Introduction
Environmental pollution is one of the biggest problems facing humanity, and its effects on living organisms have reached devastating proportions. Evaluating the toxicity of specific substances or products is often difficult. By the time we determine what the harmful effects are, pollutants and chemicals have already begun to accumulate in living organisms, and their effects are transmitted across generations.1,2 Therefore, it is very important to find suitable animal models that can be used as toxicology bioindicators to detect the harmful effects of pollutants and develop cost-effective and easily manipulated sensitive techniques. Mytilus galloprovincialis, also known as the Mediterranean mussel, stands out as an important model organism in ecotoxicology research. The role of Mytilus galloprovincialis in ecotoxicological studies is primarily related to its prevalence and biological characteristics. This species, commonly found in the Mediterranean and the Atlantic Ocean, is an excellent indicator organism for monitoring pollution levels. Mussels have the capacity to accumulate pollutants that directly affect the physical and chemical properties of water. This accumulation provides a critical database for understanding the cycling of pollutants in ecosystems and the processes of bioaccumulation.3 Thus, analyzing mussels provides important information about the presence and concentrations of toxic components in aquatic ecosystems and is also notable for its sensitivity to toxicological effects. Its responses to various pollutants serve as an important indicator when evaluating ecosystem health. Research on this species not only reveals the adverse effects of pollutants but also contributes to the development of protection strategies. The final destination of many wastes, pollutants, and chemicals is the seas and oceans. The significant consumption of fish, seafood, and aquatic products by a substantial portion of the global population has made marine organisms an exemplary model for assessing the detrimental effects of human activities, pollution, and chemicals on biodiversity. Marine organisms, particularly those within the invertebrate class, serve as sensitive indicators of environmental health. Their responses to pollutants, such as Bisphenols, plastics, and agricultural run-off, provide invaluable insights into the broader impacts of human behavior on aquatic ecosystems. The intricate relationships between these organisms and their habitats make them ideal subjects for studying the ecological consequences of environmental contamination.2 Research on the developmental and genotoxic effects of Bisphenol-A and its analogues, which are emerging compounds, on the commercial mussel species Mytilus galloprovincialis remains a relatively unexplored area. Although previous studies have addressed bisphenol contamination and mussel sensitivity, the novelty and necessity of this particular research highlight a significant gap in current knowledge. The effects of these compounds on marine ecosystems, and particularly the risks to the health of commercially important organisms, are critical for sustainable fisheries and the conservation of marine resources. Therefore, the identification and execution of this study will contribute to both the scientific literature and the development of environmental health policies.
The Food and Agriculture Organization of the United Nations (FAO) states that aquaculture will play a crucial role in meeting the food needs of the world population. Mediterranean mussel (Mytilus galloprovincialis) production, which was 907 tons in 2018, increased to 4,585 tons in 2021.4 Mussel (Mytilus spp.) farms, which have increased in number as a food source today, are undoubtedly the most effective way to convert organic matter produced by marine organisms in the first ring of the food chain into delicious and nutritious human food.5 Especially in newly established mussel farms, a large number of juveniles are needed, and initially, these are collected from natural beds instead of using collectors.4 The quality of the juvenile mussels collected from nature directly affects the quality of the product to be sold in the market. Aquatic ecosystems can contain a wide variety and number of different chemicals produced for human consumption and use. These substances, which enter aquatic ecosystems and whose number continues to increase day by day, can pose a danger to every element of natural ecosystems, especially aquatic ones. These can lead to many undesirable conditions, such as cancer, genotoxic damage, or damage to reproduction and development in organisms. Chemical contamination from discharges or leakages resulting from various anthropogenic activities in aquatic environments can bioaccumulate in aquatic organisms and reach levels that can cause physiological damage to humans via the food chain. The application of early life-stage toxicity tests, involving exposure during the most sensitive stages of the organisms to environmental stress, would greatly help in the identification of those chemicals that represent a major threat to marine species.6
