Introduction
The Chinese cyclina (Cyclina sinensis) is widely distributed in coastal areas, estuaries, and intertidal zones with sandy and muddy substrates in China. It is a eurythermal and euryhaline burrowing bivalve. The substrate environment has a significant impact on this clam and other burrowing bivalves, as their survival, behavior, physiology, and reproductive activities are closely related to the characteristics of the substrate.1 Because C. sinensis in the wild are most accustomed to living in sediment consisting of mixed sand and silt, mud and sand are the traditional substrates used for C. sinensis culture. However, the sand content, which differs among substrates, has a significant impact on the survival rate of C. sinensis.2 Therefore, there are drawbacks to using sediment as the substrate when developing a factory-based aquaculture model for C. sinensis, and it is of great significance to identify new substrates to replace the traditional sediment substrate.
Both physical and chemical aspects of the substrate impact bivalves. For example, physical properties of the substrate determine whether shellfish can effectively excavate and burrow, and they also affect foraging efficiency, growth rate, and survival rate.3 Sand diving is a physiological behavior used by C. sinensis to burrow in the substrate.4 Therefore, for new substrate material to be practical, C. sinensis must be able to sand dive effectively. He et al.5 reported that C. sinensis exhibited good sand-submergence ability in three new substrate materials: quartz sand, glass balls, and shell powder. They also found that the high density and high shear strength of shell powder and quartz sand helped increase the sand-submergence rate of this clam. Compared with the traditional sedimentary bottom, these three new types of sediment have significant advantages from the perspective of sustainability and resource recycling. The traditional sandy and muddy substrate is difficult to clean after breeding, and each replacement requires labor costs. The new substrate is low in price and reusable, thereby reducing the breeding cost. Shell powder has the function of adsorbing impurities in water quality. Experiments have shown that shell powder has a significant adsorption effect on total phosphorus and total nitrogen in reservoir water quality. The content of nitrogen and phosphorus elements in the original water body has decreased significantly.6 Quartz sand is a kind of hard, wear-resistant, and chemically stable silicate mineral. Quartz sand can remove turbidity, ammonia nitrogen, and total phosphorus in seawater.7 Under normal conditions, it does not react with most chemical substances. The particle size range is wide, ranging from a few micrometers to several millimeters. Common specifications include those between 0.1 millimeters and 3 millimeters. Experiments have shown that the removal rate of TAN and NO₂⁻-N by a glass bead filter with a particle size of 0.2 - 0.4 mm is significantly higher than that of a filter with a particle size of 0.4 - 0.6 mm.8 However, the effects of these new substrates on the growth and development of C. sinensis have not been reported to date.
A series of chemical processes that occur in the substrate itself are equally important for buried shellfish.9 Different substrates affect the chemical characteristics of the water environment, including organic matter content, nutrient levels, pH, salinity, and potential contaminants such as heavy metals and organic pollutants. These chemical factors directly or indirectly affect the growth, reproduction, and overall health of shellfish.10 Organic pollutants commonly affect the substrate, and the main sources of organic matter are leaf litter, dead aquatic plants and animals, excreta, and anthropogenically added organic wastes.11 Organic pollutants can significantly change the water quality and affect physicochemical factors in the water, such as nitrogen and phosphorus contents and oxygen-consuming organic pollutants, which in turn can cause eutrophication of the water body and lead to various problems, such as red tides.12 Because high concentrations of nitrogen and phosphorus can affect phytoplankton density and ecosystem stability and lead to metabolic disorders and even mass mortality of shellfish, monitoring of these nutrients is crucial for successful shellfish aquaculture.13
Clearly, when evaluating the applicability of new substrates for use in the culture of C. sinensis, the impact of these substrates on water quality and clam growth and health must be analyzed. The health of shellfish can be evaluated by measuring antioxidant levels. Total antioxidant capacity (T-AOC) is an indicator of the overall antioxidant defense capacity of the organism, as it includes the antioxidant effects of both enzymatic and non-enzymatic factors. Among them, catalase (CAT) and superoxide dismutase (SOD) play central roles, and their activities can be used to evaluate the strength of the organism’s immunocompetence. The combined function of these two enzymes helps maintain the free radical balance, thus reducing oxidative damage.14 Monitoring the activities of CAT and SOD can provide important information about how the organism’s antioxidant system adjusts when it is subjected to environmental stresses.15 Malondialdehyde (MDA) assays are often performed in conjunction with the measurement of SOD and CAT activities to provide a comprehensive assessment of the oxidative status and structural integrity of cells.16 Enzyme activity measurements also provide a good assessment of the health status of C. sinensis, which is an important parameter in the selection of new substrates.
