Introduction
Carp (Cyprinus carpio) is an important freshwater economic fish in China, known for its strong environmental adaptability, excellent growth performance, and significant financial benefits. It has become one of the dominant species in aquaculture, providing high-quality animal protein for human consumption. However, viral diseases caused by pathogens such as Spring viremia of carp virus (SVCV) severely restrict the development of the aquaculture industry. Therefore, research on the epidemiology, immune evasion, and control measures of SVCV is of great significance.1 SVCV belongs to the genus Sprivivirus within the family Rhabdoviridae,2 and its single-stranded negative-sense RNA genome encodes five structural proteins.3 SVCV is primarily transmitted horizontally, with fish in the viral latency period, infected fish, and dead fish as sources of SVCV infection.4 Epidemiological studies have shown that the virus can infect multiple economically essential fish species within the Cyprinidae family, including the “four major domestic fish” (black carp and grass carp) and carp.5 Viral infection can cause clinical symptoms in farmed carp, such as slow growth and decreased immunity, leading to severe mortality in severe cases.6 SVCV has a high mortality rate in cyprinid fish, causing significant economic losses to the aquaculture industry. However, no effective drugs are currently available to prevent or treat SVCV effectively. Timely diagnosis of the pathogenic virus and implementation of corresponding preventive and control measures are crucial.
Traditional Chinese medicine (TCM) plays an important role in aquaculture, characterized by its natural origin, strong biological activity, low toxicity, minimal side effects, and lack of drug resistance.7 As a representative TCM herb, Houttuynia cordata (fish mint) has been widely adopted in aquaponics. Houttuynia cordata can not only regulate the water quality of aquaculture but also has broad pharmacological activity and great potential for disease prevention and control in aquaculture. It contains various functional components, such as flavonoids and polysaccharides, which exhibit antibacterial, antiviral, anti-inflammatory, antioxidant, anticancer, and immune-regulatory activities.8 Some active components and derivatives of Houttuynia cordata can significantly inhibit lung cancer while also exerting immune-regulatory functions.9 For example, adding an appropriate amount of Houttuynia cordata in the diet can enhance the expression of anti-inflammatory factors and immunoglobulin levels in the gills after bacterial infection, thereby improving the immune capacity of koi carp.10 Houttuynia cordata also plays a role in antiviral activity. Currently, there are many studies on the antiviral effects of Houttuynia cordata against herpes simplex virus (HSV). The aqueous extract of Houttuynia cordata has good inhibitory effects on both HSV-1 and HSV-2, while the volatile oil of Houttuynia cordata mainly shows significant inhibitory effects on HSV-1.11
As the aquaculture industry moves toward green and ecological development, there is an urgent need to explore alternative solutions that can not only effectively inhibit SVCV replication but also maintain the environmental balance of water bodies and ensure the safety of aquatic products. Achieving green prevention and disease control has become a key factor, and it is even more related to the safety of aquatic product quality and the stability of the ecological environment. With this background and its advantages, TCM plays an essential role in this field. It can enhance innate immunity and regulate adaptive immunity, improving the survival rate of fish and becoming a research hotspot in aquatic disease prevention and control. In the research on SVCV, TCM has shown significant potential for application. Previous studies have found that the natural compound coumarin D5 can change the function of the SVCV G protein, interfering with the binding of the virus to the host cell surface and thus inhibiting SVCV infection within cells.12 Bupleurum saponin D significantly reduces SVCV-induced apoptosis and morphological damage in carp cells and promotes the generation of reactive oxygen species (ROS).13 Schisandrin A (SA) can inhibit substantially SVCV replication, reduce viral titer, and increase the survival rate of infected carp, enhancing the host’s innate immune response.14 Isoliquiritigenin can bind to the SVCV G protein, upregulate the expression of interferon-stimulated genes (ISGs) in host cells, and thus inhibit viral adsorption to carp epithelial cells.15
Research on the antiviral effects and mechanisms of Houttuynia cordata in carp is still insufficient. To further explore its antiviral activity, this experiment compared the effects of feeding carp with feed containing 3% Houttuynia cordata and regular feed on carp growth and antiviral capacity. Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was used to detect the expression levels of inflammatory genes in carp, verifying the immune effects of Houttuynia cordata extract on carp. This study aimed to investigate the role of Houttuynia cordata in carp farming, enhance the disease resistance of carp, stabilize carp production, and provide a solid theoretical basis and application value for SVCV research.
Materials and Methods
Cells and Virus Strain
Epithelioma papulosum cyprini cells (EPC) were stored in liquid nitrogen in our laboratory for long-term preservation. EPC cells are the most commonly used cell line for SVCV research due to their high sensitivity to the virus, making them suitable for in vitro antiviral experiments and virus amplification. These cells were cultured in M199 medium supplemented with 10% fetal bovine serum (FBS) and 1% mixture containing 100 U/mL penicillin and 100 μg/mL streptomycin at 25°C in a constant temperature incubator. The SVCV strain used in this study was stored in a -80°C ultra-low temperature freezer in our laboratory. Carp were purchased from a local market.