Several synthetic organic compounds, which have been classified as endocrine disrupters (EDCs), are transferred via municipal and industrial wastewater and they are commonly detected in the marine environment. Among these, bisphenol A (BPA), Bisphenol S, Bisphenol F, Bisphenol AF and Bisphenol B present significant research interest due to their extensive use and their physicochemical and toxicological properties. BPA, which is one of the most produced and representative of bisphenols worldwide, has been widely used in plastic production since the 1950s.7 It has been shown that BPA is slightly-to-moderately toxic to fish and invertebrates.8 This chemical, with high production and usage, has been detected in different environmental compartments and human tissues.9 Bisphenol A (BPA), although it breaks down rapidly, persists in the environment due to continuous inputs and has been reported in aquatic environments at concentrations between 0.0005 and 12 μg/L.8 BPA, which is in the class of endocrine disruptors, has been shown in previous studies to be associated with many negative health effects such as diabetes, obesity, cognitive dysfunction, reproductive disorders, cardiovascular diseases, breast cancer, etc.10 Research on the effect of BPA on model aquatic organisms has significant regulatory implications for ecosystem health. In this context, studies involving exposure during early life stages in invertebrates, which can be highly sensitive to environmental degradation, have reported the potential adverse effects of BPA as an endocrine-disrupting chemical (EDC), similar to those reported in vertebrates.6 Recently, it has been shown that exposure of fertilized eggs to BPA at environmentally relevant concentrations in the marine mussel Mytilus galloprovincialis negatively affects the formation of developed D-larvae 48 hours after fertilization,6 but there are no studies on the effects of alternatives used instead of BPA. In mussels, larval development occurs chronologically, with the activation of controlled mechanisms involved in tissue distribution and organ system differentiation. In Mytilus, deformations occurring in embryos after external oocyte fertilization due to contamination lead to adverse effects on the continuation of viability.6 Gatidou et al.11 reported that BPA concentrations in bivalve molluscs found in the Aegean Sea (Greece) ranged between <LOD and 612 ng/g (d.w.). The researchers also noted that laboratory experiments conducted with 300 μg/L of BPA resulted in significant accumulation by Mytilus galloprovincialis. At the end of the experiment, the level of BPA in the soft tissue reached 1159 ng/g (d.w.), and the residual concentrations of BPA at the end of the depuration phase (28th day) after the mussels were placed in clean water were higher than the initial concentrations.
According to the ECHA12 report, there are 17 bisphenols with a bisphenol structure and 148 substances, including bisphenol derivatives with common structural properties in bisphenols. These analogues that replace BPA are used as raw materials in industrial products such as epoxy resins, plastic bags, food packaging, cigarette filters, microwave oven containers, compact discs, toys, feeding bottles, thermal papers, sulfites and pesticides, leather tanning agents, fiber additives.13 BPA and its substitutes present in the aquatic environment are a result of human activity and hydrolysis in high-temperature and acidic/alkaline conditions of materials that originate from Bisphenols, contaminating the aquatic ecosystems.13 BPS, BPF, BPAF are widely used in BPA-free products for thermal paper, plastic packaging.14 Increasing industrial applications of BPA analogs in various products, including plastics and coatings, have raised significant environmental concerns. In a study conducted by Huang et al.,13 the levels of BPA and its replacements in the inflow and outflow of wastewater were investigated, and it was noted that their removal was ineffective (ΣBPs in the inflow and outflow as 665.3-5449.5 and 38.0-2841.5 µg/L, respectively). Huang et al.13 stated that hospital wastewater and landfill leachate significantly contribute to the contamination of water environments with BPs (concentrations in hospital wastewater between 973.0 and 27600.0 µg/L). Concerns about the negative effects of analogues on aquatic ecosystems and living things are increasing day by day. Bivalve mollusks include species of high ecological and commercial importance and are used worldwide as sentinel organisms of pollution in coastal marine environments. Bivalve embryo toxicity tests are one of the widely used test methods to assess water quality and to test the developmental effects of pollutants.6 Assessing the potential impact of emerging pollutants is particularly important for species native to the Mediterranean, which has been identified as one of the most vulnerable regions to climatic and anthropogenic changes and is affected by the world’s seas.
