
Survival Rate
Shrimp survival rate (SR) was determined by counting the number of surviving shrimps in each tank at each sampling. The counting method involved: (1) draining 80% of the tank water, (2) photographing the remaining shrimp, and (3) counting the shrimp from the photograph using an erasable marker on a screen.
Shrimp survival rate (SR): SR (%) = [N2 / N1] x 100 (1)
N2 and N1 are number of survival shrimp at current time and previous time (that measurements were taken), respectively.
Growth performance
At each sampling, 10 individuals were randomly selected from each tank (n = 50 per treatment) for individual measurements of total length (TL) and body weight (BW). Total length was measured from the tip of the rostrum to the end of the telson using a caliper (accuracy: 0.1 mm), while body weight was recorded using an electronic balance (Viet Nhat, accuracy: 0.01 g).
The following formulas were used to calculate growth parameters:
Where: W2 and W1 (g) represent shrimp body weight at the current and previous measurement times, respectively; t (days) is the duration between the two measurement times.
Feed Utilization Efficiency
Feed utilization (FI - feed intake and FCR - feed conversion ratio) was determined for each period between two samling times
FI (g) is the average of individual feed consumption daily within time (t) between two sampling: FI = (m1 - m2)/N (5)
The Feed Conversion Ratio (FCR): FCR = FI x t / WG (6)
m1 is the amount of feed assigned for each tank before each feeding; m2 is the uneaten feed sunk to the bottom of the tank. This uneaten feed was siphoned out of the tank immediately after feeding, filtered through a net, collected in a container then dried in the dryer to remove the excess absorbed water and then determined the weight after calibrating with moisture content (11%) in initial feed.
N is total number of individual in each tank at measure time.
t (days) is the time between two samplings.
WG (g) is the average of individual weight gain, as defined in formula (2).
2.3. Gut microbiota analysis
Entire Intestinal Sample Collection
Prior to the start of the experiment (Day 0 - F0), a baseline sample of gut microbiota was established. For this, approximately 300 acclimated individuals were dissected, and their pooled intestines were collected. Throughout the experiment, gut microbiota samples were collected on the same days as those for survival, growth performance, and feed utilization assessments (i.e. on days 10, 24, 36, 48, 63, 74 and 84). The number of individuals sampled are detailed in Supplementary Table S1. To ensure sufficient DNA yield for subsequent analyses, the number of individuals sampled was adjusted based on shrimp size. All samples were immediately transported to the Molecular Biology Laboratory at Nha Trang University under chilled conditions and processed within two hours of collection.
To reduce the risk of external bacterial contamination, shrimp were surface-sterilized using 75% ethanol before dissection. Using sterile forceps and scissors, intestines were aseptically removed and pooled from individuals in the five replicate tanks of each treatment, generating one composite sample per treatment per time point. Each intestinal sample was then suspended in 1 mL of phosphate-buffered saline (PBS) and stored at 4°C for subsequent analysis.
DNA extraction and library preparation, sequencing
Entire intestinal samples were homogenized in PBS using a tissue grinder, and the supernatant was obtained by centrifugation at 13,000 × g for 2 minutes using a Hettich® MIKRO 200/200R centrifuge. Total genomic DNA was subsequently extracted from the supernatant using the DNeasy PowerSoil Pro Kit (Qiagen, Germany), following the manufacturer’s protocol.
DNA integrity was assessed via 1% agarose gel electrophoresis, while DNA concentration was measured using a Qubit 2.0 Fluorometer (ThermoFisher Scientific). DNA purity (OD260/OD280 ratio) was evaluated using a Nanodrop™ 2000C spectrophotometer (ThermoFisher Scientific). Only DNA samples exhibiting high molecular weight bands without smearing on the gel, concentrations of ≥ 12.00 ng/μL, and OD260/OD280 ratios ≥ 1.70 were selected for downstream library preparation.
All nine hypervariable regions (V1-V9) of the 16S rRNA gene were amplified using the xGen™ 16S Amplicon Panel v2 (Integrated DNA Technologies, Coralville, IA, USA). Normalase™ (supplied with the kit) was used for enzymatic library normalization. The final library size was quantified by qPCR. A total of 29 libraries (F0, 7-time points x 4 treatments) were sequenced using paired-end 150 bp reads on the Illumina NovaSeq platform at KTest Science Company, Ho Chi Minh City, Vietnam.
