1. INTRODUCTION
Crayfish species native to Europe face numerous threats, such as disease, habitat loss, deteriorating water quality, overfishing and predation.1,2 However, the main cause of declines and local extinctions in European crayfish populations is the widespread presence of invasive, non-native crayfish species originating from North America.1,3–5
In recent years, a significant effort has been devoted to artificially incubating crayfish eggs for intensive culture of crayfish to increase the production of different crayfish species or to maintain the populations of local species. The artificial incubation technique has some advantages, such as preventing maternal diseases,6 reducing maternal, predator, and other water quality-related losses.7 To increase the success of artificial incubation, researchers have developed incubation devices in which crayfish eggs are either mobile7,8 or immobile,7,9,10 incubated eggs at different periods9 and different egg densities,7 tested different chemicals4,11,12 or herbal agents8,13 against fungal egg diseases.
P. leptodactylus species is naturally and widely distributed in lakes, ponds and rivers in Türkiye.14 P. leptodactylus is a species that is not mass-cultured in aquaculture settings in Türkiye. The capture production of this species was 5719 tons in 1984, but the stocks rapidly declined after 1985 due to the crayfish plague (the causal fungus Aphanomyces astaci), and the production was only 662 tons in 2022.15 The population has not recovered since the disease outbreak in 1984. The amount of crayfish, which began to decline after 1977, dropped to as low as 12 tons in 1986 due to crayfish plague and unregulated fishing, and its harvesting was prohibited until the late 1990s. With the removal of the crayfish fishing prohibition, which had been in effect since 1986, in 1999, fishing activities in the lake were revitalized, and the number of boats reached 617 in 2001.16 Therefore, crayfish aquaculture is needed to replenish the domestic stocks and increase production. However, when egg-carrying broodstock was transferred from nature into the culture conditions, mass mortality occurred due to disease and stress. Therefore, artificial incubation studies were carried out after the application of disinfectants to reduce maternal diseases and other problems.5,9 However, artificial incubation studies in this species with a long incubation period are inadequate in many aspects in terms of incubators used, different temperatures,17,18 and disinfectant applications.17 However, hatching protocols have not been developed so far in this species.17 This study investigates the effects of different incubator designs and two temperature levels on the hatching and stage II survival rates of P. leptodactylus eggs. We hypothesize that water-flow incubators that allow egg movement will enhance both hatching and survival compared to static incubators.
2. MATERIAL AND METHOD
2.1. Broodstock supply and egg stripping
Female crayfish broodstock with eggs was obtained from Lake Eğirdir, Isparta, Türkiye, by pinter fishing in April 2023. Eggs were taken from a total of 150 broodstock with an average weight of 52.59 ± 12.26 g and length of 13.23 ± 1.48 cm. Before the stripping collection of the eggs, the abdomens of the broodstock were washed with alcohol (75 % İsopropyl alcohol for 15s.).8 Then, the eggs were stripped from the broodstocks into a container filled with clean water at a temperature of 16 oC, which was equal to the Eğirdir lake water temperature at the time of fishing. The eggs were harvested using flame-sterilized pliers into a basin and treated with 3000 ppm formalin for 15 minutes.11,19 The harvested eggs were at stage XII,8,20 the heartbeat had just started, and the eye pigment was not visible, while 470 eggs were placed in each incubator.
2.2. Artificial incubation conditions
Four different incubator systems were used in the experiment (Table 1): 1. Biolife Turbojet Star X6, (biolife), water valume 350 ml, egg placement area 350 ml (commercial), 2. Jar containing a flat fly wire under to hold the eggs, water volume 770 ml, egg placement area 63.585 cm2 (original), 3. Platform with a flat screen floated with styrofoam, water volume 160 ml, egg placement area 49 cm2 (original) and 4. Aquarium water volume 7.23L, egg placement area 94,985 cm212 (Figure 1). Only biolife incubator was allowed to move the egg depending on the water current whereas the other incubators (2, 3 and 4 systems) held the eggs on the flat screen attached to different systems as outlined above. Each treatment was replicated four times (n = 4). All incubators were tested at two different temperatures: 15.5 °C (selected to suppress fungal growth) and 19.5 °C (the optimum temperature for egg incubation in this species,21). Throughout the experiment, dissolved oxygen was measured every two days (5.8 ± 0.4 mg/L at 19.5 °C and 6.9 ± 0.8 mg/L at 15.5 °C), and temperature was recorded daily.