Genotoxicity tests are widely used for chemical risk assessment or monitoring of aquatic species. Micronuclei (MN) occurs because of DNA damage caused by exposure of the organism to various mutagenic and clastogenic agents.15 The MN assay targets interphase cells of any proliferating cell population, regardless of karyotype. For this reason, it is used as a routine test method in investigating the effects of different chemicals, especially in environmental biological monitoring studies. The MN test can now be applied to different aquatic organisms.16 Determining the changes in interphase cells is important both for the health and continuity of the population and the integrity of the aquatic ecosystem. Bivalves form an important part of the aquatic ecosystem. These creatures, which are in the status of bioindicator species, have been used in laboratory studies as test creatures in the determination and evaluation of the effects of different chemicals, especially used in pollution monitoring studies due to their biological characteristics. Numerous studies have shown that the effects of chemicals can be detected by the MN test applied in the hemolymph and gill tissues of mussels (Mytilus galloprovincialis), which is an important step in the trophic level. This toxicological information is needed to assess the degree of environmental risk posed by Bisphenols.
Previous studies have noted that BPA poses a hazard to both nature and public health; however, the lack of studies regarding the analogs currently used as substitutes raises concerns. Consequently, it is necessary to regularly monitor the accumulation and effects of BPA, particularly in the economically important and edible bivalve species Mytilus galloprovincialis.
Materials and Methods
Chemicals
Analytical grade bisphenol-a [(CH3)2C(C6H4OH), Cas No: 80-05-7], Analytical grade bisphenol-s [(O2S(C6H4OH)2, Cas no: 80-09-1 (4,4’-Sulfonylbisphenol)], Analytical grade bisphenol-F [(CH2(C6H4OH)2, Cas no: 620-92-8] and Analytical grade bisphenol-AF [(CF3)2C(C6H4OH)2, Cas no: 1478-61-1] were purchased from Sigma-Aldrich, Germany. Test chemicals were dissolved in dimethylsulphoxide (DMSO) (Sigma, Cat. No: 67-68-5) as 100 µg-BPA, BPS, BPF, BPAF/L. Test concentrations of the chemicals were meticulously selected based on insights from Czarny-Krzymińska et al.14 Test concentrations for M. galloprovincialis were 0.3, 0.5, 0.8, 1.0, 1.5, 2.3 and 3.5 µg-BPA/L, 0.2, 0.5, 0.6, 0.8, 1.0, 1.5, 2.0, 3.0, 6.0 and 12.0 µg/L of BPS, BPF, BPAF and BPB in 10 mL of final test volume. Controls accompanying the experiments were untreated negative controls and 3x10-4M CdCl2 as a positive control. Embryos were also exposed to DMSO as solvent control, being added to the medium in the same volume as the highest test concentration of chemicals, and no effect was detected on the development of mussel embryos. The European Directive 67/548/EEC reports DMSO to be an environmentally familiar solvent, and according to this report, DMSO is not classified as unsafe either. Test concentrations for micronucleus test were prepared by adding the chemicals from stock solution directly to the test medium (0.1, 1.5, 3, 6, 12 and 25 μg/L).
Animals
For larval developmental assay, Sexually mature mussels (M. galloprovincialis Lam.), purchased weekly from an aquaculture farm in the in the İzmir-Çeşme (Aegean coast of Turkey 38°27′ 00.01 N″–26°37′ 27.60 E″), were transferred to the laboratory and acclimatized in static tanks containing aerated artificial sea water-ASW (ASTM, 2004), pH 7.9–8.1, 36 ppt salinity (1 L/animal), at 16 ± 1 °C. Mussels were utilized within 2 days for gamete collection. When mussels beginning to spontaneously spawn were observed, individuals were immediately placed in a 250 ml beaker containing 200 ml of aerated ASW until complete gamete emission.
For MN test, Mussels were purchased from an aquaculture farm in the in the İzmir-Çeşme (Aegean coast of Turkey 38°27′ 00.01 N″–26°37′ 27.60 E″). Before the experiment, all mussels were acclimatized (one week) in the laboratory in tanks of artificial seawater. All treatments were tested as three replicates in glass aquariums containing 10 mussels. Mussels were fed daily with the phytoplankton Chlorella sp.