Beta diversity, representing between-sample community variation, was assessed using principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity. These distance-based metrics enabled the visualization of microbial community differences among treatments and time points.
2.4. Statistical analysis
Statistical analysis of survival rate, growth performance and feed utilisation
The effects of probiotic supplementation across sampling days (fixed factors) on final body weight (BW), total length (TL), and daily weight gain (DWG) were assessed using a linear mixed model. The impact on specific growth rate (SGR) and survival rate (SR) was analyzed with a generalized linear mixed model, treating tank replicate as a random factor.
Two-way ANOVA was conducted to evaluate the effects of probiotic treatments and sampling days for each model. Pairwise comparisons among treatments at each sampling day were performed using the Tukey HSD post-hoc test, with statistical significance defined as p < 0.05. After fitting these models, the model assumptions were checked by examining the residuals for normality and homogeneity.
All statistical analyses and figure plotting were performed in Rstudio 2023.09.1. The packages utilized included rstatix,17 Rmisc,18 performance,19 emmeans,20 and ggplot2.21
Analysis of metagenomic data
Raw paired-end reads (forward and reverse) were trimmed with barcode and low-quality bases (Q = 30) using Trimmomatic v0.33.22 High-quality denoised sequences were reconstructed from paired reads using the “mergePairs” function in the DADA2.23 Chimeric sequences, which result from the erroneous recombination of unrelated reads, were identified and removed using the “removeBimeraDenovo” function in DADA2 to ensure sequence accuracy and integrity.
Operational taxonomic units (OTUs) were then clustered based on sequence similarity thresholds ranging from 80% to 97% across seven taxonomic ranks, utilizing the Blastn algorithm within the QIIME2 platform.24 The relative abundance of each OTU (%) was calculated by dividing the number of reads in each OTU cluster by the total number of high-quality reads obtained from each sample.
To visualize the microbial community structure, taxa bar plots were generated in QIIME2 at the phylum, class, genus, and species levels, highlighting the top 30 most abundant taxa. Rarefaction curves were also constructed to evaluate OTU richness and sequencing depth across samples. Alpha diversity metrics, including Observed OTUs, Chao1, Shannon, and Simpson indices, were calculated using the alpha-rarefaction function in QIIME2 to assess within-sample (intra-group) microbial diversity.
3. Results
3.1. Survival rate
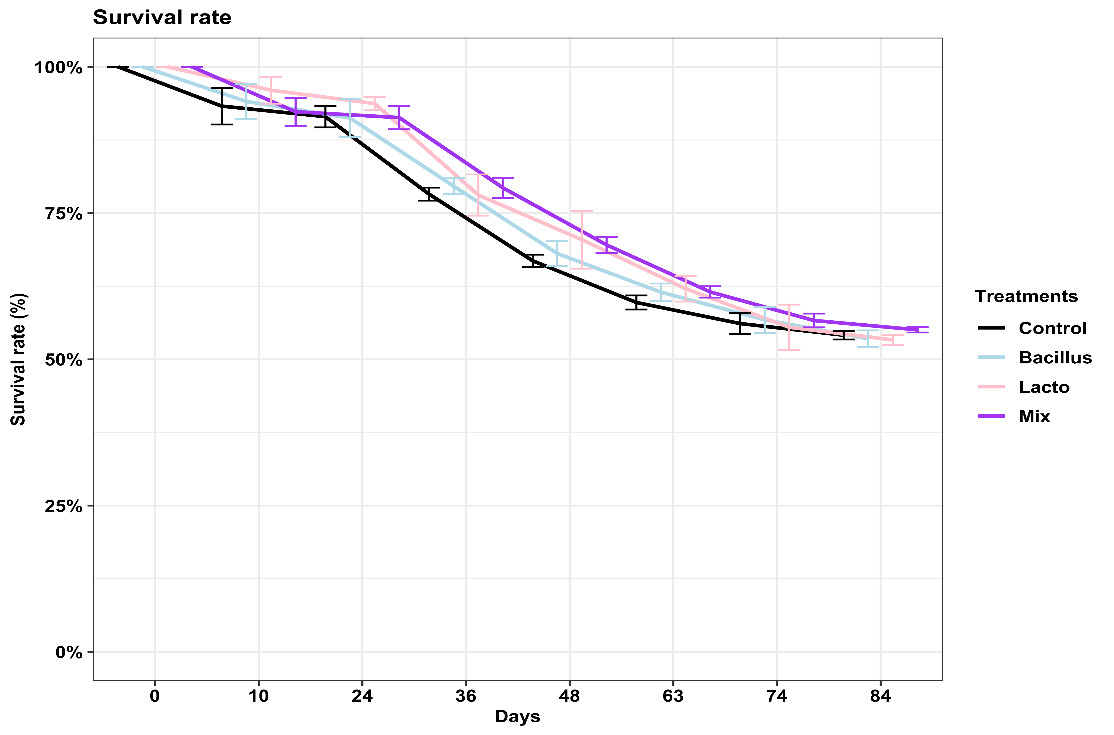
A similar downward trajectory in survival rates was observed in all treatments over the experimental period (Figure 2). Specifically, by day 36, survival rates for most treatments were around 70-80%, with the control group slightly lower, from the initial 100%. By the end of the experiment on day 84, survival rates approached approximately 50-55%, meaning an overall reduction of around 45-50%.