New substrates are needed for the future development of factory farming of C. sinensis. Previously, He et al.5 found that quartz sand, glass balls, and shell powder did not affect the sand-diving behavior of C. sinensis, but they did not evaluate the potential effects of the new substrates on the growth, health, and survival of the clams. In this study, we evaluated changes in water quality and physiological and biochemical indicators of the clams cultured in these new substrates relative to clams cultured in the traditional sediment substrate. Our results provide new ideas for the development of new substrate materials for use in culturing submerged shellfish, which are of practical use for future factory farming operations.
Materials and Methods
Experimental materials and design
The C. sinensis used in this study were reared at a breeding site located in Lianyungang, Jiangsu, China. Individuals with strong, undamaged shells were selected. Average body length was 1.50 ± 0.10 cm, and average weight was 1.2 ± 0.2 g. Sediment of moderate grain size and uniformity suitable for shellfish culture (traditional sediment substrate, hereafter referred to as silt) was selected from the shallows of the Lianyungang Sea, Jiangsu Province, China (34°40′10.88″N, 119°27′57.18″E). Shell powder was procured from the state-owned CIC Zhonglu (Shandong) Agricultural and Animal Husbandry Development Co., Ltd. (Shandong, China). Glass balls were purchased from Shandong Hengyuan New Material Co., Ltd. (Shandong, China), and quartz sand was purchased from Chengdu Taifengxue Technology Co. (Sichuan, China). Before the start of the experiments, the clams were reared in a continuously aerated substrate-free tank for 14 days. The clams were fed twice a day with an algal solution of Trichoderma lucidum (2 x 105 cells mL–1). All clams were handled in strict accordance with the Guidelines for the Care and Use of Laboratory Animals developed by the Bureau of Laboratory Animal Management of the State Council of the People’s Republic of China and approved by the Laboratory Animal Management Committee of Jiangsu Ocean University.
The four substrates (shell powder (40 mesh), quartz sand (40 mesh), glass balls (60 mesh), and silt (control)). Before use, the three types of substrates other than silt need to be filtered through a 40-mesh filter screen to avoid the presence of large particles that may affect the foraging behavior of shellfish in the shallow sand. Three replicate experimental groups were set up for each substrate, and 30 C. sinensis were placed in each tank. The thickness of the substrate was set at 7 cm, and the experiment lasted for 90 days. The water level in the tanks was maintained at 30 cm, the water temperature was controlled at 16 ± 9°C. Due to the long experimental period, the water temperature fluctuated greatly due to weather conditions, but the water temperature in each breeding tank was the same at the same time. The salinity was maintained at 24 ppt, and the pH was 8.1 ± 0.2. To ensure good water quality, the aquaculture system was oxygenated 24 h a day to keep the dissolved oxygen concentration > 6 mg L–1. Clams were fed with Platymonas subcordiformis (2 x 105 cells mL–1) and Chaetoceros calcitrans (4.5 x 105 cells mL–1) twice a day.
Analysis of survival and growth
All C. sinensis in each replicate tank from each experimental group were cleaned and dried of surface moisture with absorbent paper at the end of the training period. Each clam was weighed using an analytical balance (accurate to 0.001 g), and the length, width, and height of the shell were measured using digital Vernier calipers (accurate to 0.01 mm). At the end of the 3-month experiment, the survivors in each group were counted, and the survival rate was calculated as follows: (number of C. sinensis alive at the end of the experiment/30) x 100. Ten C. sinensis were randomly selected from each tank for dissection. The shells were removed, and the intact mollusk parts were collected for subsequent enzyme activity analysis.