Toxicity tests
General methods followed Fabbri et al.6 mussels (M. galloprovincialis), where spawning was thermally induced. After spawning, mussels were removed from beakers and sperm and eggs were meshed to remove debris. All procedures were carried out in aerated, filtered ASW made of analytical grade salts, at a constant temperature of 16 ± 1 °C. For each experiment, eggs and sperm were obtained from two individual specimens. Spawning animals were transferred individually to separate beakers, with each female paired with sperm from a single male. Fertilization was then conducted under gentle stirring conditions. After the fertilization, eggs were transferred into 10 ml test media. In each trial, there were five replicates per treatment. Aliquots of the egg suspension (a minimum of about 250 eggs/mL) were transferred to 20 ml ASW medium and fertilized with an aliquot of sperm suspension in order to obtain an egg: sperm ratio of approximately 1:10 in a final volume of 10 ml. After 30 min, fertilization success was verified by microscopical observation. Wells were then incubated at 16 ± 1 °C for 48 h, with a photoperiod of 16 h light: 8 h dark. At the end of the incubation time, samples were fixed with buffered formalin (4%). One hundred larvae were counted, distinguishing between normal larvae (D-shape) and abnormalities (malformed larvae and prelarval stages). Test results’ acceptability was based on negative control for a percentage of normal D-shape larvae ≥80% (ASTM, 2004). The toxicity of each chemical was assessed by calculating the median effective concentration (EC50).
Micronucleus Test
BPA, BPS, BPF, BPAF and BPB exposure concentrations were determined as 1,1,5,3, 6, 12 and 25 µg/L. After the end of acclimation, ten mussels in each aquarium were tested at five concentrations in addition to the control group and positive control (CdCl2) for 72 h. For the micronucleus test, hemolymph and gill tissues were sampled every day for 24,48,72 hours. To determine MN ratios, hemolymph was taken from the mussels with a thin-tipped syringe and put into a mixed fixative (acetic acid: ethanol (1:3)) solution. Then it was centrifuged at ×1000 rpm, and the supernatant was removed. The remaining pellet was spread on the slides. Fixation was performed with ethanol, and the slides were allowed to dry. Slides stained with 5% Giemsa were closed with the help of entellan and a coverslip to avoid exposure to the air.1 In the preparation slides for microscopic observations, a minimum of 1000 cells for each mussel were examined, and 2000 cells were counted from each mussel. Then, the micronucleus and abnormal cell frequencies were calculated. For the gill MN assay, after cutting the adductor muscle, the gills were removed and placed in tubes containing acetic acid. Shedding epithelial tissue from the gills was achieved with the help of a pipette. Then, the sample was centrifuged at ×1000 rpm, and the supernatant was removed. The remaining pellet was spread on the slides. Fixation was performed with ethanol, and the slides were allowed to dry. Slides stained with 5% Giemsa were closed with the help of entellan and a coverslip to avoid exposure to the air. In the preparation slides for microscopic observations, a minimum of 1000 cells for each mussel were examined, and 2000 cells were counted from each mussel. Then, the micronucleus and abnormal cell frequencies were calculated.
Statistical Analysis
The BPs concentrations reduced embryogenesis success to 50% and 90% of the control, respectively. These parameters were calculated using the probit method using the EPA Probit Analysis Program to calculate EC50 Values Version 1.5. Dunnett’s tests were used to compare the differences in the frequency distribution of the evaluated parameters between the control and treatment groups by applying the logarithmic transformation to normalize distributions.
