Introduction
Microplastic pollution has emerged as a global environmental crisis in aquatic ecosystems.1 Among common environmental MPs, polystyrene (PS) is frequently detected and widely studied due to its persistence and toxicity.2 The hazards of microplastics to the marine environment cannot be underestimated, and the environmental behaviour and toxicity generation mechanisms of microplastics include physical damage and release of chemical additives. Microplastics and nanoplastics are recalcitrant materials in marine exposure conditions, and although they represent only a small fraction of the particles and nanoparticles in seawater, the tendency of plastics to absorb and concentrate persistent organic pollutants (POPs) has proved to be a serious problem.3 Therefore, the safety of microplastics in the marine environment for marine organisms is a major concern.
MPs ingested by marine organisms, including fishes,4 birds,5 and invertebrates,6 can disrupt intestinal homeostasis by inducing histopathological damage and microbial dysbiosis.7 The gut microbiota, essential for digestion, immunity, and barrier function, is highly sensitive to MP exposure.8 several studies have shown for the first time that polystyrene microplastics of different sizes can affect the amount of mucus in the gut of adult zebrafish and induce microbiota dysbiosis, notably, MP toxicity exhibits size-dependent effects, as demonstrated in zebrafish (Danio rerio), where 0.5 μm MPs caused more severe toxicity than 50 μm MPs.4Some people have demonstrated that microplastics induce oxidative stress and alter intestinal calcium levels in vertebrates. The toxic effects of microplastic particles—varying in chemical composition and size—primarily manifest through intestinal damage. Notably, these adverse effects depend more significantly on the size of the microplastics than on their chemical composition.7
Thamnaconus septentrionalis is a demersal marine fish species of warm temperate waters, primarily distributed in coastal areas of China, Japan, and the Korean Peninsula.9 Recognized for its tender flesh and comprehensive nutritional profile with high protein content, it serves as an ideal food fish and has gained widespread popularity. In recent years, however, wild populations of T. septentrionalis have shown significant decline due to marine environmental pollution and overfishing, among other factors9,it is critically important to promote the sustainable expansion of T.septentrionalis puffer aquaculture. In recent years, T. septentrionalis puffer farming has primarily relied on offshore systems, where microplastic pollution is more severe. T. septentrionalis was selected because: (1) its benthic feeding behavior increases MP exposure risk, (2) as an emerging aquaculture species, its MP sensitivity has direct economic implications, and (3) its gut physiology differs markedly from model species like zebrafish. Therefore, investigating the effects of microplastics on this species holds significant practical relevance.
Existing research on this species focuses on parasitic diseases10 and heavy metal toxicity,9 with limited data on MP effects. Therefore, the novelty of this study lies in its experimental design, which incorporates (1) microplastics (MPs) of varying sizes and (2) a combined analysis of microbiota and enzymatic responses. This approach enables a comprehensive assessment of MP effects while providing deeper insights into the mechanisms underlying microplastic toxicity. In this study, T. septentrionalis were exposed to 1μm and 5μm polystyrene microplastics for 30 days to investigate: (1) gut microbiota alterations via 16S ribosomal RNA (rRNA) sequencing, and (2) intestinal antioxidant and digestive enzyme activities. This dual approach provides comprehensive data on marine fish microplastic toxicity, supporting ecological risk assessment and aquaculture monitoring.
Materials and Methods
Materials and Experimental Fish
Polystyrene microplastics (MPs,1μm and 5μm; spherical, monodisperse) were purchased from Jingcheng Plastic Raw Material Co., Ltd. (Guangdong, China). The microplastics used as substrate were polystyrene (PS) with a density of approximately 1.1g/cm³, slightly higher than seawater density (1.02 g/cm³). In static water, these particles settle slowly at rates influenced by their size and shape (larger or irregularly shaped particles settle faster). However, in flowing water, they may remain suspended or undergo resuspension, delaying sedimentation. Since aerators operated continuously in the aquaculture system, creating water movement, the PS microplastics could maintain uniform dispersion throughout the water column. Particle size and morphology of MPs were verified via fluorescence microscopy (DM4 B, Leica Microsystems, Germany). MPs were sonicated (30 min) and washed with deionized water prior to use.