Primers
All primers used in this experiment were purchased from Wuhan Genecreate Biotechnology Co., Ltd. and stored in a refrigerator at 4 °C (Table 1).
Cell Transfection and Infection
EPC cells were routinely added to 12-well cell plates. The cell infection experiment was initiated when the cell density reached 90%. This experiment included two groups: a control group and an experimental group. The control group received no special treatment and represented the normal cell state. For the experimental group, 1 μL of Houttuynia cordata injection (concentration of 2 g/mL) was added after the cells adhered to the plate. After cells were co-incubated with the Houttuynia cordata extract for 12 hours, 5 μL of SVCV virus was added to each well for infection. After 24 hours of infection, cell samples were collected, and the expression of inflammatory genes in EPC cells was detected using RT-qPCR to investigate Houttuynia cordata’s effects on these genes’ expression.
In Vivo Experiment
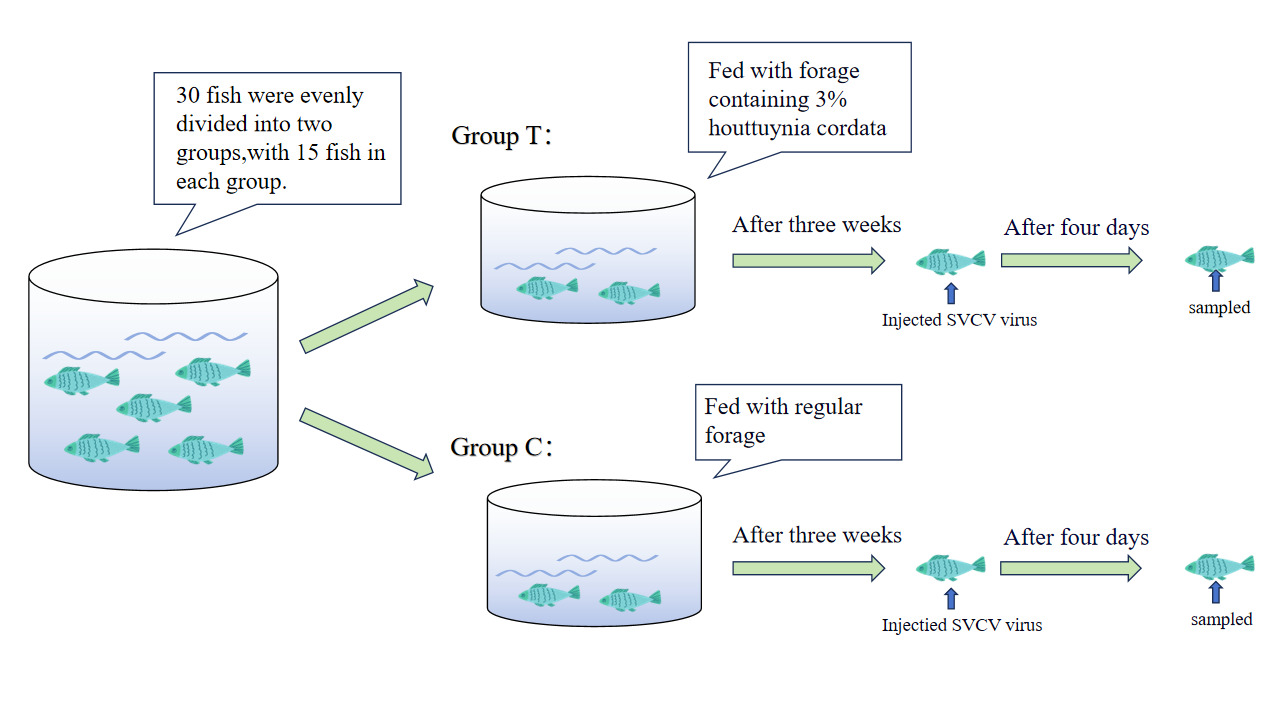
The in vivo experiment aimed to investigate further Houttuynia cordata’s effects on carp’s antiviral capabilities and the immune response under different viral challenge conditions. The experimental design included two groups, each with 15 carp. Group C (control group) was fed regular feed without any Houttuynia cordata components. In contrast, Group T (experimental group) was fed feed supplemented with 3% Houttuynia cordata to observe the effects of moderate Houttuynia cordata intake on carp. The feeding process began with a three-week acclimatization period for both groups to adapt to their respective feeds. After three weeks, carp in both groups were intraperitoneally injected with SVCV virus at 0.2 mL per fish. The feeding regimen continued as planned to observe the effects of Houttuynia cordata on carp during the viral infection process. Sampling was conducted on day 4 post-infection. Multiple tissue samples were collected from the carp, including gills, liver, spleen, head kidney, muscle, and intestine (Figure 1). These tissues cover various parts of the carp related to immune response and viral infection. Detection of these tissue samples provides comprehensive insights into the immune response and viral distribution in different tissues of carp during infection, offering rich data support for further analysis of the antiviral effects of Houttuynia cordata.
