1. Introduction
Didymozoidae is a family of parasites classified under the class Trematoda and the order Digenea, with most species being hermaphroditic and predominantly distributed in tropical and subtropical marine waters.1 Typically, didymozoids use small fish and cephalopods in the upper layers of the ocean as intermediate hosts and large fish species as their definitive hosts.2,3 In the Atlantic bluefin tuna (Thunnus thynnus), the majority of Didymozoidae parasites are found parasitizing the gill arches, pharyngeal region, and visceral organs,4 causing a range of disease symptoms in their hosts, such as gill tissue inflammation, cysts, and necrosis.5,6 Mele and colleagues conducted an analysis of metazoan gill parasites in wild longfin tuna (Thunnus alalunga), identifying Didymozoidae as the dominant taxon, comprising 95.8% of the collected specimens. This study establishes longfin tuna as the most recently documented host for Didymozoidae.7 Additionally, a significant accumulation of Didymozoidae trematode eggs was discovered within the connective tissue of Atlantic mackerel (Scomber scombrus) in northern Spain, mainly between skeletal muscle fibers, with severely infected mackerel showing symptoms of muscular inflammation and anorexia.8 In a comprehensive survey of fish trematodes in Hawaii, Yamaguti9 documented Didymozoidae parasites parasitizing various fish species, all of which exhibit distinct anterior region and posterior region morphology. Additionally, a new species, Didymocystis lamotheargumedoi n. sp., has been described from three tuna species (Thunnus atlanticus, T. albacares, and Katsuwonus pelamis). This species is characterized by cysts containing two nearly identical hermaphroditic worms. The cysts are rounded and feature hard, sclerotized walls of host origin. The anterior region is slender and attached to the ventral side of the posterior region, which is ventrally concave. The oral sucker is terminal and pyriform, followed by a globular pharynx. The esophagus is relatively long, and the caeca are narrow in the anterior region but inflated and twisted in the posterior region, containing dark ingesta. The eggs are bean-shaped, measuring 14–17 μm in length and 9–10 μm in width. These morphological features provide crucial insights into the biology of this parasitic organism.1,10 These challenges primarily stem from the complex life cycle of Didymozoidae, the diversity of their hosts, and their specific parasitic locations within the host, which make it particularly difficult to isolate and purify mitochondrial DNA from parasitized tissues.11 Moreover, these parasites often form close associations with host tissues and may occur in relatively low numbers, further complicating the extraction of sufficient quantities and quality of mitochondrial DNA. Despite the mitochondrial genome being a unique and accessible genetic marker,12 and being a small circular or linear DNA molecule located in the mitochondria, independent of the nuclear genome, it has unique characteristics. The mitochondrial genome is frequently utilized in biological classification, phylogenetic analyses, and population genetics studies. It plays a crucial role in distinguishing parasite species and genera, as well as in conducting phylogenetic and population genetic structure analyses.13
The species of interest, the yellowfin tuna (Thunnus albacares), is a member of the mackerel family Scombridae, characterized by its pelagic, warm-water nature, high migratory behavior, and rapid swimming capabilities.14 Yellowfin tuna are mainly found in the tropical and subtropical waters of the Pacific, Indian, and Atlantic Oceans, including the South China Sea and near Taiwan.15 The extensive migratory patterns of tuna expose them to a wide range of parasitic infections.16 Currently, parasitic diseases in tuna aquaculture have become a focal point of concern, necessitating the identification of the parasites infecting them to enhance our understanding of fish species susceptible to parasitic infections. While tuna can be cultivated through aquaculture, industry’s development has been continually hampered by low survival rates, challenges in seed breeding, and disease issues. Most fry still originate from wild juveniles, which may carry pathogens and parasite infections. Therefore, supplementing information on the parasites of yellowfin tuna is an urgent research priority.
In this study, we acknowledge the importance of robust morphological data for accurate species identification. To address this, we supplemented our morphological observations with detailed molecular identification methods to accurately determine the species of parasites infecting yellowfin tuna. Our comprehensive approach aims to establish a solid foundation for the identification and management of yellowfin tuna parasites. Moreover, by focusing on the evolution and diversity of the Didymozoidae mitogenome, we hope to contribute valuable insights to the field and enhance research efforts in population genetics and species identification.