While the mixed probiotic group (Lactobacillus + Bacillus) generally displayed a slightly higher numerical survival rate throughout the experiment, particularly noticeable from day 36 onwards, no statistically significant differences in overall survival were evident between the treatment groups (p>0.05), as indicated by the overlapping error bars across all data points.
3.2. Growth performance
Dietary supplementation with probiotics (Bacillus and Lactobacillus) demonstrably influenced the growth of Pacific white shrimp, initiated from PL10 (Figure 3). Notably, shrimp exclusively fed Bacillus exhibited superior growth, evidenced by significantly higher body weight and length at day 74 (Figure 3A&B) compared to those receiving Lactobacillus alone, a mix of both probiotics, and the control diet (p<0.05). However, by the end of the experiment on day 84, no significant differences in body weight and length were observed among the treatment groups (p>0.05).
Regarding growth rates, both SGR and DWG exhibited distinct trends across the developmental stages (Figure 3C&D). Initially, during the post-larval stage (up to day 24), both SGR and DWG showed an increasing trend across all treatments. As shrimp transitioned into the juvenile stage around day 48, a notable shift occurred: both SGR and DWG generally decreased significantly across all groups. This downward trend continued into the adult stage (from day 63 onwards), where the rates became relatively stable with less fluctuation.
Specifically, at day 48, an interesting observation was made: the mixed probiotic group displayed a significantly the highest specific growth rate (SGR) compared to the others (p<0.05), hinting at a potential early growth spurt associated with this combination (Figure 3C). A similar trend of significantly higher DWG in the mixed probiotic group was also observed at day 48 (Figure 3D) compared to other groups (p<0.05).
However, this advantage in the mixed probiotic group was short-lived. By day 63, both SGR and DWG in the mixed probiotic group decreased significantly and were the lowest among all treatments (p<0.05) (Figure 3C&D). Moving towards day 84, both SGR and DWG showed a slight increase again across most groups. Importantly, at this point, the SGR in the mixed probiotic group remained significantly different from the Bacillus group, while no significant differences in DWG were observed among the treatment groups (Figure 3C&D).