Real-Time Quantitative PCR (RT-qPCR)
RNA was extracted from both cell and carp tissue samples using TRIzol reagent. RNA concentration was measured using a nucleic acid detector. Reverse transcription was performed to obtain cDNA from cells and various carp organs according to the instructions of the HiScript II qRT Super Mix reverse transcription kit (Vazyme Biotech Co., Ltd.). The obtained cDNA was diluted fivefold with ddH₂O for subsequent experiments. The RT-qPCR reaction system included: 5 μL of 2×Taq Pro Universal SYBR qPCR Master Mix, 0.2 μL of forward primer (10 μmol/L), 0.2 μL of reverse primer (10 μmol/L), 3.6 μL of ddH₂O, and 1 μL of cDNA. The RT-qPCR reaction conditions were as follows: initial denaturation at 95°C for 30 s, followed by 45 cycles of 95°C for 10 s and 60°C for 30 s, and a melting curve analysis at 95°C for 15 s, 60°C for 60 s, and 95°C for 15 s. β-actin was used as the reference gene for both cells and inflammatory genes, and the gene expression differences were calculated using the 2-ΔΔCT method.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA). The statistical significance of the data was determined using Student’s t-test. A p-value of less than 0.05 was considered statistically significant. The symbols *p < 0.05, **p < 0.01, and ***p < 0.001 were used to indicate levels of significance.
Results
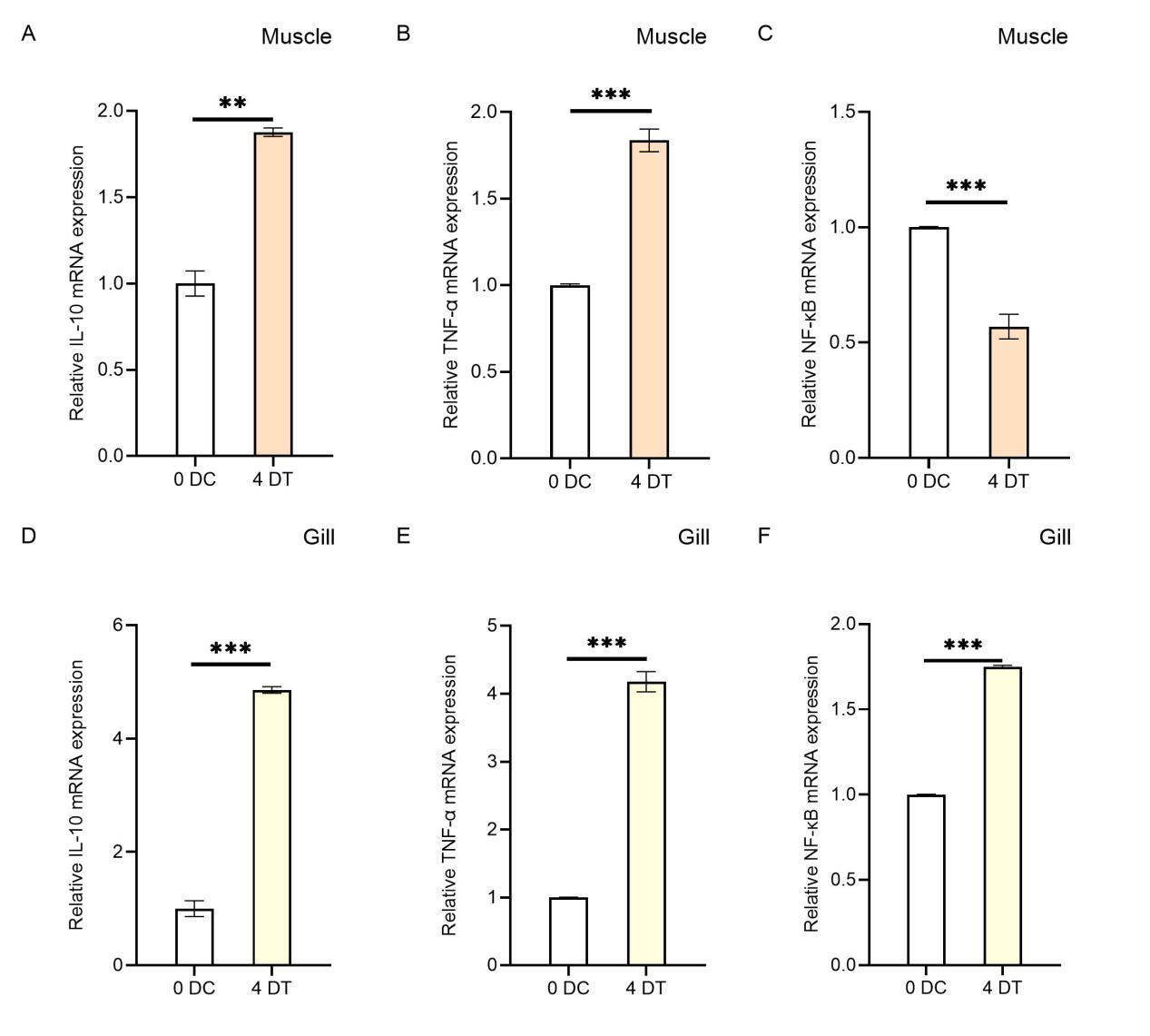
Effects of Houttuynia cordata on Inflammatory Cytokines in External Organs of Carp
Interleukin-10 (IL-10), tumor necrosis factor-alpha (TNF-α), and nuclear factor kappa-B (NF-κB) play important roles in the immune response triggered by SVCV infection. IL-10 has strong immunosuppressive and anti-inflammatory properties,16 while TNF-α is a key mediator in local and systemic inflammatory responses. NF-κB activation regulates the expression of inflammatory cytokines and antiviral genes.17 To investigate the effects of Houttuynia cordata on carp’s resistance to SVCV infection, this study selected three different types of carp organs: external organs, internal organs, and immune-related organs, and quantitatively detected the expression levels of the three key immune-related genes (interleukin-10 (IL-10), tumor necrosis factor-alpha (TNF-α), and nuclear factor kappa-B (NF-κB)).