Six-month-old individuals of T. septentrionalis were obtained from the Jiangsu Ganyu Research Center (East China Sea Fisheries Research Institute, Shanghai, China). All the individuals were transported to East China Sea Fisheries Research Institute (Shanghai, China) by truck and acclimated in aerated tanks (20± 1°C, pH 7.0–7.5, dissolved oxygen >5.0 mg L−1) for 7 days before performing experiments. During experiments, fish were fed commercial pellets (3% body weight daily; Qingdao Saigelin Marine Feed Co., Ltd.).
Experimental Design
Three groups were set up for MPs exposure of T. septentrionalis, which were named CA (control group), CB (1μm PS-MPs ,1×107microspheres·L−1) and CC (5μm PS-MPs ,1×107microspheres·L−1). The selected concentration was based on three key considerations: first, environmental monitoring data indicate that polystyrene microplastics in coastal waters typically occur at concentrations around 107microspheres·L−1, making this level environmentally relevant; second, some studies showed that higher concentrations (>107microspheres·L−1) caused significant fish mortality while lower concentrations had minimal biological effects; and third, this concentration range aligns with established ecotoxicological protocols and has been widely used in previous studies of MP effects on aquatic organisms.11,12 This approach ensures both environmental realism and comparability with existing literature. Each group included three replicates (20 fish per replicate), and the exposure lasted 30 days. After exposure, the test fish were anesthetized using MS-222 (Sigma, 10 mg/L). Once the fish were unresponsive, they were rinsed with distilled water three times and dissected on ice. From each replicate, three fish were randomly selected, yielding a total of 18 fish per treatment group. The outer walls of their intestines were wiped with 75% ethanol (by volume, same below), and any attached fat was removed using tweezers. The intestines were then rinsed three times with physiological saline. The intestines of three fish were pooled as one sample, resulting in six samples per treatment group. Each sample was placed in a pre-labeled and sterilized Eppendorf tube. All samples were stored immediately in a refrigerator at −80 °C for further analysis.
16S rRNA sequencing
Microbial 16S rRNA gene sequencing was used to detect the flora of the individually preserved gut contents. Microbial DNA was extracted using the HiPure Soil DNA Kit (or HiPure Stool DNA Kit) (Magen, China) following the manufacturer’s protocols. 16S rRNA target region amplification conditions: 95°C for 5 min; 30 cycles of 95°C 1min, 60°C 1min, 72°C 1min; final extension at 72°C for 7min. Microbial 16S rRNA gene sequencing was used to detect the flora of the individually preserved gut contents. Forward primer 341F (5′-CCTACGGGNGGCWGCAG-3′) and reverse primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′),6 were selected to amplify the V3–V4 region of 16S rRNA gene. Qualified libraries were sequenced on Novaseq 6000 using PE250 mode. PCR products were quality-checked using 2% agarose gel, purified with AMPure XP Beads (Beckman, USA), and quantified with Qubit 3.0. Libraries were prepared using the Illumina DNA Prep Kit (Illumina, USA) and qualified with the ABI StepOnePlus Real-Time PCR System.
Experimental analysis of enzyme activity assays
The frozen intestinal samples were thawed on ice, precisely weighed using analytical paper, and homogenized in ice-cold physiological saline at a 9:1 (v/w) ratio. Mechanical grinding was performed in an ice-water bath to prepare 10% (w/v) tissue homogenates, followed by centrifugation at 3,000 × g for 15 min at 4°C. The resulting supernatant was aliquoted for subsequent biochemical analyses, including:(1) Digestive enzymes: amylase (AMS) and lipase (LPS);(2) Antioxidant enzymes: superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT);(3) Total protein content (for data normalization). All assay kits were obtained from Nanjing Jiancheng Bioengineering Institute (China), with analytical procedures strictly following the manufacturer’s protocols. Enzyme activities were expressed as units per milligram protein (U/mg prot), except GSH (μmol/mg prot) and LPS (U/g prot).
Data Analysis
Data were analyzed using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA) to calculate mean values and standard deviations (SD). Statistical analyses were performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) was conducted to determine significant differences in biomarkers among groups, followed by post-hoc multiple comparisons using both Tukey’s Honestly Significant Difference (HSD) test and Duncan’s new multiple range test. Differences were considered statistically significant at P < 0.05. Figures were generated using Origin 2018 (OriginLab Corporation, Northampton, MA, USA).