A recirculation system was used to carry out the experiment at 19.5 oC, and the system had a 2200 L total volume with daily 1% water renewal, a particle screen filter, a biological filter, a thermostatic heater, and a store tank. A total of 4 tanks, each with equal volume (80 L per tank), were used in the experiment (Figure 2). The experiment at 15.5 oC temperature was run in a flow-through water system (Figure 3). In both temperature regimens, a 19 ml/sec water flow was provided to tanks (no. 5 in Figure 2, and no. 6 in Figure 3) containing the jars, platforms, aquarium, and biolife incubators. All incubators were treated with 3000 ppm formalin for 15 minutes every two days to prevent fungal growth until the first hatching of the eggs. Dead eggs were removed from the aquarium and platform incubators every day, but dead eggs were removed from the jars and biolife incubators on the days of formaldehyde treatment. Once formaldehyde treatment was stopped, all systems were checked daily for dead eggs and larvae. When the eggs started to hatch (period I), 10 sponges (approximately 1.5 × 1.5 cm) were placed in small incubators and 15 sponges in large incubators8 (Figure 1). In the experiment, the performance of incubators was evalauted based on the hatching rate and survival rates of the stage II juvenile (with no telson or uropods16,22). The juveniles that reached the stage II were transferred to their respective tanks at the same temperature.
2.3. Statistical analysis
Data were checked for normality and homogeneity by Shapiro-Wilk’s and Bartlett tests, respectively. A two-way ANOVA was used to determine the influences of two experimental factors (temperature and incubators) on hatching performance and survival at stage II. Moreover, each incubator tested at each temperature was separately compared using one-way ANOVA. The significant treatments were discriminated using the Tukey HSD post hoc test at the hatching and stage II. The statistical analysis was performed using JMP v.8.0 for Windows (SAS Institute Inc., Cary NC, USA).
3. RESULTS
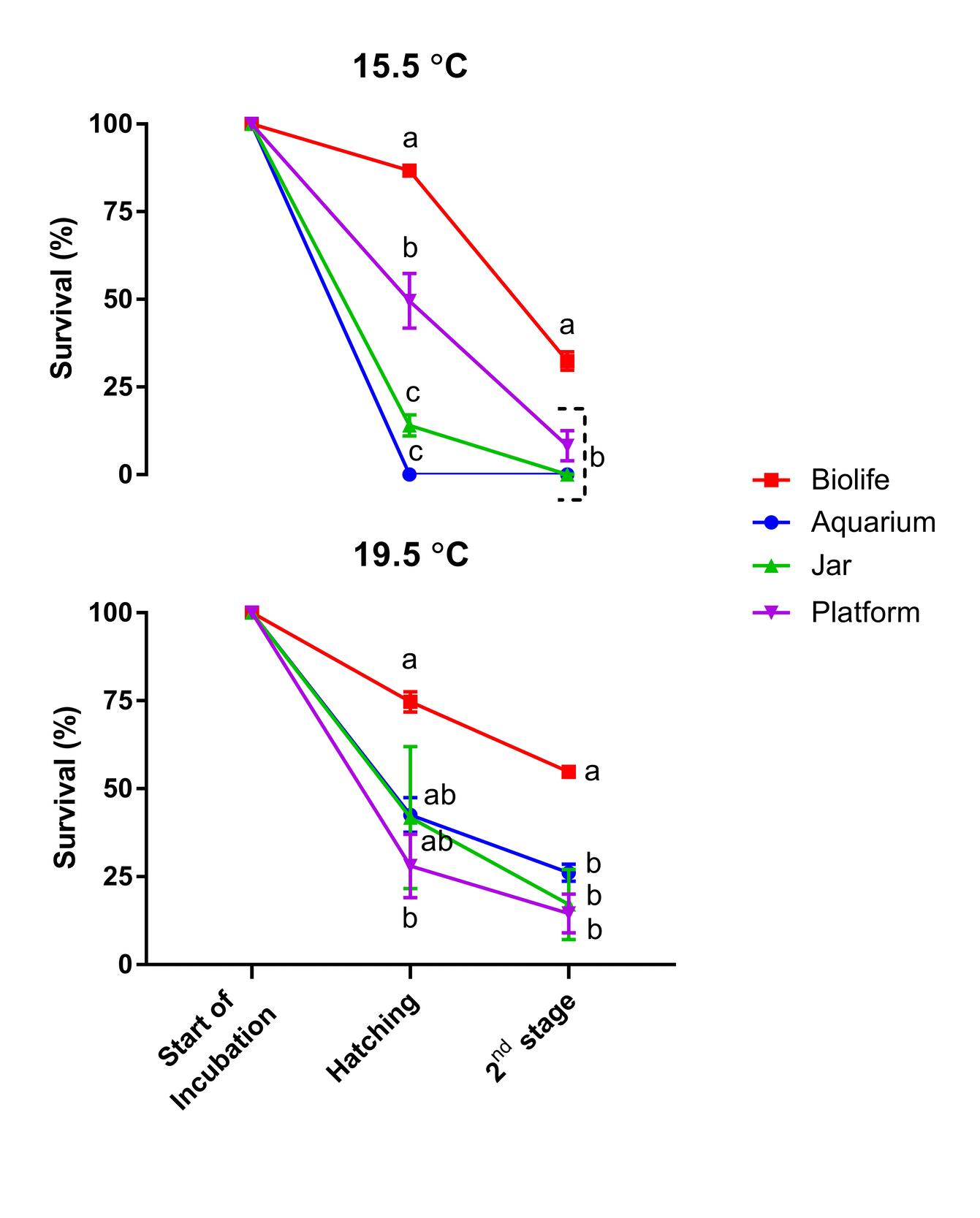