Effects of Houttuynia cordata on Inflammatory Cytokines in Internal Organs of Carp
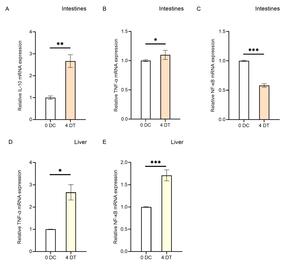
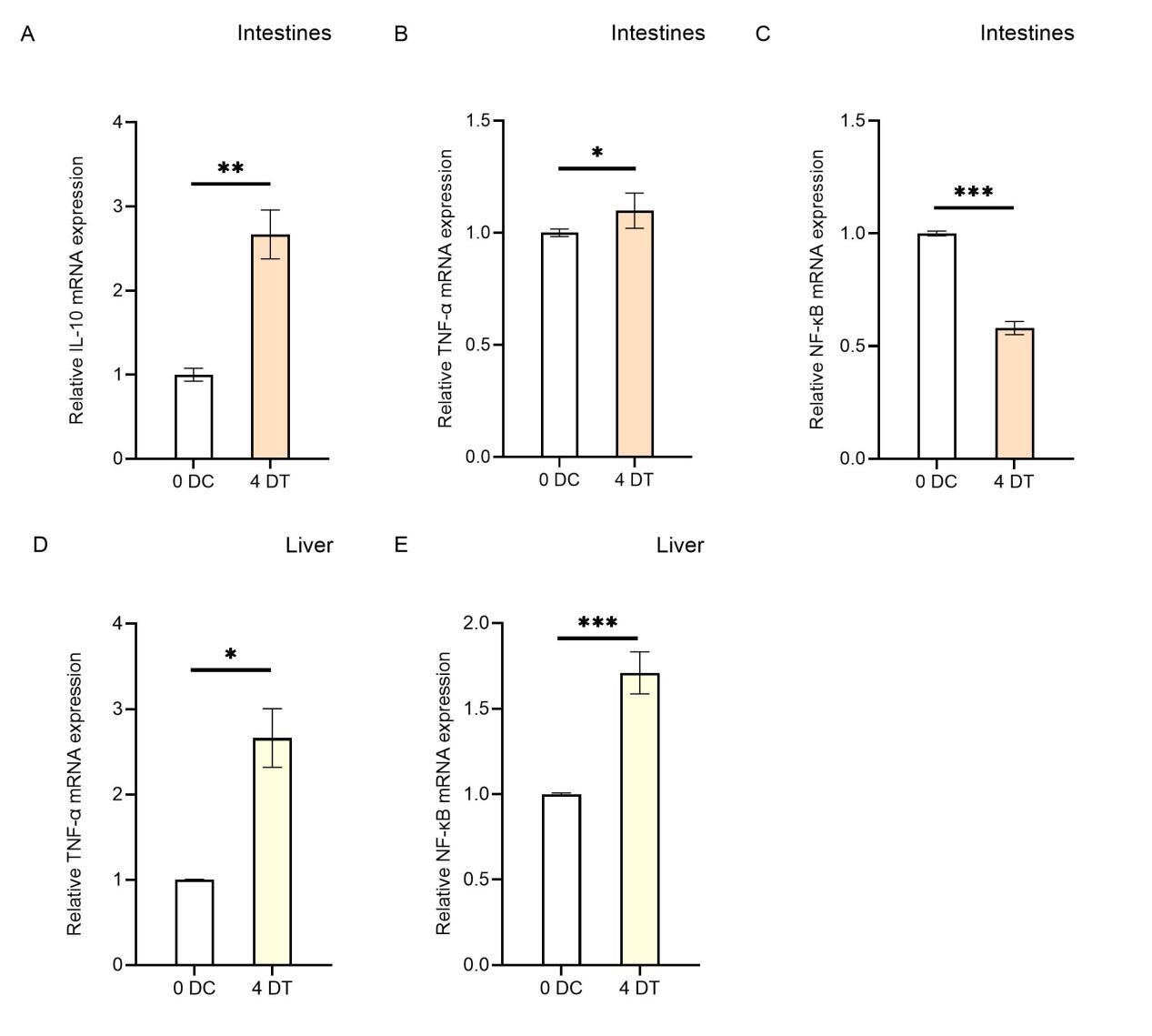
To investigate the effects of Houttuynia cordata on the expression of immune-related genes in carp’s internal organs (intestine and liver), this study analyzed the regulatory effects on gene expression in these organs using RT-qPCR technology (Figure 3). The results showed that compared to the control group (0D), the expression levels of IL-10 and TNF-α in the intestines of carp in the Houttuynia cordata group (4D) increased, with IL-10 showing a more significant increase than TNF-α (Figure 3A, B), showing a 2.6-fold increase (*P < 0.01). In the liver, the expression level of TNF-α increased by 2.6-fold (Figure 3D), while IL-10 expression data were unavailable. The findings indicate that Houttuynia cordata generally enhances the resistance of carp internal organs to SVCV infection.