Results
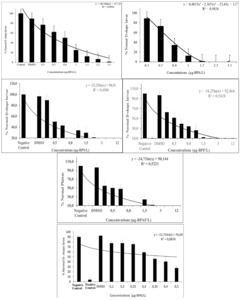
When embryos were exposed to BPA and analogs, which are BPS, BPF, BPAF, and BPB, throughout embryogenesis, significant effects of these compounds were observed at concentrations ranging from 0.2-12 µg /L. The embryotoxicity tests show the classic dose-response curve, indicating a decreased percentage of normal D-shape development with increasing bisphenols (Figure 1). The reduction in embryogenesis success, assessed as the percentage of normally developed D-larvae. From these data, the EC50 of BPA, BPS, BPF, BPAF, and BPB on the embryogenesis of M. galloprovincialis were calculated and shown in Table 1. The species of mussels tested in the present study show results consistent with the values mentioned above (Table 1). Significant differences in d-yields and inhibition of growth of mussel larvae were observed between treatment and controls at the end of the 48h exposure. Results of M. galloprovincialis embryotoxicity probit analyses estimated the impact of BPA on exposed embryos to be EC50 0.476 µg-BPA/L concentration (Table 1). Concentrations of 3.0 and 5.0 µg /L of BPA caused D-shape deformity rates of 7.5% and 22.89 %, respectively. Although no larval malformations were observed at 2.3 and 3.5 µg/L BPA, differentiation was arrested at the prelarval stage by approximately 63%. It has been seen that the lower concentrations of BPA caused developmental deformities, while the higher concentrations of BPA adversely affected the embryos at earlier stages of development (Figure 1). The impact of BPS on exposed embryos was estimated to have an EC50 of 0.558 µg-BPS/L (Table 1). The lower concentrations of BPS generally caused malformations in the D-shape. It has been seen that the lower concentrations of BPS caused deformities, while the higher concentrations of BPS adversely affected the embryos at earlier stages of development (Figure 1). The embryotoxicity tests show the classic dose-response curve, indicating a decreased percentage of normal mussel development with increasing BPF (Figure 1). The lower concentrations of the BPF generally caused malformations. When compared with the negative control group, concentrations of BPF between 0.2-12.0 µg-BPF/L caused deformities of 36% and 73 %, respectively (p<0.001). The impact of BPF on exposed embryos was estimated as EC50 0.410 µg /L BPF concentration by probit analyses Table 1. As a result of the embryotoxicity tests, the dose-response curve (Figure 1) showed a decreased percentage of normal larvae at increasing BPF concentrations. According to the dose-response curve, the lowest concentrations of BPAF influenced the development of embryos. The scores of developmental defects of larvae generated by BPAF-exposed embryos showed that developmental hazards were significantly increased at all concentrations tested (p<0.01) (Figure 1). By probit analyses, the effect of BPAF on exposed embryos was estimated to be EC50 0.509 µg/L BPAF concentration Table 1. According to the dose-response curve, the lowest concentrations of BPB influenced the development of embryos. The scores of developmental defects of larvae generated by BPB-exposed embryos showed that developmental hazards were significantly increased at all concentrations tested (p<0.0001) (Table 1, Figure 1). By probit analyses, the effect of BPB on exposed embryos was estimated to be EC50 0.419 µg/L BPB concentration.
This study was performed to identify the genotoxic potential of BPA and analogues (BPS, BPF, BPAF, BPB), sampling times 24, 48, 72h using the MN test. The frequency of MN was determined, and other nuclear abnormalities were observed, such as nuclear buds, apoptotic cells (Table 2, Figure 1). It was observed that the MN values formed in the hemolymph cells exposed to BPS concentrations increased at 24, 48, and 72 h compared with the control. The highest concentration, 25 µg BPS/L, was MN ‰24,4 at 72 h. In the gill cells, nuclear bud and apoptotic cell formation were observed from other nuclear anomalies apart from the micronucleus formation. MN values in gill cells exposed to BPS concentrations increased at 24, 48, and 72 h compared with control, but were lower compared to those in hemolymph cells (Table 2). This situation is thought to be caused by the formation of nuclear buds and apoptotic cells in gill cells (Table 3). At the highest concentration, 25 µg BPS/L, MN was ‰19.7 at 72 h, while apoptotic cells and nuclear buds were ‰9,33 and ‰ 8,33, respectively. MN values formed in hemolymph and gill cells exposed to BPF concentrations increased at 24, 48, and 72 h compared with control. Similar with BPS, nuclear bud and apoptotic cell