2. Materials and Methods
2.1. Source of Materials
The experimental animal of this study was yellowfin tuna (Thunnus albacares) captured on 11 October 2023 from the waters at geographic coordinates 110°2’42"E, 17°25’23"N. The capture was conducted using longline fishing. The water temperature at the time of capture was 28°with a salinity of 35‰ and a dissolved oxygen level of 9.5mg/L. After capture, the specimen was immediately transported to the Tropical Aquaculture Research and Development Center, affiliated with the South China Sea Fisheries Research Institute of the Chinese Academy of Fishery Sciences. Due to the poor condition of the tuna upon capture, an urgent analysis was required. Prior to dissection for parasite extraction, an overdose of MS 222 was administered to reduce stress. A total of five parasites were found and removed from the gill arches and intestinal cecum and prepared for further observation in a sterile environment.
2.2. Morphological Observation of Didymozoidae
Initial morphological assessments were performed with an Olympus SZ40 dissecting microscope, followed by an in-depth histological examination. The specimens were preserved in 4% paraformaldehyde and then subjected to a series of dehydration and embedding processes. Longitudinal sections (4 μm thick) were prepared using a Leica RM 2016 rotary microtome (Shanghai Leica Instruments Co., Shanghai, China) and stained with hematoxylin and eosin (HE) for general histological analysis. Each slide containing tissue sections was permanently mounted with neutral balsam. The sections were observed under a Nikon Eclipse Ni-U upright microscope (Nikon Instruments Inc., Tokyo, Japan). This thorough procedure was designed to precisely characterize the external and internal morphological features of the Didymozoidae parasites.
2.3. Whole Mitochondrial Genome Sequencing of Didymozoidae
To conduct genomic analysis, parasite samples were immediately flash-frozen in liquid nitrogen and stored at -80°C until DNA extraction. Genomic DNA was extracted using the TIANGEN Marine Animal Tissue DNA Extraction Kit (Tiangen Biotech Co., Beijing, China) according to the manufacturer’s instructions, which include tissue lysis, protein removal, DNA binding, washing, and elution steps. The quality and concentration of the extracted DNA were assessed using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, Massachusetts, USA) and by 1% agarose gel electrophoresis. Prior to mitochondrial genome sequencing, we amplified a fragment of the 18S rRNA gene using universal primers commonly employed for marine organisms: forward primer 5’-CTTCCGCAGGTTCACCTACGG-3’ and reverse primer 5’-ACCTGGTTGATCCTGCCAG-3’.17 Additionally, a fragment of the 28S rRNA gene was amplified using universal primers: forward primer 5’-TCGATGAAGAGCGCAGCAAA-3’ and reverse primer 5’-AGTTTCTTTTCCTCCGCTTG-3’.18 These amplifications were conducted to support taxonomic identification and phylogenetic positioning of the specimen. Mitochondrial genome sequencing was performed by Shanghai Ling En Biotechnology Company.
High-quality DNA samples were used to construct sequencing libraries with the NEBNext Ultra DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, Massachusetts, USA) following the standard protocol. This process included DNA fragmentation, end repair, adapter ligation, and PCR amplification. The constructed libraries were subjected to high-throughput sequencing on the Illumina NovaSeq 6000 platform, generating 150 bp paired-end reads.19
Raw sequencing data were quality-filtered using Trimmomatic v0.39 to remove low-quality reads and adapter sequences. The clean reads obtained after quality control were assembled into the mitochondrial genome using SPAdes v3.14.1 software with the “–meta” parameter to accommodate potential mixed genomes20; other parameters were set to default. The assembled mitochondrial genome was annotated using the MITOS web server21 and visualized with CGView software.