Effects of Houttuynia cordata on Inflammatory Cytokines in Immune Organs of Carp
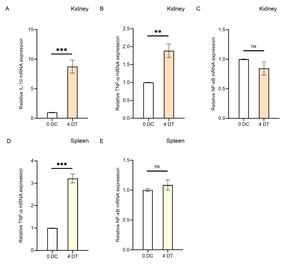
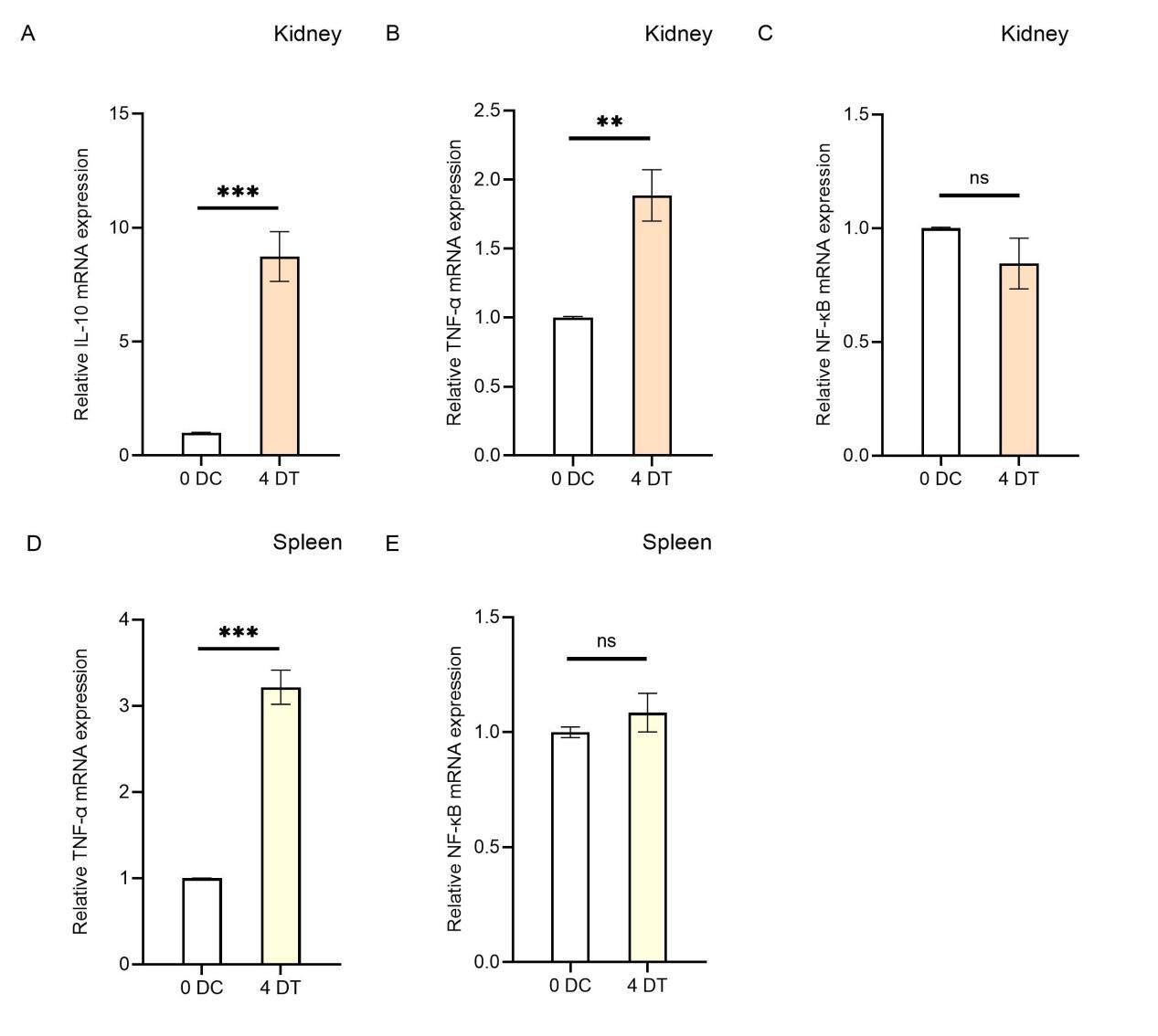
To investigate the effects of Houttuynia cordata on the expression of immune-related genes in carp’s immune organs (kidney and spleen), this study analyzed the regulatory effects on gene expression in these organs using RT-qPCR technology (Figure 4). The results showed that compared to the control group (0D), the expression levels of IL-10 and TNF-α in the kidneys of carp in the Houttuynia cordata group (4D) increased significantly, with IL-10 expression showing an 8.7-fold increase (P < 0.001) and TNF-α expression by 1.8-fold (P < 0.01)(Figure 4A, B). This indicates that the increase in IL-10 expression was more pronounced than that of TNF-α.