Enzyme activity assays
Intact soft parts were extracted from the hepatopancreas of each clam to determine enzyme activity. An analytical balance with an accuracy of 0.0001 g was used to measure 0.1 g of tissue. Next, 0.75% sterile saline (0.75 g NaCl/100 mL sterile water) was added (w/v, 1:9) to the samples using an automatic homogenizer (FastPrep-24, Shanghai Boyi Biotechnology Co., Ltd., Shanghai, China), and the samples were centrifuged at 590 g for 15 min at 4°C. SOD and CAT activities, T-AOC, and MDA content were measured using biochemical kits purchased from Nanjing Jianjian Biotechnology Co., Ltd. (Nanjing, China). All experimental steps were carried out by strictly following the guidelines of the instruction manual.17
Water quality testing
On the last day of the experiment, water samples were collected. Collecting water samples at this time enables us to conduct correlation analysis between water quality conditions and the final growth and physiological state of the C. sinensis. Before taking the samples, the water circulation and the oxygen supply from the air stone were temporarily stopped. Water samples (25 mL) were taken from the surface layer, middle layer, and bottom layer in each of the three replicate tanks of each experimental group. The suspended particles in each sample were removed using a filter membrane. The samples were stored in a freezer at –20°C until used for water quality analysis (Wang et al., 2015). Nitrite nitrogen (NO2–-N) content was measured by visible spectrophotometry (GB/T7493-1987),18 total protein (TP) and total nitrogen (TN) contents were measured by quantitative UV spectrophotometry (GB 3838-2002),19 and ammonium nitrogen (NH4+-N) was determined using Nasher’s reagent method (HJ 535-2009).20
Data processing
SPSS (v.24.0, IBM, Armonk, NY, USA) was used for data analysis in this study. The data are presented in the form of (mean ± SE). One-way analysis of variance and Duncan’s analysis were used to compare data among groups. All graphs were created using Excel (2024, Microsoft, Redmond, WA, USA). Differences were considered significant at P < 0.05.
Results
In the experimental results, the survival rate of the second control group of the shell powder was abnormally lower than that of the other groups. Besides, the other values had reasonable error ranges.
Survival of C. sinensis cultured on different substrate types
Table 1 shows the survival rates of C. sinensis cultured on different substrates after 90 days. The survival rate of clams in the shell powder group was the lowest(P < 0.05), but there were no significant differences among the silt, glass ball, and quartz sand groups (P > 0.05).
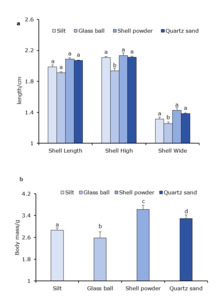
Growth of C. sinensis cultured on different sediment types
Shell length of C. sinensis did not differ significantly among the different substrate groups (Fig. 1a). Shell height and shell width of clams in the glass ball group were significantly lower than those of the other groups (P < 0.05), but the values in the shell powder and quartz sand groups did not differ significantly from those of the control group (P > 0.05). At the end of the experiment (Fig. 1b), the body mass of C. sinensis was significantly higher (P < 0.05) in the shell powder group than in the other three groups, and it was lowest in the glass ball group.
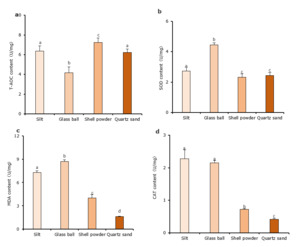
Effect of different sediment types on antioxidant performance
The T-AOC of C. sinensis from the shell powder group was significantly higher than that of clams cultured on the other substrates (P < 0.05, Fig. 2a). The T-AOC was lowest in clams from the glass ball group, and the value was significantly lower than that of the other three substrate types (P < 0.05).