Results
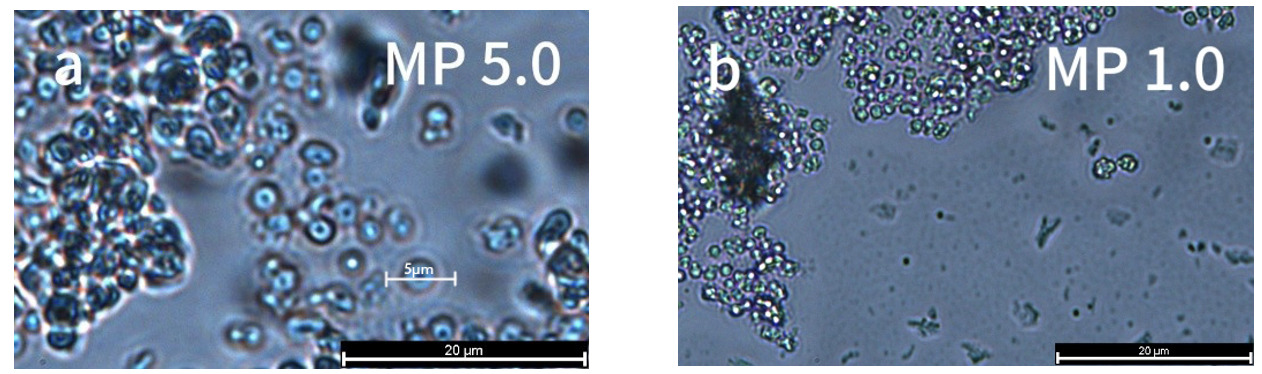
The morphology of MPs
The morphologies of the tested plastic samples are shown in Figure 1. The micron particle sizes were 1μm and 5μm. The PS-MPs with both 5 (Figure 1a) and 1μm (Figure 1b) particle size showed a regular spherical shape.
Sequencing Outcomes
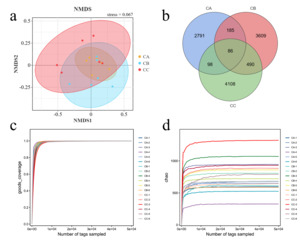
High-throughput sequencing of gut microbiota in T. septentrionalis (groups CA, CB, and CC) exposed to microplastics of varying sizes revealed high-quality effective data, with >95% of sequences meeting quality thresholds (Figure 2), indicating robust sequencing reliability. Beta diversity analysis demonstrated clear separation between experimental groups, with intra-group samples clustering tightly (reflecting strong biological reproducibility) and inter-group samples distinctly distributed in their respective regions, confirming significant microbial community divergence under different microplastic treatments. Non-metric multidimensional scaling (NMDS) analysis represented the β-diversity between different treatment groups (Figure 3a). The results showed that the community structure of all groups was significantly different from the control (Stress <0.2). The shared and unique OTUs of different treatment groups were represented differently by Venn diagrams (Figure 3b). We observed 11367 OTUs from 18 samples. The number of OTUs shared by CA and CB was 271, accounting for 1.61% and 2.38% of the total sequences, respectively. Moreover, the number of OTUs shared by CA and CC groups was 184. The number of OTUs in CB and CC groups was higher than that in CA. The Goods_coverage index, representing microbial community coverage, approached 1 in all samples (Figure 3c), indicating that the probability of undetected novel species was minimal and confirming sufficient sequencing depth. The rarefaction curve (Chao1 index), which reflects both sequencing adequacy and species richness, plateaued as shown in Figure 3d. This asymptotic trend demonstrates that the sequencing volume was appropriate, and additional replicates would unlikely reveal new microbial taxa, confirming that our gut microbiota data accurately represent the true biological reality.