In the spleen, the expression level of TNF-α increased significantly by 3.2-fold (Figure 4D), while data for IL-10 were missing. These findings suggest that feeding Houttuynia cordata generally enhances the resistance of carp immune organs to SVCV infection.
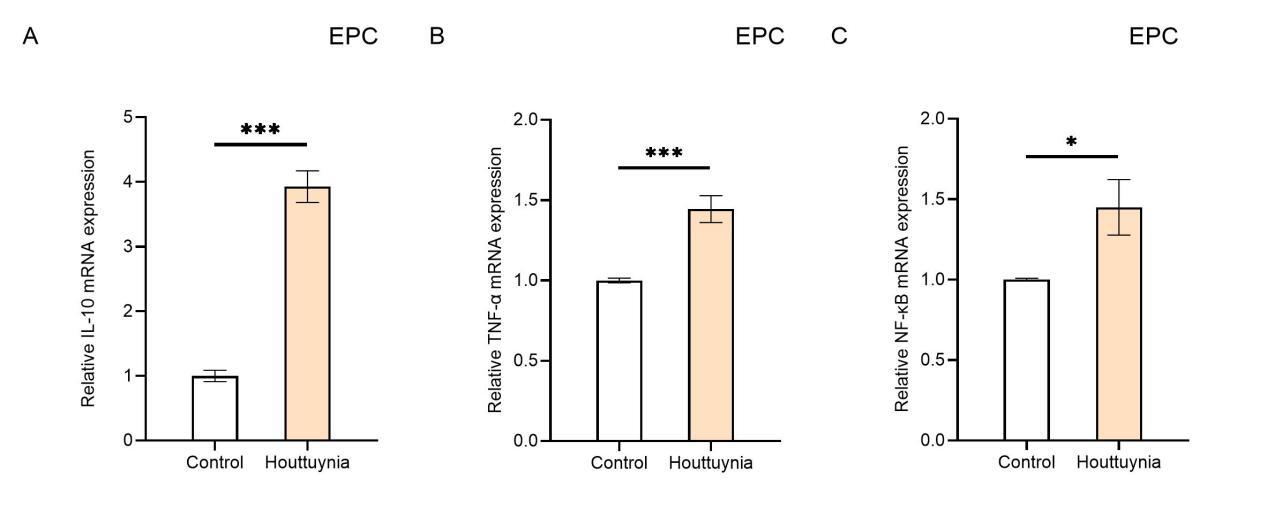
Effects of Houttuynia cordata Extract on Inflammatory Cytokines in Carp EPC Cells
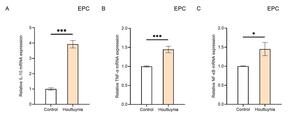
To investigate the effects of Houttuynia cordata extract on the immune response of carp EPC cells, this study examined EPC cells infected with SVCV for 24 hours using RT-qPCR to detect the expression levels of IL-10, TNF-α, and NF-κB genes (Figure 5). The results showed that compared to the control group, the relative expression levels of IL-10, TNF-α, and NF-κB in the experimental group of EPC cells increased significantly. Specifically, the expression level of IL-10 increased nearly 4-fold (P< 0.001) (Figure 5A), while the expression levels of TNF-α and NF-κB both increased by 1.4-fold (P< 0.001) (Figure 5B, C). These findings indicate that Houttuynia cordata extract enhances the resistance of carp EPC cells to SVCV infection and promotes immune function.
Discussion
Spring viremia of carp virus (SVCV) is a highly contagious pathogen that poses a severe threat to cyprinid fish, with distinct seasonal characteristics, primarily erupting in spring when water temperatures rise.18 The virus can be transmitted through various routes, including water, fishing tools, and excretions from infected fish, often causing acute infections and leading to large-scale mortality in farmed fish populations, posing a significant threat to the aquaculture industry.19 The substantial economic losses caused by SVCV outbreaks have become one of the main obstacles to the sustainable development of the aquaculture industry.20 However, there are currently no highly effective treatments available for this virus.21 In traditional aquaculture practices, chemical drugs and antibiotics can disrupt aquatic ecosystems. Against this backdrop, our team proposed using Houttuynia cordata as a potential plant-based solution. As a traditional Chinese medicinal herb, Houttuynia cordata has pharmacological properties such as heat-clearing and detoxifying, anti-inflammatory, and antibacterial effects.22 When used as a feed additive, it has the advantages of low toxicity, minimal residue, and no detectable drug resistance.23 Therefore, our team believes that Houttuynia cordata can be a candidate drug for combating carp spring viremia.