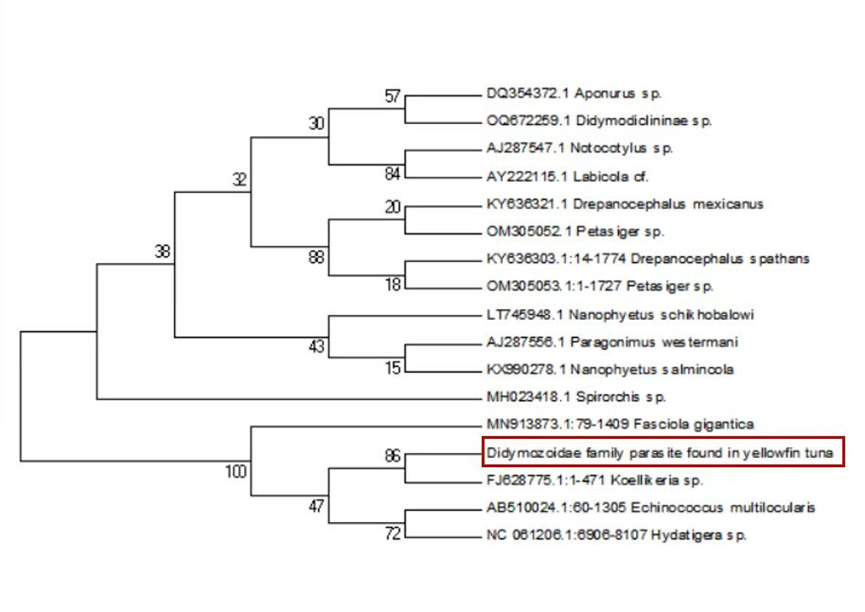
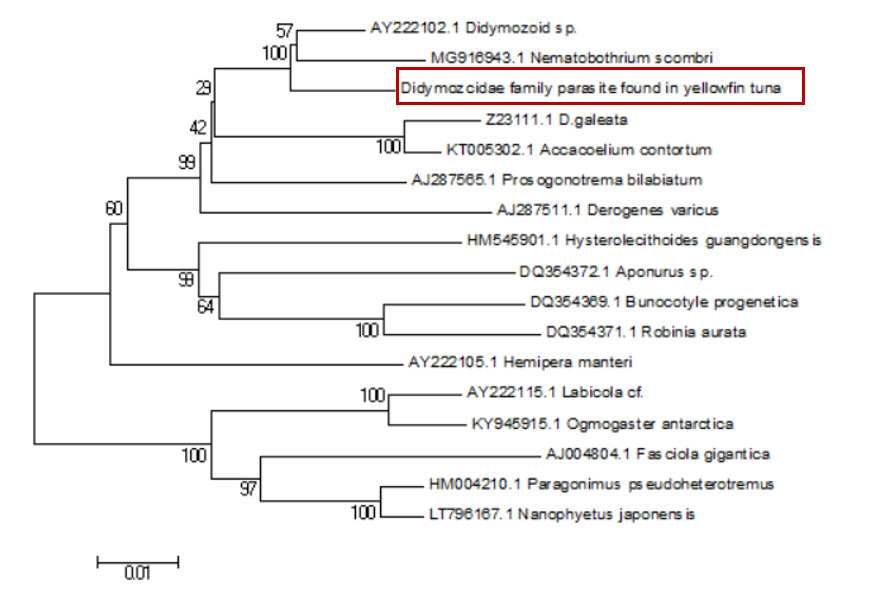
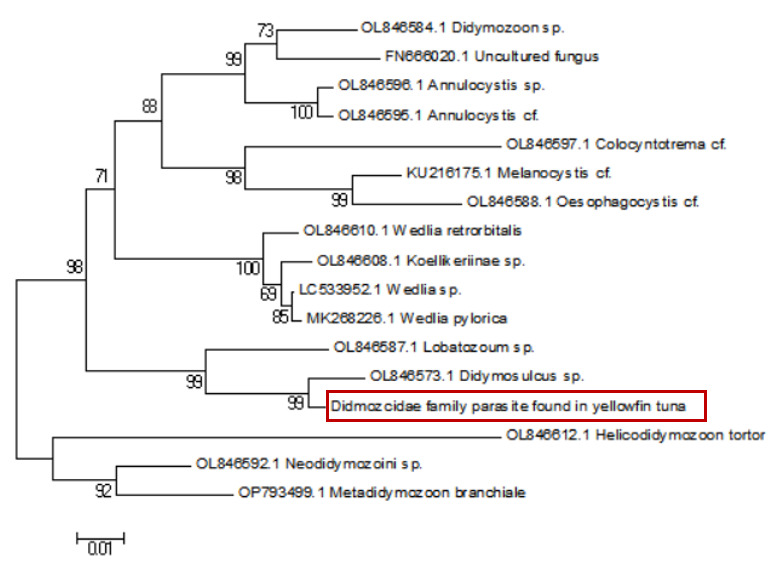
To place the Didymozoidae parasite within the broader context of trematode phylogeny, we conducted comparative mitogenomic and phylogenetic analyses. First, a phylogenetic tree was constructed using concatenated amino acid sequences of the COX1 gene in MEGA 5.2 software, employing the Maximum Likelihood method with 1,000 bootstrap replicates. The Kimura 2-parameter model was used for this analysis. Echinococcus multilocularis and Hydatigera sp. were included as outgroups to provide a comparative framework. At the nuclear gene level, we also constructed phylogenetic trees based on 18S rRNA and 28S rRNA gene sequences to further verify the taxonomic position of the parasite. Both trees were generated using the Neighbor-Joining method.22
3. Results
3.1. Morphology and tissue tropism of the parasite
Upon dissection of the anesthetized yellowfin tuna (Thunnus albacares), a total of five Didymozoid parasites were observed. Three were attached to the gill arches (Fig. 1B), and two were found on the surface of the intestinal cecum (Fig. 1C). The parasites exhibited a pale-yellow coloration and measured approximately 1.5 to 4 mm in total length. All specimens, regardless of attachment site, displayed a characteristic two-part body structure comprising an anterior anterior region and a posterior region, both encapsulated within a cyst. The anterior region was elongated and flattened, capable of free movement.
The parasites attached to the gill arches had a smooth and swollen posterior region, resembling an oval shape with a relatively symmetrical appearance (Fig. 1D). In contrast, the parasites attached to the surface of the intestinal cecum showed a flattened posterior region, with a slender and elongated anterior region, and a distinct demarcation between the anterior region and posterior region (Fig. 1E).
3.2. Histological Analysis