Gut Microbial Composition
Dominant species critically shape the ecological and functional architecture of microbial communities. Analyzing taxonomic composition across hierarchical levels enables robust interpretation of community assembly, dynamics, and ecological implications. Here, we characterized species composition at multiple taxonomic ranks (phylum to species) and visualized abundance variations via stacked bar plots. Microplastic particle size significantly altered gut microbial composition across all taxonomic levels (phylum to species) in T. septentrionalis. At the phylum level, Pseudomonadota exhibited its highest relative abundance in the 5μm microplastic exposure group CC and lowest in the control group CA. Conversely, Campylobacterota and Bacillota showed an inverse pattern, dominating in microplastic-free controls (Group CA) while reaching minimal levels under 5μm microplastic exposure (Group CC).In T.septentrionalis gut microbiota, five phyla exhibited high relative abundance: Pseudomonadota (34.61%), Bacillota (23.25%), Campylobacterota (29.36%), Bacteroidota (6.13%), and Actinomycetota (1.60%) in the CA group, demonstrating moderate evenness (Figure 4a). In contrast, the CC group displayed pronounced dominance of Pseudomonadota (61.66%), indicating reduced diversity. This homogenization pattern persisted across taxonomic levels (Figure 4b-f).At the class level, Gammaproteobacteria dominated in both CB (47.21%) and CC (50.48%) groups, suggesting size-dependent microplastic exposure favors oligotrophic taxa (Figure 4b). Genus-level analysis revealed Vibrio as the predominant taxon, with comparable abundances across groups (CA: 17.03%, CB: 26.83%, CC: 25.66%), implying limited inter-group divergence in core taxa distribution.
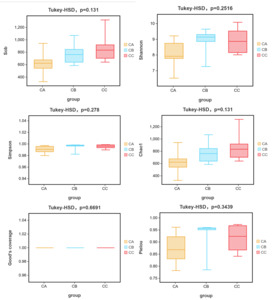
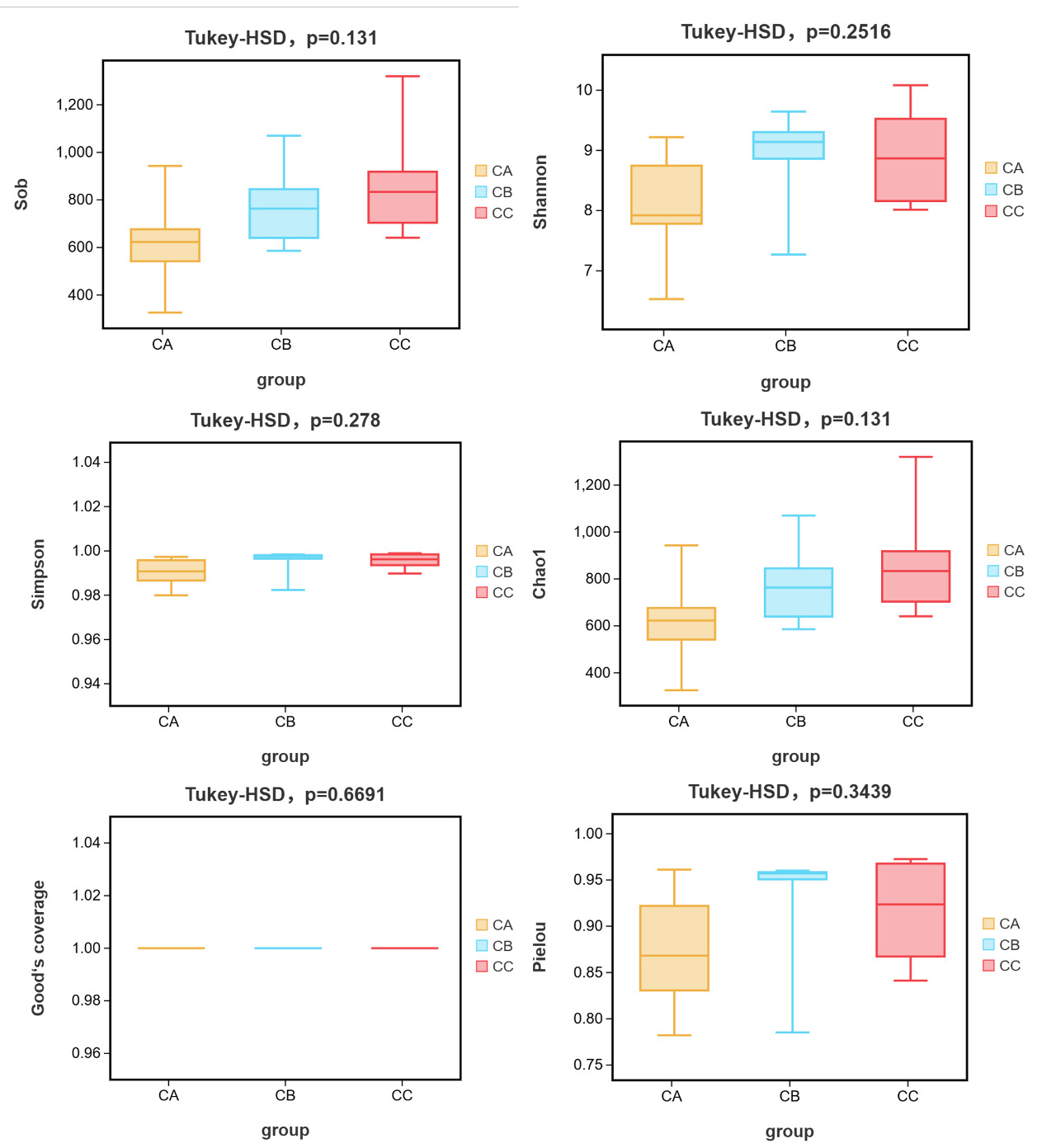
Alpha diversity analysis demonstrated significant increases in all indices (Sob, Shannon, Simpson, Chao1, Goods_coverage and Pielou) in microplastic-exposed groups (CB: 1μm; CC: 5μm) compared to controls (CA), with the 5μm group showing the most pronounced effects (Figure 5). While microbial coverage (Goods_coverage=1) remained complete across groups, larger microplastics induced greater diversity enhancement, particularly in species richness metrics (Sob and Chao1). Beta diversity analysis revealed particle size-dependent clustering patterns: CA and CB groups affiliated first at phylum/species levels, whereas CB and CC groups clustered together at order/family/genus