The experimental results indicate that the appropriate feeding of Houttuynia cordata-enriched feed can influence the expression levels of key immune genes in carp, such as the anti-inflammatory cytokine IL-10 and the tumor necrosis factor TNF-α. Decursinol angelate, a compound derived from Houttuynia cordata, has been shown to inhibit the nuclear factor kappa-B (NF-κB) signaling pathway in mouse monocytic leukemia cells (Raw264.7), significantly enhancing the phagocytic capacity of macrophages and thereby controlling the release of inflammatory cytokines.24
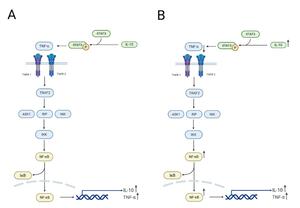
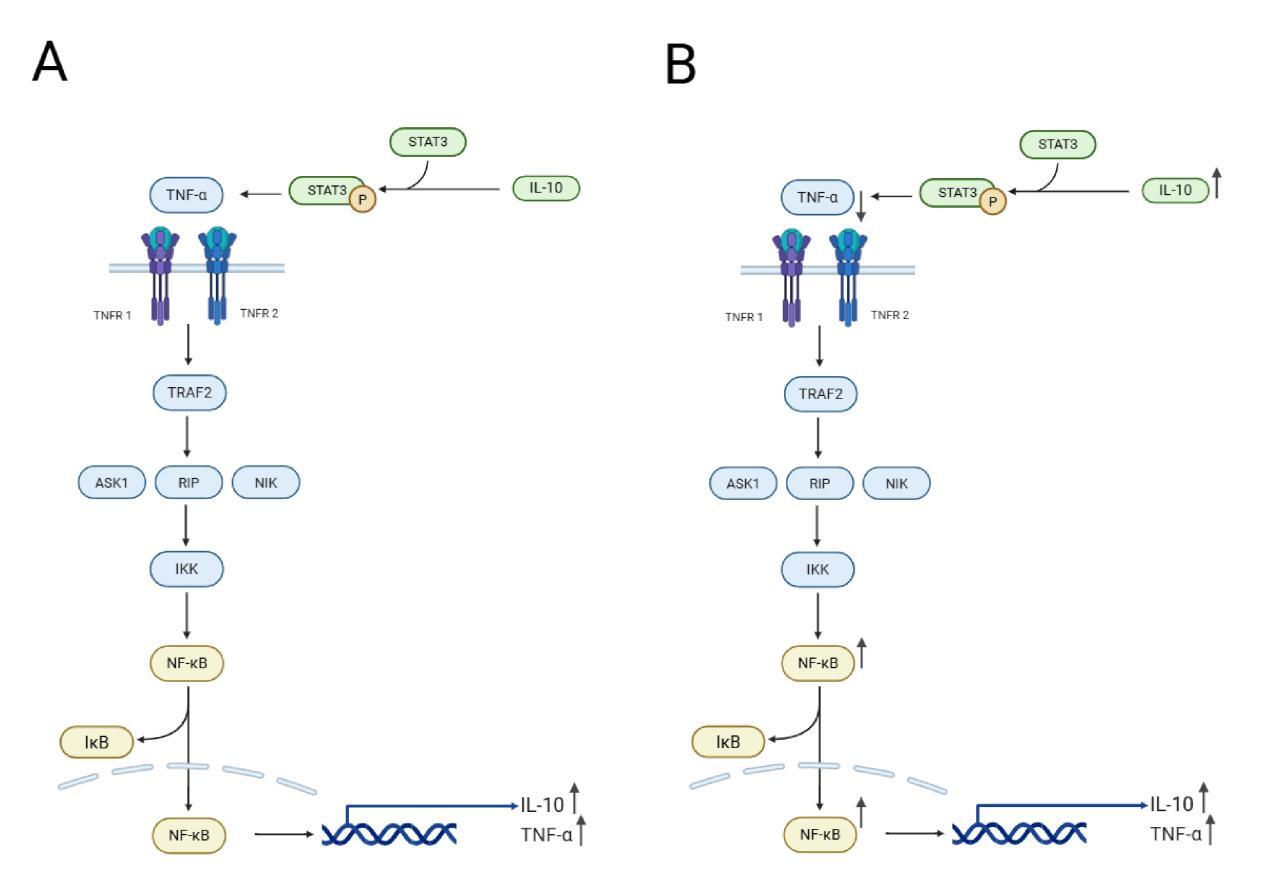
In this study, we compared the relative expression levels of immune-related genes in various tissues (gills, liver, spleen, head kidney, muscle, and intestine) of carp fed with Houttuynia cordata-enriched feed (experimental group, 4D) and those fed with regular feed (control group, 0D) after viral challenge for four days, as well as in EPC cells infected with SVCV for 24 hours. The results revealed significant differences in gene expression levels across different tissues. Apart from genetic background and environmental factors, these differences may be attributed to these organs’ varying stages of anti-inflammatory responses. For example, in the intestinal tissue of the 4D group, the relative expression levels of TNF-α and IL-10 were higher, while NF-κB expression was significantly reduced. This suggests that the anti-inflammatory response in this tissue had progressed to a later stage. At this point, the nuclear translocation of NF-κB was inhibited due to the prior activation of its receptor by IL-10, reducing the transcription of pro-inflammatory genes and thereby alleviating the inflammatory response. The intestine, being in the later stage of the anti-inflammatory response, corresponds to the “dietary” treatment of carp with Houttuynia cordata in the experiment, making it the earliest site of anti-inflammatory activity (Figure 6). Additionally, the expression of NF-κB in muscle tissue was significantly reduced in the Houttuynia cordata group (Figure 2C), while in gill tissue, NF-κB expression was significantly increased in the Houttuynia cordata group (Figure 2F). The results indicate that the effects of Houttuynia cordata on the NF-κB signaling pathway in carp muscle and gills may involve different mechanisms. The expression analysis of NF-κB showed that Houttuynia cordata exerted significant tissue-specific regulatory effects in other tissues. In intestinal tissue, NF-κB expression was downregulated by 0.5-fold compared to the control group (Figure 3C), while in liver tissue, the expression of this factor was significantly upregulated, increasing by 1.8-fold (Figure 3E). This tissue-specific expression pattern suggests that Houttuynia cordata may regulate the NF-κB signaling pathway’s activity in carp’s intestines and liver through different molecular mechanisms.