formation were observed in gill cells (Table 3). At the highest concentration of 25 µg BPF/L, the MN in the hemolymph and gill cells at 72 h was ‰17.3 and ‰15.8, respectively (Table 4). In gill cells, apoptotic cell BPF started to be observed at 72 h and the highest concentration (25 µg BPF/L) at 72 h was ‰ 9.5, while the nuclear bud was ‰11.3. Compared to BPS, BPF seems to have a similar effect. MN values formed in hemolymph and gill cells exposed to BPAF concentrations increased at 24, 48, and 72 h compared with the control. At the highest concentration of 25 µg BPAF/L, the MN in the hemolymph and gill cells at 72 h were ‰25.3 and ‰26.5, respectively (Table 5). Nuclear bud and apoptotic cell formation were not observed when BPAF was compared with BPS and BPF, but higher MN formation was observed compared to BPS and BPF. MN formation was also detected at close values in hemolymph and gill cells. It was found that MN formation observed at 24, 48, and 72 h for all three chemicals increased depending on the day. At the highest concentration of 25 µg BPAF/L, MN was determined as ‰25.2 and ‰ 19 in the hemolymph and gill cells at 72 h, respectively (Table 6). When BPB was compared with BPAF, MN values in hemolymph cells were found to be similar. It was observed that BPAF was more effective in MN formation in gill cells. It is seen that MN formation in mussels exposed to BPA analogues BPS, BPF, BPAF, and BPB increases depending on concentration and day. Additionally, it was determined that BPS and BPF were more effective by creating other nuclear anomalies, whereas BPAF affected gill and hemolymph cells equally, and BPB especially affected hemolymph cells similarly to BPAF.
Discussion
Our study demonstrated that BPA and its analogs can negatively affect the long-term development of the Mediterranean mussel M. galloprovincialis. The different physicochemical properties of BPs determine their environmental behavior. BPS, BPF, and BPA, which have low log Kow values, are mostly detected in the aqueous phase, whereas BPB and BPAF, with high log Kow values, are more likely to accumulate in sediments and organism tissues; this is also reflected in the bioconcentration factors (BCF). BCF values greater than 100 for BPAF and BPB indicate significant bioconcentration and, consequently, greater exposure to their harmful effects.17 The accumulation of BPs in aquatic organisms represents a significant source of these pollutants for higher trophic levels, posing risks to human health and the overall ecosystem.18 A previous study by Fabbri et al.6 reported that BPA significantly affected the 48-hour embryo development of M. galloprovincialis, showing a LOEC of 0.1 μg/L and an EC50 of 3.68 μg/L, respectively. However, there are no studies reporting the effective concentrations and effects of BPS, BPF, BPAF, and BPB on Mytilus galloprovincialis. In the present study, the EC50 values were calculated as 0.476 µg-BPA/L, 0.558 µg-BPS/L, 0.410 µg-BPF/L, 0.509 µg-BPAF/L, and 0.419 mg-BPB/L, respectively. Similar results were obtained in the study conducted by Fabbri et al.,6 which observed that BPA is more toxic than the current analogs. Since these values fall within the concentration range of bisphenols found in coastal environments, this data supports the hypothesis that mussel embryos are sensitive to environmental BPs concentrations and that this compound may cause developmental abnormalities through toxic effects in marine bivalves. The effects of BPA on mussel embryos were compared with those of the natural estrogen 17β-estradiol (E2). E2 significantly affected larval development in the 48 h embryotoxicity tests (EC50 = 6.904 μg/L), with effects similar to those of BPA.6 The effects of BPA on mussel embryos were compared with the natural estrogen 17β-estradiol (E2). E2 significantly affected larval development in 48-hour embryotoxicity tests (EC50 = 6.904 μg/L) and showed effects similar to those of BPA.6 Compared to the EC50 values in our study, BPA analogs were found to be more toxic than 17β-estradiol. Fabbri et al.6 reported that BPA and E2 caused morphological effects at a concentration of 10 μg/L, similar to our study, and that the presence of immature, not fully developed D-shell larvae began to be observed at these concentrations. In our study, the presence of immature D-shell larvae began to be observed at 0.2. Compared to the previous study, it was found that BPF, BPAF, BPS, and BPB damaged embryo development more than BPA and E at low concentrations. These data indicate that the transition from the trochophore stage to the first D-shell larva represents a critical developmental step affected by bisphenols.