According to the HE-stained tissue section shown in Fig. 2, the parasite is hermaphroditic, with the testes located at the anterior end of the posterior region. The vas deferens and oviduct are positioned in the middle part of the posterior region. Vitellaria are situated on both sides of the posterior region, and the uterus is well-developed, thick, and curved, occupying a substantial portion of the posterior region. The uterus is filled with mature, bean-shaped mature egg cells, approximately 15 μm in diameter. The parasite has both oral and ventral suckers, with the oral sucker located at the anterior end and the pharynx not distinctly visible. The ventral sucker is positioned in the middle of the posterior region; these suckers are used to attach to the host’s tissue surfaces and to extract blood and nutrients. The common genital pore opens near the oral sucker, and the digestive organs are degenerated, characterized by a straight and slender esophagus. Granular plasmacytoid cells can be observed in the esophagus and around the ventral sucker.
3.3. Complete Mitogenome and Molecular Identification
In the mitochondrial genome map depicted in Fig.3, the parasite’s mitochondrial DNA spans 16,468 base pairs and is characterized by a linear arrangement. This mitogenome includes 12 protein-coding genes, but it notably lacks the ATP8 gene. It contains 19 tRNA coding regions; however, three tRNA genes (trnC, trnG, and trnS1) are absent. Additionally, there is one rRNA coding region, which does not include the rrnL gene. The total length of the protein-coding genes amounts to 10,194 bp, whereas the combined length of the tRNA and rRNA genes is 1,225 bp and 760 bp, respectively. Furthermore, regions that remain uncharacterized or unannotated add up to 4,289 bp. The nucleotide composition, with a distribution of 27,36% adenine (A), 40.9% thymine (T), 12.44% cytosine (C), and 19.3% guanine (G), reflects a modest C+G content bias of 31.74%.