levels, with distinct class-level groupings (Figure 6a-f). Taxonomic shifts were most apparent at genus level, where control communities (CA) were dominated by Arcobacter (37.7%) and exhibited lower richness (10 genera >1% abundance), while exposed groups (CB/CC) showed Vibrio dominance (28.1-29.9%) with higher richness (15 genera >1% abundance) and reduced Anaerorhabdus abundance (CA:13.24% vs CB/CC:1.93-6.70%), indicating a general transition from specialist-dominated to generalist-rich communities under microplastic exposure.
The consistent alpha diversity increases, and taxon-specific beta diversity patterns demonstrate that microplastics, particularly larger particles, significantly restructure gut microbial communities in T.septentrionalis through both diversity enhancement and functional group reorganization. These effects manifest as: (1) size-dependent diversity gradients (5μm > 1μm > control), (2) hierarchical taxonomic restructuring (rank-dependent clustering), and (3) ecological strategy shifts (specialist-to-generalist transition), collectively suggesting that microplastic size mediates competitive outcomes among gut microbiota. The greater impact of 5μm particles may reflect their optimal size for both physical colonization and nutrient surface area, driving more substantial community changes than smaller (1μm) particles, while the control group’s low diversity and Arcobacter dominance likely represent baseline conditions unaffected by microplastic-induced competition.
Effects of polystyrene microplastics on intestinal antioxidant and digestive enzyme activities in T. septentrionalis
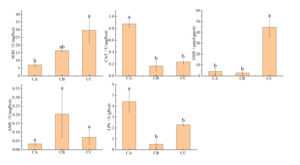
Exposure to polystyrene microplastics induced significant particle size-dependent alterations in antioxidant enzyme activities (Figure 7). Superoxide dismutase (SOD) activity exhibited a general increasing trend, with the 5μm exposure group (CC) showing a 4.2-fold elevation (29.75±7.84 U/mg prot) compared to controls (CA: 7.16±1.22 U/mg prot; P < 0.05), while the 1μm group (CB: 16.43 ± 0.69 U/mg prot) showed no statistical difference (P > 0.05). Conversely, catalase (CAT) activity demonstrated significant suppression across exposure groups (CC: 0.24±0.04 U/mg prot; CB: 0.17±0.08 U/mg prot) relative to controls (0.88±0.06 U/mg prot; P < 0.05). Glutathione (GSH) activity displayed a pronounced increase in the 5μm group (44.68 ±9.04μmol/g prot vs control 4.17±2.70μmol/g prot; P < 0.05), with no significant change in the 1μm group (2.70±1.04μmol/g prot).