3.3. Feed utilization efficiency
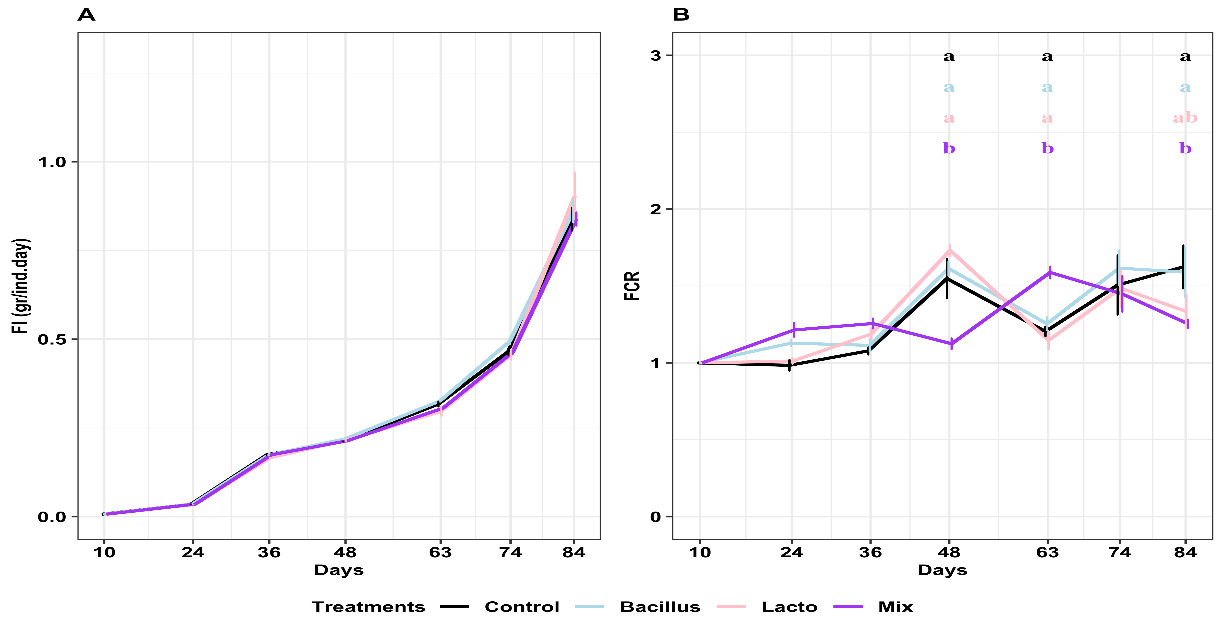
While daily feed intake (FI) increased with shrimp growth, probiotic supplementation did not significantly influence this parameter (p > 0.05) (Figure 4A). Conversely, the feed conversion ratio (FCR) was significantly influenced by probiotic treatments (Figure 4B). Specifically, the mixed probiotics treatment exhibited the lowest FCR at day 48, the highest at day 63, and a subsequent decrease to the lowest value again at day 84 (p < 0.05). The FCRs of the other three treatments did not differ significantly from each other (p > 0.05).
3.4. Intestinal Microbiota
Day 84 samples for 16S rRNA gene sequencing (2 out of 4 total samples) exhibited substantial host DNA contamination, leading to low-quality sequencing and their subsequent exclusion from the analysis. A total of 8,413,280 high-quality sequence reads were obtained from 25 pooled intestinal samples, corresponding to 3,783 operational taxonomic units (OTUs). The number of OTUs per sample ranged from 2,332 to 4,828 (Supplementary Table S2). Across all developmental stages, probiotic treatments did not significantly affect alpha diversity metrics, including Observed OTUs (p = 0.9307), Chao1 (p = 0.9827), Shannon (p = 0.8527), and Simpson (p = 0.8275) indices (Supplementary Tables S3 and S4).
Beta diversity patterns were evaluated using principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity (Supplementary Figure S1). The resulting ordination plot did not reveal distinct clustering among treatment groups (Control, Bacillus, Lactobacillus, Mixed, and baseline sample), indicating substantial overlap in microbial community composition across treatments and time points. Samples were dispersed without clear separation along the first two principal coordinates, which accounted for a moderate portion of the variance.
To statistically assess differences in microbial community structure, PERMANOVA tests were conducted on all pairwise treatment comparisons (Supplementary Table S5). All comparisons yielded non-significant results (p > 0.682, q > 0.99), with pseudo-F values ranging from 0.1355 to 0.5135. These findings indicate that probiotic supplementation did not significantly alter the overall gut microbial community structure at the beta diversity level.
3.5. Gut Microbiota Composition
Phylum- and Class-Level Analysis
Overall, probiotic supplementation induced discernible phylum and class-level reorganizations in the gut microbiota, leading to specific proportional changes that differed from the initial F0 composition and varied among treatments and over time (Figure 5A & B).
At the phylum level (Figure 5A), the baseline F0 sample was primarily dominated by Pseudomonadota. Across all treatments and time points, Pseudomonadota consistently remained the most abundant phylum. Compared to F0, all probiotic treatments (Bacillus, Lactobacillus, Mix) and the Control group exhibited shifts in the relative abundance of other phyla, notably Bacteroidota, Actinomycetota, and Bacillota, which varied considerably over time.