Previous studies reported reduced or delayed fertilization after BPA exposure in sea urchins.8 Throughout the experiments, we observed consistent decreases in the normal development of embryos exposed to concentrations of both BPA and its analogs. The present study results showed that abnormalities and a dose-dependent increase in abnormalities occurred with increasing concentrations of the chemical. No data is available on the possible toxic effects of BPA analogues on the Mediterranean mussel M. galloprovincialis, an important part of the aquatic community. As a result of the experiments, it was shown that toxic levels of the bisphenol analogs, as BPAF>BPB>BPF>BPS>BPA, for M. galloprovincialis. Similar findings and ranking were reported by Zhu et al.19 In an in vitro study, the cytotoxic effects were identified in the order of BPAF > BPB > BPA > BPF > BPS. It has been reported by several researchers that BPA is acutely toxic to aquatic animals (LC50 ranged from 2.5 to 6900 µg/L).8 Reports vary on the sensitivity of aquatic invertebrate organisms to BPA. This study determined the genotoxic potential of BPS, BPF, BPAF, and BPB using the micronuclei test of indicator organisms. The MN analysis of hemolymph and gill cells of M. galloprovincialis has been reported in several studies.2,20–22 The hemocytes of mussels can be used to determine cytogenetic damage,23 and the open vascular system of mussels consists of hemocytes. Additionally, MN analysis has been used in different cell types and organisms (fish, sea urchin) such as hepatic cells, kidneys, and fins, especially in gills. Mussels are great model organisms for the detection of the effects of pollutants on the marine environment. Since the gills in mussels are the first organ to be exposed to pollutants/chemicals, hemocytes are target tissues for MN testing, as they are constantly circulating cells. The gills are the tissues where accumulation occurs. A fraction of the cell population is composed of hemocytes moved into the tissue from the circulatory system. A function of the hemolymph is the removal of harmful substances and small particles.24 It has also been suggested that hemocytes, which play a role in immune defense,18 make them more vulnerable than other cells to pollutants/chemicals such as genotoxic xenobiotics.25
In this context, BPS, BPF, BPAF, and BPB affected hemolymph cells more, which is consistent with previous studies. There are many studies in which the genotoxic potentials of different pollutants/chemicals are determined by examining the micronucleus formation in M. galloprovincialis hemolymph and gill cells. Romdhani et al.,26 in their study, exposed Mytilus galloprovincialis species to microplastic (50µg/L), benzo [a] pyrene (1 µg/L), and their mixtures, collected after 3 days. It was reported that the MN frequency increased in hemocytes at the end of the third day compared to the control. The highest MN frequency was determined for benzo [a] pyrene (‰ 20). It was also detected for microplastics (‰18.66) and their mixture (17.33‰). The MN determined for microplastics collected from the environment in the study is close to the value found for BPF (17‰) hemolymph cells in the present study. Nalbantlar and Arslan (2017) investigated the genotoxic effect of PFOS, one of the persistent organic pollutants, on the M. galloprovincialis species. They reported that MN values increased depending on the concentration (2, 3,4,5, and 6 mg PFOS/L). The frequencies of MN with the highest concentration were 31.26±1.63‰ and 31.84±0.96‰ for gill and hemolymph, respectively. Biomonitoring studies were conducted with wild mussels in the Venice lagoon, where several sites were selected representing different contamination levels. Significant MN increases (1.4- to 5.3-fold compared with the related reference area) were found in hemocytes and gill cells of mussels from polluted sites associated with increased DNA adducts and with high levels of polycyclic aromatic hydrocarbon (PAH), polychlorinated biphenyl (PCB), heavy metals and organochlorinated compounds.27 In a study in which micronucleus testing was performed to determine the genetic damage in the hemolymph, liver and gills of M. galloprovincialis in the Izmir Bay (West Coast of Turkey), they reported that the frequency of MN was high in the stations where the dockyard wastes were found and that there could be high pollution in these regions.2 In an in vitro study conducted by Zhu et al.,19 the cytotoxic effects were identified in the order of BPAF > BPB > BPA > BPF > BPS. Similar findings and ranking are similar to the current study. Based on the results obtained, BPAF showed the highest toxicity and was reported to bind more strongly to estrogen receptors than other BPs, causing adverse reproductive and developmental effects in different organisms. It was found that BPF has a higher potential ecological risk than BPA and BPS. Considering that numerous studies in literature emphasize that BPA analogs are not completely safe, despite the abbreviation “BPA-free” implying otherwise, their widespread use poses a threat to humans, animals, and entire ecosystems.