The amylase (AMS) activity showed a non-significant increasing tendency with microplastic size (CA: 0.03±0.01 U/mg prot; CB: 0.20±0.14 U/mg prot; CC: 0.07±0.04 U/mg prot; P > 0.05) (Figure 7). In contrast, lipase (LPS) activity was markedly inhibited in both exposure groups (CB: 0.51±0.38 U/g prot; CC: 2.29±0.13 U/g prot) compared to controls (4.42±0.98 U/g prot; P < 0.05), exhibiting an 88.5% and 48.2% reduction for 1μm and 5μm exposures, respectively.
Discussion
Impact of Microplastics on Gut Microbiota at the Phylum Level
The gut microbiota plays a critical role in host physiology, including nutrient metabolism, immune regulation, and intestinal health maintenance.13–15 Our sequencing data (coverage >0.99) revealed distinct microplastic (MP)-induced shifts in the gut microbiota of T. septentrionalis. While alpha diversity indices (Shannon/Simpson) showed modest increases in both 1μm and 5μm MP-exposed groups compared to controls (CA), the 5μm group exhibited more pronounced changes (Figure 5). Notably, Pseudomonadota became the dominant phylum in MP-exposed fish, with significantly higher abundance in the 5μm group (52.3% vs. 38.1% in 1μm), suggesting particle-size-dependent dysbiosis. This aligns with established patterns of microbial community adaptation to environmental stressors.16,17
Bray-Curtis clustering further demonstrated that microbiota composition correlated with MP exposure levels: controls (CA) clustered separately from low-dose (CB) and high-dose (5μm, CC) groups. The distinct CC cluster implies dose-dependent reorganization of microbial communities, consistent with prior observations in Mytilus coruscus exposed to MPs.18 However, unlike M. coruscus—which showed resilience to 10μm MPs after 21 days—T. septentrionalis exhibited marked Pseudomonadota proliferation, particularly with 5μm MPs. This divergence highlights species-specific vulnerability to MP-induced dysbiosis.
The ecological implications of Pseudomonadota dominance warrant attention. In healthy hosts, gut microbial equilibrium is maintained through competitive interactions,19,20 with pathogens suppressed by commensals and host immunity. When a single gut microbe proliferates extensively, it may disrupt the microbial balance and affect the host’s health.21,22 The disproportionate expansion of Pseudomonadota—a phylum containing opportunistic pathogens like Pseudomonas plecoglossicida —suggests MP exposure may compromise this balance. The greater Pseudomonadota abundance in 5μm-exposed fish implies that larger particles pose higher toxicity risks, potentially due to prolonged gut retention or enhanced surface area for microbial colonization. This is corroborated by reports linking P. plecoglossicida to intestinal epithelial necrosis, though further histopathological validation is needed.23 Nevertheless, some scholars have noted that after exposing Mytilus coruscus to a 10μm microplastic environment for 21 days, their gut microbiota community and abundance showed no significant changes. This indicates that the impact of microplastics on gut microbiota may vary across species.24
Genus-Level Microbial Shifts Under MP Exposure
At the genus level, MPs exposure increased Vibrio (26.83% in exposed groups) and Acinetobacter abundances while reducing Anaerorhabdus (13.24% to 1.93%) and Lactobacillus. Vibrio’s proliferation may relate to biofilm formation on MPs, exploiting adsorbed organics for growth.25,26 Though its overabundance could trigger inflammation, consistent with observed SOD upregulation and MDA reduction.27 Anaerorhabdus decline likely impairs short-chain fatty acid (e.g., butyrate) synthesis, compromising gut barrier function,26 which aligns with suppressed lipase activity.25 The sharper reduction of Lactobacillus in the 5μm group suggests enhanced physical damage from larger MPs, destabilizing commensal populations.
Physiological Toxicity Mechanisms: Oxidative Stress and Digestive Dysfunction
Our results demonstrate that MPs exert size-dependent effects on host physiology through two interconnected pathways: oxidative stress imbalance and digestive enzyme disruption.