At the class level (Figure 5B), Gammaproteobacteria, reflecting the phylum-level dominance of Pseudomonadota, was the most abundant class in the F0 sample (approximately 40%) and consistently remained the most dominant class across all treatments and time points (ranging from 37–68%). Gammaproteobacteria abundance generally increased from F0 (40.33%) to over 60% by Day 24 in most treatments (e.g., C24, B24, L24, M24), followed by fluctuations and a secondary peak around Day 74 (up to 60.46%). Bacteroidia consistently formed a substantial portion of the microbiota across all groups. Alphaproteobacteria (8–36%) and Flavobacteriia (typically >10%) were also consistently abundant. In some treatments, Alphaproteobacteria decreased at Day 24 but increased again at Days 36 and 48. A marked rise in Actinomycetes (>10%) was observed around Day 63 in certain groups (e.g., C63, B63). Fusobacteriia became prominent by Day 74 (up to 7.08%) in some treatments (e.g., C74, B74).
Genus- and Species-Level Analysis
Following specific proportional changes in phylum and Class levels among treatments and over time (Figure 5A&B). These shifts were further characterized at the genus and species levels (Figure 6A&B), revealing a complex and dynamically responsive microbial community.
At the baseline (F0), the gut microbiota was characterized by a diverse genus composition. Across all treatments and time points, Vibrio was the most abundant genus (18.06%–48.28%), although its relative abundance fluctuated over time and across treatments compared to F0. Other consistently abundant genera included Yersinia (2.39%–13.71%) and Salmonella (0.98%–7.95%), both belonging to Enterobacteriaceae. Photobacterium (2.82%–21.17%), a genus within Vibrionaceae, also appeared prominently. A notable specific observation was a peak in Photobacterium abundance in the Bacillus-treated group at Day 74 (B74). Other genera, including Algoriphagus and Flavobacterium, generally accounted for less than 10% of the microbiota but showed temporal shifts. For instance, later-stage samples revealed increased relative abundances of Algoriphagus, Flavobacterium, and Halioglobus, indicating dynamic microbial responses beyond F0 (Figure 6A).
At the species level (Figure 6B), the shrimp gut microbiota exhibited complex and variable compositions compared to F0, influenced by both probiotic treatment and culture duration. Key species such as Salmonella enterica, Vibrio parahaemolyticus, and Yersinia pestis each displayed distinct temporal and treatment-specific trends that reflected the dynamics of their respective genera. For example, S. enterica increased notably in the Lactobacillus-treated group by Day 74 (L74, 13.09%), while V. parahaemolyticus (a species of Vibrio) maintained relatively stable abundance throughout the study. Y. pestis (a species of Yersinia) peaked during mid-culture (17.36% at L36), indicating potential transient colonization. Additional species, including Algoriphagus machipongonensis, Thalassoglobus polymorphus, Ilumatobacter coccineus, Capnocytophaga canimorsus, and Vibrio taketomensis, displayed distinct distribution patterns. For instance, A. machipongonensis surged on Day 63 in both the control and mixed probiotic groups, while T. polymorphus exhibited a transient presence. V. taketomensis (another Vibrio species) increased toward the later stages in the Lactobacillus-treated group. These findings demonstrate that probiotic supplementation and culture duration drive specific compositional shifts down to the species level, often mirroring the trends observed at the genus level but with finer resolution and unique temporal patterns.
4. Discussion
The present study thoroughly examined the long-term impact of dietary probiotic supplementation, encompassing single Bacillus subtilis, single Lactobacillus acidophilus, and a mixed probiotic formulation, on Litopenaeus vannamei from post-larval to adult stages under controlled culture conditions. Our findings illuminate the intricate and dynamic interplay between probiotic intervention, host development, and gut microbial community shifts, providing crucial insights for advancing sustainable aquaculture practices.