The SOD activity of clams in the glass ball group was significantly higher than that of the other three groups (P < 0.05, Fig. 2b). The shell powder substrate had the lowest SOD activity, but the difference between it and the quartz sand substrate was not statistically significant (P > 0.05).
Clams in the glass ball group also had the highest MDA content (Fig. 2c), and it was significantly higher than that of the other three groups (P < 0.05). The MDA content was lowest in the quartz sand group, and it was significantly different from the values of the other three groups (P < 0.05).
Clams in the silt (control) substrate had the highest CAT activity (Fig. 2d). The activity was significantly higher (P < 0.05) than that of the shell powder and quartz sand substrates, but it did not differ significantly from the value of the glass ball group. The CAT activity of the quartz sand substrate was significantly lower than that of the other three substrates (P < 0.05).
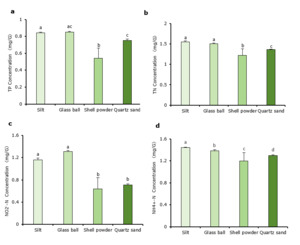
Influence of different substrates on water quality
Among the different treatments, the TP concentration was highest in water from the tanks containing the glass ball substrate (Fig. 3a), but there was no significant difference (P > 0.05) between it and the tanks containing silt and quartz sand substrates. The tanks containing the shell powder substrate had a significantly lower (P < 0.05) TP concentration compared to those containing the other three substrates.
The TN concentration differed significantly among the different substrate types (P < 0.05, Fig. 3b). The value for the quartz sand substrate was significantly lower (P < 0.05) compared to those of the silt and glass ball substrates. The tanks containing shell powder substrate had significantly lower (P < 0.05) TN concentration compared to the other three substrate types.
The NO2–-N concentration differed significantly among the different substrate types (Fig. 3c). Its concentration was significantly higher (P < 0.05) in the tanks containing glass ball substrate than in those containing shell powder or quartz sand Tanks with shell powder substrate had the lowest nitrite concentration, and the value was significantly lower (P < 0.05) than that in tanks containing silt or glass ball substrates.
NH4+-N concentrations also differed among treatments (Fig. 3d). Tanks containing silt substrates had significantly higher (P < 0.05) NH4+-N concentrations compared to tanks with the other three substrates. In contrast, culture water from the shell powder substrate tanks had significantly lower (P < 0.05) NH4+-N concentration compared to the other three groups.
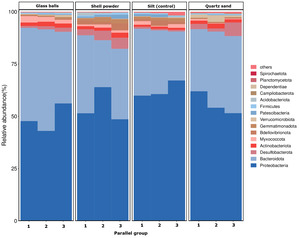
The microbial community structure is presented in Fig. 4.
Discussion
Survival and reproduction of buried shellfish are closely related to the substrate environment in which they live. The type of substrate directly affects the physiology and behavioral habits of buried shellfish, thereby influencing their growth and survival.21–23 Results of the present study showed that C. sinensis cultured on shell powder or quartz sand substrates had better growth and antioxidant capacity compared to clams cultured on silt or glass ball substrates. This finding suggests that C. sinensis is subjected to less environmental stress in shell powder or quartz sand environments, and both substrates have adsorptive properties that can improve the water quality of the culture environment.24,25 Among the four substrates tested, the growth of C. sinensis was worse in the glass bead substrate, likely because this substrate impaired the sand diving effect.5 Additionally, the water quality in the tanks containing the glass bead substrate and the antioxidant capacity of the clams in these tanks were poorer, which suggests that being in a state of stress led to their slower growth. The survival rate of C. sinensis was lowest in the shell powder group, possibly due to infection by pathogens during water change and feeding. We have subsequent experiments that have confirmed that there is an abnormal number of Proteobacteria in the shell powder group in one parallel group. Proteobacteria includes many pathogenic bacteria such as Escherichia coli, Helicobacter pylori, Salmonella, Vibrio, etc., and has been confirmed to be a potential indicator of various intestinal dysfunctions in animals. Especially when intestinal inflammation and hyperglycemia occurs, the Proteobacteria phylum is often the dominant phylum. Regarding the survival of C. sinensis, a large number of deaths occurred in the second parallel group of the shell powder substrate, which might be caused by the excessive pathogenic bacteria,26 as also illustrated in the microbial community structure diagram (Fig. 4). The sediment-carrying capacity of sessile bivalves is one of the indicators reflecting their vitality and is also an important factor influencing the effect of bivalve seeding and rearing. The faster the sediment-carrying speed, the stronger the adaptability of the bivalves, the higher the survival rate, and the higher the recovery rate.3 A study has found that when compared with the traditional muddy bottom sediment as the control group, the C. sinensis performs better in terms of the duration and depth of sediment burial when using shell powder, glass beads, and quartz sand as the sediment instead of muddy sediment.5