Oxidative stress imbalance: Antioxidant enzymes (SOD, CAT, GSH) are pivotal in mitigating oxidative stress.28 Studies have demonstrated that microplastics can alter the activities of antioxidant enzymes in various species, including coral Anthozoan, zebrafish, mussels, Litopenaeus vannamei, and Carassius auratus, thereby inducing oxidative stress in these organisms.29–32 In the 5μm MP group, we observed a significant surge in SOD activity coupled with suppressed CAT function, likely due to limited leaching of antioxidants (e.g., BHT) from larger particles.33,34 This compensatory SOD response, consistent with observations in MP-exposed corals and zebrafish.35,36 This suggests an impaired ROS scavenging capacity. Conversely, 5μm MPs likely exacerbated physical abrasion, intensifying oxidative damage.37
Digestive enzyme disruption: In aquatic organisms, it has been proven that digestion rate affects nutrient absorption efficiency, with digestive enzyme activities serving as key indicators of both the digestion rate and energy absorption.38Among these enzymes, AMS and LPS play significant roles in the digestion of carbohydrates and lipid cellulose, respectively,39 and are crucial for the absorption of nutrients. Digestive enzymes (AMS, LPS) were also impacted. Although amylase (AMS) showed non-significant changes, LPS activity was consistently inhibited in both MP groups, potentially due to competitive protease secretion by Vibrio.26The marginal AMS shift in the 1μm group might reflect compensatory carbohydrate metabolism by Proteobacteria, warranting further validation. Similar findings in fish and mussels40–42 confirm MPs’ broad disruption of digestive efficiency, irrespective of enzyme activity trends.
These physiological disruptions appear to initiate a self-perpetuating vicious cycle: microbial dysbiosis leads to impaired digestion, resulting in energy deficits that further weaken stress resistance. Notably, the depletion of Anaerorhabdus and consequent butyrate deficiency may exacerbate this cycle by increasing intestinal permeability and facilitating MP translocation,26 highlighting the complex interplay between MP-induced microbial shifts and host physiological responses.
Ecological Implications and Future Perspectives
Our findings highlight several critical ecological considerations that warrant further investigation. The species-specific vulnerability, where T. septentrionalis exhibited greater sensitivity to 5μm MPs than M. coruscus, likely stems from fundamental differences in feeding ecology (carnivorous versus filter-feeding strategies) and inherent gut microbiota composition. This interspecies variation underscores the need for future studies to incorporate diverse trophic groups to develop comprehensive predictive models of MP toxicity across aquatic taxa.
While short-term microbiota shifts may be partially mitigated through functional redundancy mechanisms, such as the metabolic flexibility of Proteobacteria, our results suggest that chronic MPs exposure could overwhelm these compensatory systems, potentially leading to irreversible health consequences at both individual and population levels.
Notably, the Pseudomonadota/Anaerorhabdus ratio emerged as a promising biomarker for MP exposure. However, its ecological applicability requires rigorous field validation to account for natural environmental variability and complex ecosystem interactions. These findings collectively emphasize the need for a more nuanced, species-specific approach in assessing the ecological impacts of microplastic pollution.
Acknowledgments
This study is financially supported by Central Public-interest Scientific Institution Basal Research Fund, ECSFR, CAFS(No. 2024TD07)and Science and Technology Commission of Shanghai Municipality (Shanghai Natural Science Foundation No. 19ZR1470100).
Authors’ Contribution
Data curation: Xin Chen (Equal), Qiqun Cheng (Equal). Formal Analysis: Xin Chen (Equal), Qiqun Cheng (Equal), Qiqun Cheng (Lead). Funding acquisition: Xin Chen (Lead). Investigation: Xin Chen (Equal), Qiqun Cheng (Equal), Di Peng (Equal), Sumaiye MIERADILI (Equal). Methodology: Xin Chen (Equal), Qiqun Cheng (Equal), Di Peng (Equal), Guoyang Liu (Equal). Resources: Xin Chen (Equal), Qiqun Cheng (Equal), Yanqing Wu (Equal). Software: Xin Chen (Equal), Qiqun Cheng (Equal). Validation: Xin Chen (Equal), Qiqun Cheng (Equal), Di Peng (Equal), Sumaiye MIERADILI (Equal), Guoyang Liu (Equal), Yanqing Wu (Equal). Writing – original draft: Xin Chen (Lead). Writing – review & editing: Xin Chen (Equal), Qiqun Cheng (Equal), Di Peng (Equal). Conceptualization: Qiqun Cheng (Lead). Project administration: Qiqun Cheng (Lead). Supervision: Qiqun Cheng (Lead). Visualization: Qiqun Cheng (Equal).
Competing Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
All animal experiments in this study were conducted in accordance with the relevant national and international guidelines. Our project was approved by Institutional Animal Care & Use Committee (IACUC) of East China Sea Fisheries Research Institute.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.