This research prominently demonstrates that probiotic supplementation significantly influences shrimp growth, with distinct effects depending on the specific strain or combination used. A standout finding is the superior and sustained growth performance consistently observed in shrimp fed Bacillus subtilis alone, signifying its potent growth-promoting capabilities in this extended culture system. This aligns with established literature on Bacillus species’ ability to improve nutrient utilization through enzyme secretion.25 While the mixed probiotic group initially showed a promising “early growth spurt” that coincided with the juvenile transition period, suggesting a transient synergistic effect, this advantage was not maintained in later adult stages. This highlights the critical need to consider the timing and duration of probiotic application, as efficacy can vary significantly with host developmental stage. The observed temporal variations in growth rates across all groups further emphasize that probiotic effects are not static but dynamically interact with the shrimp’s inherent physiological changes. In contrast, the impact of Lactobacillus acidophilus was more modest, reflecting the context-dependent efficacy often reported for Lactobacillus strains in aquaculture.7,26
Despite clear growth modulations, probiotic supplementation in this study did not lead to statistically significant improvements in overall shrimp survival. While a slight numerical advantage was noted in the mixed probiotic group, the overarching decline in survival rates across all treatments underscores the dominance of other multifactorial elements inherent in long-term aquaculture, such as environmental conditions, water quality, or background pathogen pressure. This suggests that while probiotics contribute to gut health, their direct impact on survival under complex farming conditions may be limited, or they function best as part of a broader, integrated health management strategy (Hoseinifar et al., 2018). Besides, in typical shrimp farming practices, the application of probiotics for environmental stabilization is a widely adopted and often preferred strategy. However, to isolate the specific effects of the various dietary probiotics on the shrimp’s gut microbiota and minimize confounding influences from waterborne microorganisms, we deliberately omitted probiotic water treatments in our experimental setup. While this decision may have influenced the overall survival rate observed across the entire trial, it is crucial to note that this potential impact was uniformly distributed across all experimental treatments. We believe the observed mortality rate is explainable for our lab-scale experiment. For instance, Kim et al.27 reported mortality rates between 35% and 45% when rearing shrimp from PL12 for 112 days in 500L RAS tanks at 150 PLs/m3. Our study used a significantly higher density of 500 PLs/m3, yet our mortality figures were comparable to theirs.
Our in-depth analysis of the shrimp gut microbiota provides a comprehensive understanding of its composition and the profound, dynamic modulations induced by probiotics. The study notably details how probiotic interventions drive specific taxonomic shifts, moving beyond the baseline (F0) composition and exhibiting distinct patterns across treatments and developmental stages. The consistent dominance of Pseudomonadota (especially Gammaproteobacteria) signifies its foundational role in the shrimp gut, consistent with previous research.16,28 However, within this general stability, probiotics orchestrated nuanced changes: the relative abundances of other key phyla like Bacteroidota, Actinomycetota, and Bacillota were significantly altered; at the class level, even within the dominant Pseudomonadota, classes like Alphaproteobacteria showed contrasting temporal dynamics compared to Gammaproteobacteria, demonstrating fine-tuned microbial responses; and crucially, at the genus and species levels, specific probiotic treatments led to distinct shifts. The Bacillus-treated group, correlating with its superior growth, showed a specific enrichment of Photobacterium, suggesting a potential mechanistic link. The enrichment of Photobacterium in the Bacillus-treated group, which also demonstrated superior growth, suggests its potential role in nutrient metabolism or gut health. This aligns with studies indicating Photobacterium can contribute to digestive efficiency in marine organisms.29 Conversely, the Lactobacillus group saw an increase in Salmonella enterica and a transient peak of Yersinia pestis, highlighting the strain-specific and temporal impacts of different probiotics on potential commensals or opportunistic taxa. The dynamic presence of Vibrio species throughout the study, a common inhabitant in crustacean systems,30,31 further illustrates the complex environment in which probiotics operate. These intricate microbial reorganizations demonstrate that probiotics don’t merely add beneficial bacteria; they actively reshape the existing microbial ecosystem, influencing host physiology.
The improved Feed Conversion Ratio (FCR) specifically observed in the mixed probiotic treatment signifies enhanced feed utilization efficiency, consistent with reports on Bacillus spp. and combined probiotic applications.25 This likely stems from the complementary roles of the two probiotic strains in promoting nutrient digestion (via Bacillus’ enzymatic activity) and improving gut health and nutrient assimilation (Lactobacillus).7,26 This enhanced FCR implies a more functionally efficient gut microbial ecosystem, directly translating to better conversion of feed into shrimp biomass.