Among the various antioxidant properties of organisms, T-AOC represents the overall level of enzymatic and non-enzymatic antioxidants in the body, which can be used to evaluate the antioxidant capacity of the organism.27 In the current study, clams cultured on shell powder substrate had the highest T-AOC. SOD can convert superoxide into CAT through catalytic stress, which plays an important role in the regulation of oxidative stress in organisms.28,29 As a peroxidized end-product, MDA reflects damage to cell membranes caused by the attack of free radicals.30,31 Changes in CAT activity and MDA content can be used to determine the antioxidant capacity of the organism under stressful conditions. Lower values of CAT and MDA indicate less environmental stress, which allows most of the available energy to be used for growth.32,33 In their experiments, the shell meal group and the quartz sand group had higher growth rates compared to the silt group (control). The experimental results showed that the expression of CAT and MDA was lower than that of the silt group (control). The glass beads are hard in texture and have a special surface condition. During the movement or filter-feeding activities of the C. sinensis, their shells, gills, mantle, and other parts are prone to friction and collision with the glass beads. This mechanical stimulation can cause tissue damage to the mussels, such as cell rupture and tissue tearing. The antioxidant enzyme system (such as superoxide dismutase SOD) within the mussels’ bodies will be activated to remove the excessive reactive oxygen species produced during the inflammatory process, thereby alleviating the oxidative stress-induced damage to the cells.34
In aquaculture, the substrate provides a habitat for benthic organisms such as shellfish, and it is also a key area for the accumulation of nutrients and organic matter.35 Through rational selection of substrate, water quality can be effectively optimized to promote the sustainable development of aquaculture.36 In the current study, there was little difference in water quality between the glass bead group and the control group. However, the water quality of the shell powder and quartz sand groups was better than that of the control group, and the shell powder group performed better compared to the quartz sand group. Among the substrates tested, the clams showed better adaptability to the shell powder substrate, which, together with the improvement of water quality by the shellfish themselves, contributed to a more stable and high-quality water environment.37 Quartz sand is characterized by a large surface area and strong adsorption capacity, which allows it to filter organic salts and NH4+-N and other pollutants in the water.38 In contrast, the uniform and smooth surface of glass beads does not readily react with the residues and excreta in the water, which leads to the accumulation of pollutants in the absence of timely cleanup.
In summary, the C. sinensis cultured on the shell powder substrate had the fastest growth rate and the best antioxidant capacity. Compared with the traditional muddy sediment (silt), the shell powder and quartz sand substrates also improved water quality, and the water purification ability of the shell powder was greater than that of the quartz sand. Based on these results, we propose that shell powder can replace the traditional C. sinensis cultivation sediment.
Authors’ Contribution
Conceptualization: Zhengyi Li; Methodology: Guojun He; Formal analysis and investigation: Chaohua Wang; Data curation: Shizhao Du; Writing - original draft preparation: Shiyu Yan; Writing - review and editing: Yihua Chen; Funding acquisition: Zhiguo Dong; Resources: Zhiguo Dong; Supervision: Zhiguo Dong.
Competing Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
We confirm that all efforts were made to ameliorate any suffering of the clam Cyclina sinensis. During the experiment, all authors complied with the Convention on Biological Diversity and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.