An overall increase in DNA damage in both hemocytes and spermatozoa has been reported in marine amphipod Gammarus aequicauda exposed to 0.25, 0.5, and 1 mg/L BPA, BPF, or BPS for 24 h. It has been reported that 1mg/L concentration of BPF caused a significant increase in both hemocytes and spermatozoa compared to the control, while BPS did not cause a significant increase.28 Arslan et al.1 found similar results to the present study in their study, in which they exposed Mytilus galloprovincialis to increasing BPA concentrations (12.5, 25, 50, 75, 100 µg/L) for 21 days.
In this study, the increase in MN frequency depending on the concentration, even in short-term exposure, indicates the genotoxic potential of bisphenol analogues. The observation of a lower frequency of MN than bisphenol A suggests that they are related to both the selected concentrations and the duration. However, the results of our study show that these analogues (BPS, BPF, BPAF, BPB) produced instead of bisphenol A are potential genotoxic chemicals and our study is compatible with previous studies. The findings revealed that the parameter of developmental effects in mussel embryos raised concerns about the toxicity of BPA and analogues. Both the present study results and the other related studies showed that contamination with BPA and its analogs caused developmental effects. The results of the investigations on the developmental effects of BPs on M. galloprovincialis are not available. In addition to the micronucleus formation in hemolymph and gill cells, other anomalies such as nuclear bud, apoptotic cells, and nucleoplasmic bridge were reported in the study. It has been reported that the MN is ‰14.67 and ‰22.17, respectively, in hemolymph and gill cells at a concentration of 25 µg BPA/L. The MN values determined for the highest concentration of 25 µg/L BPS, BPF, BPAF, and BPB in our study were similar. Additionally, nuclear bud and apoptotic cell formation were observed in gill cells in BPS and BPF, similar BPA. These results revealed the genotoxic potential of these analogues (BPS, BPF, BPAF, BPB), and further studies must be conducted in terms of the sustainability and safety of the aquatic ecosystem.
These types of studies are important for predicting the toxic effects of chemicals on living organisms. The present study results show that BPA and its alternative concentrations in the environment are hazardous at present; therefore, unless the environmental concentration of BPA is kept under control, it can pose a potential danger to the sustainability of aquatic organisms and aquaculture. The biological and ecological sensitivity of the species Mytilus galloprovincialis, particularly with regard to bisphenol exposure, is not sufficiently known. This species is of vital importance for ecosystem balance and marine biodiversity. However, more research is needed on its sensitivity to endocrine-disrupting chemicals such as bisphenol. This deficiency weakens the effectiveness of environmental protection policies and puts the overall health of marine organisms at risk. Therefore, comprehensive studies are essential to protect Mytilus galloprovincialis and to better understand its effects on the ecosystem.
Acknowledgments
The present study was supported in the context of Scientific Research Project of TUBITAK (Project No: 119Y246). Grant by Özlem ÇAKAL ARSLAN
Authors’ Contribution
Conceptualization: Özlem Çakal Arslan (Lead). Methodology: Özlem Çakal Arslan (Lead). Formal Analysis: Özlem Çakal Arslan (Lead). Investigation: Özlem Çakal Arslan (Lead). Writing – original draft: Özlem Çakal Arslan (Lead). Writing – review & editing: Özlem Çakal Arslan (Lead). Funding acquisition: Özlem Çakal Arslan (Lead). Resources: Özlem Çakal Arslan (Lead). Supervision: Özlem Çakal Arslan (Lead).
Competing of Interest – COPE
‘No competing interests were disclosed’.
Ethical Conduct Approval – IACUC
Research involving animals
Since experiments involving invertebrates were used, no unethical practices were committed. All experiments were conducted within ethical guidelines. Ethical approval was not required for the study.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.