The observation that the mixed probiotic group exhibited an early growth advantage that was not sustained warrants further discussion. One plausible explanation for this transient effect could involve antagonistic or competitive interactions between the two probiotic strains, Bacillus subtilis and Lactobacillus acidophilus, within the shrimp gut. While individually beneficial, their combined presence might have led to competition for colonization sites or nutrient resources, or even cross-inhibition through antimicrobial compounds produced by one strain against the other, thereby diminishing their long-term synergistic potential. For example, Bacillus species are often spore-formers and can produce various antimicrobial compounds, while Lactobacillus species produce lactic acid and other organic acids. These different mechanisms could lead to an initial “shock” or synergistic effect that later fades as one strain outcompetes the other, or as the shrimp’s gut environment adapts to the combined presence.
Furthermore, initial positive shifts induced by the mixed probiotics in the resident gut microbiota may have been temporarily counteracted by the subsequent dynamics of the endogenous microbial community. As the shrimp matured, the gut environment and existing microbial populations could have re-established a new equilibrium, potentially diminishing the sustained impact of the mixed probiotic intervention. This links to your finding of specific taxonomic changes (e.g., Salmonella enterica or Yersinia pestis in Lactobacillus group, even if transient, could play a role).
While previous studies often report sustained benefits from mixed probiotics including improved growth performance, immune response, and pathogen resistance,32 our findings revealed that such effects may be transient. Notably, the administered probiotic strains did not become dominant members of the intestinal microbiota and their influence diminished after cessation of supplementation, as also observed by Kewcharoen and Srisapoome.33 This suggests that the long-term persistence and colonization ability of probiotics remain limited, warranting further investigation into the durability and functional stability of these microbial interventions. This outcome could potentially stem from inter-strain competition between Bacillus subtilis and Lactobacillus acidophilus within the shrimp gut,34 or be a result of dynamic host-microbe adaptations where the shrimp’s gut environment or immune responses evolve over time, diminishing the initial probiotic impact29,35
In conclusion, our study, alongside findings from Vu et al.28 and Holt et al.,16 demonstrates that the gut microbiota of L. vannamei is a dynamic and critical component of shrimp health, influenced by developmental stage, environmental conditions, and probiotic supplementation. The distinct growth outcomes and specific microbial shifts associated with different probiotic formulations provide compelling evidence for the importance of targeted probiotic strategies. The observed fluctuations in dominant taxa like Vibrio and Gammaproteobacteria underscore the microbiome’s sensitivity to internal and external stressors. While overall diversity metrics may not always reflect dysbiosis,16,28 the specific changes in key taxa, as detailed here, are paramount. Future research should prioritize elucidating the precise functional roles of these specific microbial taxa, focusing on how the microbiome mediates host responses to various environmental and management challenges. A deeper understanding of these host-microbe interactions will be essential for developing highly effective, microbiome-informed interventions to optimize shrimp health, growth, and resilience in intensive aquaculture systems.
Acknowledgments
This research was financially supported by the Ministry of Education and Training under the grant number B2023-TSN-14.
Authors’ Contribution
Conceptualization: Quyen Dang Ha Vu, Binh Thuy Dang; Methodology: Quyen Dang Ha Vu, Trung Duc Vo, Le Thi Bich Nguyen; Formal analysis and investigation: Quyen Dang Ha Vu, Linh Phuong Pham, Trung Duc Vo, Le Thi Bich Nguyen, Sang Quang Tran; Writing - original draft preparation: Quyen Dang Ha Vu, Trung Duc Vo; Writing - review and editing: Linh Phuong Pham, Binh Thuy Dang, Oanh Thi Truong, Hoang-Minh Le; Funding acquisition: Quyen Dang Ha Vu, Hoang-Minh Le.
Competing Interest – COPE
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Conduct Approval – IACUC
In accordance with Vietnam’s National Regulations on the Use of Animals in Research (Decree 32/2006/ND-CP, 2006), Pacific white shrimp (Litopenaeus vannamei) is not listed under Vietnamese endangered species classifications; therefore, ethical approval was not required. All procedures complied with institutional animal care standards to ensure their humane treatment throughout the experiment.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
Raw sequence data in the FASTQ format used in this research was uploaded to Genbank under BioSample (Accession numbers: SAMN49080030 - SAMN49080053, SAMN48529804) and BioProject number PRJNA1276678.