The effects of different temperatures and incubators on the hatching rate and survival rate in stage II of P. leptodactylus are shown in Table 2 and Figure 4. The two ANOVA analyses displayed a significant effect of incubator types (p < 0.0001) but not temperature (p = 0.2481) on the hatching rate. However, there was a significant interactive influence of incubation types and temperature on the hatching rate (p = 0.0025), indicating incubator performance changed depending on temperature. Indeed, no hatching in the aquarium-type incubator was recorded at 15.5 °C, while 42.55% of hatching was the case at 19.5 °C. Similarly, a significantly higher hatching rate was observed in the jar at 19.5 °C than at 15.5 °C (Table 2). Although numerical differences were the case in incubator types of biolife and platform at different temperatures, they were not statistically significant (p > 0.05).
The highest hatching rate was recorded in the biolife type incubator at 15.5°C (86.74%), followed by 74.68% at 19.5 °C (p > 0.05), while the lowest hatching rate was recorded in the aquarium type incubator at 15.5°C (0%).
Interestingly, both incubator types and the temperature had significant influences on the survival at the stage II (p < 0.0001), but their interactions were insignificant (p = 0.1083). The best survival rate was found in biolife (54.82%) at 19.5 °C, while the worst survival rate was found in jars (0.07%) at 15.5 °C. All incubator types performed better at 19.5 °C compared with 15.5 °C (Table 2).
A summary of information on incubation and stage II transition times in experimental groups is displayed in Table 3. At 19.5 oC, a total of 6 formalin disinfections were applied to the incubated eggs, while 10 formalin applications were made at 15.5 oC. The onset of egg hatching was around 20 days at 19.5 °C, but it took 30 days at 15.5 °C. Completion of hatching was between 24 and 30 days at 19.5 °C and 40 and 48 days at 15.5 °C. The time between the beginning and the end of the opening was 4-5 days in biolife, aquarium, and jar at 19.5 °C, but it extended up to 10 days in the platform. The corresponding time at 15.5 °C was 9 days in the Biolife and 17 days in the jar and platform incubators. When the incubation period was standardized based on the degree days, it was between 390 and 410 days at 19.5 °C and 481 days at 15.5 °C. The time from the eggs to the completion of stage II was 30-35 days at 19.5 °C and 47-48 days at 15.5 °C (Table 3).