Within the protein-coding genes, eight are initiated with an ATG codon, covering COX3, COB, NAD4L, NAD4, ATP6, NAD2, NAD1 and NAD5, while a group of four genes starts with a GTG codon, including NAD3, COX1, COX2, and NAD6. The termination of these genes is indicated by a TAG stop codon for eight genes (COX3, COB, NAD4L, NAD4, ATP6, NAD2, NAD3, and COX2), and a TAA stop codon for the remaining four (NAD1, COX1, NAD6, and NAD5), showcasing a specific pattern of codon usage for ending translation in these protein-coding regions.
3.4. Phylogenetic tree construction
Sequence alignment revealed a 98% similarity between the parasites collected from the two sampling sites. Following the completion of gene sequencing, we focused on the COX1 gene. A comprehensive comparison of this sequence against the NCBI database was conducted using BLAST (Basic Local Alignment Search Tool) on March 23, 2025 (Table 1). The results showed an 88.54% genetic similarity to Koellikeria sp., a member of the family Didymozoidae, suggesting a potential affiliation with an as-yet-unnamed genus within this family. In addition, we amplified and sequenced a fragment of the 18S rRNA gene from the same parasite, and BLAST analysis revealed 97.24% similarity to Didymozoid sp. (Table 2), another member of Didymozoidae. Furthermore, a comparison of the 28S rRNA gene sequence revealed 96.81% similarity to Didymosulcus sp. (Table 3), also within the same family. To further investigate the phylogenetic relationships of this parasite with its taxonomic relatives, phylogenetic trees were constructed using the neighbor-joining method (Fig. 4, Fig. 5 and Fig. 6). However, despite these efforts, the precise taxonomic placement of the parasite at the genus or species level remains unresolved.
4. Discussion
According to pertinent studies, this type of parasite is predominantly found parasitizing the gills, connective tissues, and visceral organs of bluefin tuna (Thunnus thynnus), mackerel (Scomber scombrus), and skipjack tuna (Katsuwonus pelamis).23–25 Their life cycle is notably complex, with most adhering to the host’s gills and viscera, subsisting on the host’s blood and nutrients.26 Two species of Didymozoidae, namely Didymosulcus palati and Didymosulcus philobranchiarca, were initially reported off the coasts of South America and the Atlantic, parasitizing the gill arches of Thunnus atlanticus, Thunnus albacares, and Thunnus obesus. They manifest as yellow cyst-like structures, partitioned into an anterior and a posterior section, with convoluted seminal and vitelline ducts, and a developed uterus filling the entire post-abdominal cavity.27 This morphological and organ structure is similar to that of the parasites observed in the present study. Additionally, NCBI database comparison reveals a 77.72% genetic similarity between Didymosulcus palati and the Didymozoidae parasites we characterized. Although this similarity is substantial, there are notable morphological differences; for instance, unlike the Didymozoidae found in the gills of Katsuwonus pelamis in the Southwest Atlantic (South America), the absence of a ventral sucker in the parasite observed in this study suggests it belongs to a distinct genus. However, other organ structures remain fundamentally similar to the parasites characterized here.28 In addition to the mitochondrial COX1 gene, we also analyzed the nuclear 18S rRNA gene sequence of the parasite and compared it with known sequences in the NCBI database. The results showed a 97.24% sequence similarity to Didymozoid sp., a member of the family Didymozoidae. This high level of similarity in the nuclear rRNA gene further supports the classification of the parasite within Didymozoidae and suggests a closer phylogenetic relationship with the genus Didymozoid. Furthermore, analysis of the 28S rRNA gene revealed 96.81% sequence similarity to Didymosulcus sp., another genus within the same family. The combination of these nuclear markers provides additional molecular evidence for the parasite’s placement within Didymozoidae and suggests that it may represent a distinct lineage closely related to both Didymozoid and Didymosulcus. The presence of oral and ventral suckers is crucial for the effective adherence of parasites to host tissues, facilitating nutrient assimilation. These structures not only anchor the parasites securely but also play a significant role in the feeding process. However, the specific functions and adaptations of these suckers may vary across different species within the Didymozoidae family, reflecting their ecological niches and evolutionary pressures.29 Several studies have indicated that Didymozoidae parasites can cause tissue damage and provoke immune responses in their hosts.30 These parasites, commonly found in the gills, connective tissues, and visceral organs of marine fish, may impair respiratory efficiency by affecting gill function and reducing oxygen exchange. Additionally, their presence in digestive tissues could lead to nutrient competition, indirectly influencing host growth and immune function.31 The absence of a ventral sucker in the Didymozoidae parasite characterized in this study suggests a distinct attachment mechanism compared to other species within the family. While some Didymozoidae species use suckers for secure anchorage, others may rely on alternative adhesion strategies, such as embedding within mucosal layers or forming cyst-like structures.32 These structures provide a secure attachment point, enabling the parasite to more effectively absorb nutrients from the host’s tissues. This mode of attachment not only allows the parasite to remain in close contact with the host tissue but may also aid in its positioning within the host, thereby increasing its chances of survival and reproductive success.33 Significantly, the observed morphological characteristics and genetic similarities highlight the complex interactions between Didymozoidae parasites and their host species. Understanding these relationships not only enhances our knowledge of their ecological roles but also underscores the importance of monitoring these parasites in marine ecosystems, particularly as they can impact the health of commercially significant fish species. Continued research is essential to unravel the intricacies of their life cycles and adaptations, which will ultimately contribute to more effective management strategies for both the parasites and their hosts.