The experiment demonstrated that Houttuynia cordata can enhance the immune capacity of carp, improving their antiviral ability. Moreover, Houttuynia cordata had no negative impact on the growth performance of carp. The extract of Houttuynia cordata enhanced the antiviral immune capacity of carp without adversely affecting their normal growth, development, or health, thereby ensuring the economic benefits of carp farming in the aquaculture industry. These results provide feasible theoretical support for developing Houttuynia cordata as a natural component for antiviral agents. The practical application value of this study in the aquaculture industry is mainly reflected in three aspects: First, using Houttuynia cordata as a feed additive generally enhances the immune response of carp and significantly improves their antiviral capacity. Second, the intervention using traditional Chinese medicine avoids the problem of antibiotic misuse in aquaculture. Lastly, the expression of anti-inflammatory genes and other target genes can serve as a universal indicator of the actual antiviral efficacy of medicinal feed.
This study on the antiviral effects of Houttuynia cordata against SVCV bridges natural medicine with precision medicine. With the rapid development of modern biotechnology and the deepening of interdisciplinary research, the active components of Houttuynia cordata and their antiviral mechanisms will be systematically elucidated. Houttuynia cordata and other traditional Chinese medicines have the potential to become more effective components in the field of antiviral therapy, offering a “natural immune regulation” solution with Chinese characteristics to the world in the future.
Acknowledgments
This work was supported by the project of Hubei Key Laboratory of Animal Nutrition and Feed Science, Wuhan Polytechnic University (202405); The Research and Innovation Initiatives of WHPU (2023). Chongqing Science and Technology Commission (CSTB2023NSCQ-MSX1092) (Xianlin He).
Authors’ Contribution
Data curation: Zhixuan Li (Equal), Yingda Yang (Equal). Formal Analysis: Zhixuan Li (Equal), Yingda Yang (Equal), Jing Wang (Equal), Yake Wang (Equal), Xianlin He (Equal). Project administration: Zhixuan Li (Lead). Resources: Zhixuan Li (Equal), Yingda Yang (Equal), Jing Wang (Equal). Software: Zhixuan Li (Equal), Yingda Yang (Equal). Visualization: Zhixuan Li (Lead). Writing – original draft: Zhixuan Li (Equal), Yingda Yang (Equal), Jing Wang (Equal), Yake Wang (Equal). Supervision: Yaqian Bai (Equal), Baowei Zhang (Equal). Writing – review & editing: Yaqian Bai (Equal), Jiaqi Zhang (Equal). Funding acquisition: Jian Zheng (Equal), Chi Zhang (Equal). Investigation: Xianlin He (Lead). Conceptualization: Chi Zhang (Lead). Methodology: Chi Zhang (Lead). Validation: Baowei Zhang (Equal), Jiaqi Zhang (Equal).
Competing of Interest – COPE
‘No competing interests were disclosed’.
Ethical Conduct Approval – IACUC
This study involves animal experiments and strictly follows the “Animal Research: Reporting In Vivo Experiments” (ARRIVE) guidelines developed by the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs). It meets the requirements of the ARRIVE Essential 10 checklist, and relevant information has been included in the corresponding section of the article. The experiment has obtained ethical approval from the College of Animal Science and Technology, Wuhan Polytechnic University. During the experiment, we used analgesic drugs to alleviate the suffering of animals. Additionally, this study fully complies with the relevant provisions of the Convention on Biological Diversity and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.