4. DISCUSSION
The success of incubation and transition to the stage II was the best in Biolife Turbojet Star X6 incubators, where the egg moved in the system at both temperatures. Higher hatching rates were observed at 15.5 °C compared to 19.5 °C in the Biolife incubator, which is likely due to the increased fungal proliferation at the higher temperature. At 19.5 °C and 15.5 °C, incubation success was found to be lower in aquarium, jar, and platform type incubators in which the egg was not moving. At 19.5 °C, it was observed that the fungus prevalence of egg was more pronounced and spread more rapidly in immobile systems. Especially in immobile systems, the position of the eggs relative to each other and the distance between them affect the severity of fungal infection. For this reason, the number of dead eggs between the replication may have considerably varied. Fewer fungus infection was observed in eggs at 15.5 °C. However, more egg deformation was observed with prolonged hatching. The deformation can be characterized by the protrusion of the egg yolk, and this issue was especially more experienced in the aquarium-type incubators. The platform system used in the present study was inspired by the AquaVerde system,8 which found high hatching success (94.08%) in the incubation of Redclaw crayfish (Cherax quadricarinatus). The researchers used disinfection for eggs every other day, and the incubator had 11 vibrations/min. The same system was used for the incubation of C. quadricarinatus eggs, and a high hatching rate at high temperature (28-29 °C) was reported with a daily formalin treatment.20 Our results are inconsistent with other research,8,20 probably due to differences between the platform systems. The researchers provided the vibration system to the platform that moves the egg in their system. Also, they disinfected the eggs more frequently.
In similar aquarium incubators, other researchers9,12,19 also reported a higher hatching rate with 83% in eggs of Pacifastacus leniusculus at 10°C at the beginning and 15.5 °C after black spot eye formation by applying formalin every other day. In the same incubator, the temperature increment from 9 °C to 16 °C resulted in an 85% hatching rate with a formalin application 3 times a week.13 We speculate that the discrepancies from these studies may be due to the fact that, in the present study, incubation was carried out at a constant temperature of 15.5 °C. In our study, we primarily focused on the efficiency of different incubator systems. To achieve this, we aimed to test the incubators at two different temperatures: one was 15.5 °C, a temperature known to suppress fungal infections, and the other was within the optimal temperature range for egg incubation of the species. In nature, the eggs of this species undergo an incubation period of 5–6 months. During this period, water temperatures in the natural habitat gradually increase from around 9–10 °C to 18–20 °C, at which point hatching occurs. Another researcher also incubated the eggs of C. quadricarinatus at different densities in a moving incubator (ZISS incubator).7 Similar to the Biolife incubator in our study, they found 82.05% hatch rate and 55.12 % stage II larvae survival rate.7
The stage II survival rate was higher at 15.5 °C than at 19.5 °C . The reason for this may be the optimal transition to stage II, and the highest survival rate in stage II was observed at temperatures between 18–20 °C.6,13,17
The findings of the present study indicated that the highest hatching and stage II rates were observed in the Biolife incubator. The systems that move the eggs give better results in the incubation of crayfish eggs, and the hatching is good even if the temperature was not increased during the later part of the incubation. It can be suggested that starting incubation at low temperatures and increasing the temperature close to the observation of black eye will give more successful results in order to prevent fungal infections in long incubation in moving systems, especially in cold water species. In particular, larger versions of this incubator system can be developed and commercialized for the incubation of crayfish eggs.
Acknowledgments
The authors declare that there are no acknowledgements.
Authors’ Contribution
Funding acquisition: Mustafa AKIN (Equal), Seval BAHADIR KOCA (Equal), Kamil ATSATAN (Equal), Hasan Batuhan Emre ÖZDOĞAN (Equal), Amanda Kusuma DEWİ (Equal). Project administration: Mustafa AKIN (Equal), Seval BAHADIR KOCA (Equal), Kamil ATSATAN (Equal), Hasan Batuhan Emre ÖZDOĞAN (Equal), Amanda Kusuma DEWİ (Equal). Resources: Mustafa AKIN (Equal), Seval BAHADIR KOCA (Equal), Kamil ATSATAN (Equal), Hasan Batuhan Emre ÖZDOĞAN (Equal), Amanda Kusuma DEWİ (Equal). Conceptualization: Seval BAHADIR KOCA (Equal). Investigation: Seval BAHADIR KOCA (Equal). Writing – original draft: Seval BAHADIR KOCA (Equal). Methodology: Seval BAHADIR KOCA (Equal). Writing – review & editing: Seval BAHADIR KOCA (Equal). Visualization: Seval BAHADIR KOCA (Equal). Data curation: Hüseyin SEVGİLİ (Lead). Formal Analysis: Hüseyin SEVGİLİ (Lead). Software: Hüseyin SEVGİLİ (Lead). Validation: Hüseyin SEVGİLİ (Lead).
Competing of Interest – COPE
Authors have no competing interests, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Ethical Conduct Approval – IACUC
In this study, ethical approval was not required as live animals were not used.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.


.jpeg)
.jpeg)


.jpeg)
.jpeg)