Various studies have demonstrated that intestinal flukes (Ogmocotyle) isolated from pandas (Ailurus fulgens),34 as well as pancreatic flukes (Eurytrema coelomaticum) from ruminants,35 possess 12 protein-coding genes, 22 tRNA genes, 2 rRNA genes, and 2 non-coding region genes in their mitochondrial genomes, with lengths of 14642bp and 15831bp, respectively. In contrast, the mitochondrial genome of Paragonimus ohirai from Japan spans 14818 bp and lacks non-coding regions, distinguishing it from the aforementioned flukes.36 Numerous studies cited in literature, such as Liu et al.,37 have observed the absence of the ATP8 gene across various taxa within the Platyhelminthes phylum. This recurrent absence, although not universal, may signify an evolutionary adaptation that is characteristic of certain lineages within this diverse phylum .37 ATP8 encodes a subunit of ATP synthase, which plays a critical role in oxidative phosphorylation and ATP production. Its loss in certain parasitic flatworms suggests a shift towards an alternative energy metabolism strategy, potentially increasing reliance on host-derived ATP or anaerobic metabolic pathways.38 An analysis of the mitochondrial genome in the current study indicates the presence of only 19 tRNA genes and a single rRNA gene, reflecting a deficit of three tRNA genes and one rRNA gene relative to other platyhelminths. This reduction could signify genomic compression, leading to the loss of non-essential genes through evolutionary processes. The loss of rRNA genes, particularly rrnL (16S rRNA), may have significant implications for ribosomal assembly and mitochondrial translation efficiency.31 This suggests that the parasite might have developed mechanisms to utilize host ribosomes for protein synthesis, thereby reducing its dependence on its own translational machinery. Furthermore, the potential consequences of such genomic streamlining may include a reduced capacity for autonomous protein synthesis, resulting in a greater dependence on host resources. An analysis of the mitochondrial genome in the current study indicates the presence of only 19 tRNA genes and a single rRNA gene in the parasite, reflecting a deficit of 3 tRNA genes and one rRNA gene relative to other platyhelminths, which could signify genomic compression and the consequent loss of non-essential genes through evolutionary processes. Furthermore, the potential implications of this genomic compression may include a reduced capacity for autonomous protein synthesis in the parasite, resulting in a greater dependence on host resources. As specialized parasites evolve, they may undergo genomic streamlining, where certain genomic functions become redundant due to the ability to exploit the host’s molecular machinery, such as ribosomes, for protein synthesis. The loss of tRNA and rRNA genes as a consequence of genomic compression not only diminishes the parasite’s capacity for independent protein synthesis but also likely increases its reliance on the host’s translational system. This process of genomic simplification, particularly the loss of rRNA genes, suggests that some specialized parasites may have developed mechanisms to utilize host ribosomes for protein synthesis, further reducing their dependence on their own rRNA genes.39–41
The phenomenon of mitochondrial genomes not presenting in a circular form, while uncommon in most eukaryotes, indeed exists among certain parasitic entities.42 Typically, mitochondrial DNA manifests as a closed circular molecule.43 However, for some organisms, linear or other non-circular mitochondrial genome configurations may confer evolutionary adaptive advantages. Such adaptations could relate to replication efficiency, genomic stability, or responses to environmental stressors.44 The parasite possesses an uncharacterized genomic region spanning 4,289 bp, within which gene expression may be exceedingly low and thus undetected. These unknown regions may harbor non-coding areas and additional genes, potentially containing sequences that regulate gene expression, such as promoters, enhancers, or silencers. These regulatory elements are crucial for modulating the transcriptional activity of adjacent genes.45 Furthermore, the unknown regions may encompass pseudogenes, which are non-functional gene copies rendered inactive through mutation and incapable of encoding functional proteins.46 Consequently, the non-circular nature of this parasite’s mitochondrial genome may stem from evolutionary pressures to adapt to its parasitic niche, with certain genes undergoing degeneration. These uncharacterized gene regions could contain pseudogenes, non-coding areas, and other genes with lost signals, thus extending the length of this parasite’s genome compared to other platyhelminth parasites. However, the precise reasons warrant further investigation and discussion. Moreover, the defining characteristics of Didymozoidae remain to be elucidated through more extensive research.
5. Conclusions
Through morphological and histological examination, the Didymozoidae parasite under study is characterized by a body divided into anterior and posterior sections and exhibits hermaphroditism, with both oral and ventral suckers. Its reproductive system is well-developed, including a uterus, vitelline ducts, testes, vas deferens, and a common genital pore, with immature oocytes contained within the uterus. The digestive system is simplified, featuring a narrow esophagus without a distinct pharynx. The mitochondrial genome of this parasite is linear, spanning 16,468 bp and includes 12 protein-coding genes (excluding the ATP8 gene), 19 tRNA genes, and one rRNA gene (with a deficit of 3 tRNA genes and the rrnL gene), plus an uncharacterized segment of 4,289 bp. The COX1 gene shows 88.54% sequence identity with Koellikeria sp., while the 18S rRNA and 28S rRNA genes exhibit 97.24% and 96.81% similarity to Didymozoid sp. and Didymosulcus sp., respectively—three genera within the family Didymozoidae. These results collectively suggest that the parasite belongs to Didymozoidae and may represent a distinct, yet undescribed, lineage closely related to Didymozoid and Didymosulcus. Its precise taxonomic classification, however, remains unresolved and will require further investigation using additional molecular markers and more comprehensive phylogenetic analyses.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 32460927); the Project of Sanya Yazhou Bay Science and Technology City (grant number SKJC-2022-PTDX-015); the Central Public-Interest Scientific Institution Basal Research Fund, CAFS (grant number 2023TD58, 2024XT04, 2025XT03); the Science and Technology special fund of Hainan Province (grant number ZDYF2024XDNY247).
Authors’ Contribution
Conceptualization: Junhua Huang (Lead). Investigation: Junhua Huang (Lead), Zhengyi Fu (Lead). Writing – original draft: Junhua Huang (Lead). Methodology: Zhengyi Fu (Equal), Qing Wang (Equal). Data curation: Zhengyi Fu (Equal), Qing Wang (Equal). Formal Analysis: Zhengyi Fu (Lead). Visualization: Qing Wang (Lead). Validation: Qing Wang (Lead). Writing – review & editing: Zhenhua Ma (Equal), Wei Wang (Equal). Funding acquisition: Zhenhua Ma (Lead). Resources: Zhenhua Ma (Lead). Project administration: Zhenhua Ma (Lead). Supervision: Wei Wang (Lead).
Competing Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
The experiments were in compliance with the regulations and guidelines established by the Animal Care and Use Committee of South China Sea fisheries Research Institute, Chinese Academy of Fishery Sciences and permitted by this committee.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.

__parasite_on_the_gill_arch_of_yellowfin_tuna_(b)__parasite_on_.png)
.png)



__parasite_on_the_gill_arch_of_yellowfin_tuna_(b)__parasite_on_.png)
.png)