Introduction
Due to the high global market demand, shrimp aquaculture has been rapidly developed in Asian countries over the recent decades. Vietnam is one of the top 4 largest shrimp exporting countries in Asia, producing about 1 million tons in 2023, with an export value of 4.3 billion USD in 2022.1 Whiteleg shrimp, Litopenaeus vannamei have several excellent characteristics, including rapid growth and suitability for high stocking densities2; therefore, it has become a popular species for aquaculture in the Mekong delta, Vietnam. However, high-density cultivation under climate change conditions has significantly increased stress and disease incidences in shrimp. Among various environmental factors, salinity is considered one of the most impactful parameters in shrimp farming, especially during sudden heavy rainfall events caused by climate change.3 Although whiteleg shrimp grows well in a range of salinity from 10 to 15 ppt,4 it becomes stressed when salinity is drastically reduced to less than 10 ppt.5 Heavy rainfall suddenly decreased salinity in aquaculture ponds due to the direct entry of a substantial amount of freshwater.6 Low salinity stress may affect the shrimp immune system, leading to less resistant pathogens.7 In addition, intensive shrimp farming systems are often associated with high ammonia concentrations, which can exceed 40 mg/L.8 Excess ammonia in shrimp pond water may weaken shrimp’s immunity, thereby making them more sensitive to pathogenic microorganisms.9
Probiotics, prebiotics, and synbiotics have been widely applied in aquaculture to promote growth, immunity, and disease resistance of aquaculture species. Synbiotics are combination of probiotics and prebiotics, in which the prebiotics are non-digestible compounds that selectively stimulate the growth or activity of probiotics, produce a synergistic effect in the host.10 Moreover, polysaccharides and dietary fiber are the two primary types of prebiotics.11 Noni leaf extract, which is particularly rich in dietary fiber (24.53%) and carbohydrates (37.82%),12 can serve as an effective source of prebiotics. A previous study reported that dietary supplementation with a mixture of Lactobacillus acidophilus and Moringa oleifera leaf extract significantly enhanced the immune system of L. vannamei.13 In addition, Li et al.14 reported that a combination of Bacillus sp. and isomalt-oligosaccharides significantly increased the shrimp’s immunity resistance to WSSV diseases. Based on the previous findings, prebiotics can enhance shrimp’s immune system by providing energy to stimulate the growth of beneficial bacteria in the gut and have immunostimulatory effects.15 Probiotics can modulate the immune system through stimulating innate and adaptive immune systems, producing cytokines, and increasing the number of immune cells produced.16 The immune system closely affects the well-being of shrimp, especially under adverse conditions such as pathogen invasion and environmental stress. Kaleo et al.17 reported that dietary supplementation with 0.5% Moringa oleifera leaf extract enhanced immune functions both before and after exposure to high ammonia stress in freshwater prawn (Macrobrachium rosenbergii). In the last study, Galib et al.18 reported that shrimp fed Ocinum tenuiflorum leaf extract enhanced immune parameters after challenge with WSSV. However, little is known about the effects of environmental stress on immune response of the whiteleg shrimp after being fed with dietary probiotic, prebiotic, and synbiotic. Therefore, this study assessed the effects of probiotic, prebiotic, and synbiotic on immune indices, namely total hemocyte count, phenoloxidase, and hemocyanin levels of L. vannamei before and after exposure to environmental stress.
Materials and Methods
Preparation of diets and animals
Leaves of noni, Morinda citrifolia were collected from Ca Mau province, Vietnam. The leaves were washed, air-dried and then ground into powder for extraction. The extraction was performed as per the methodology described by Phan et al.19 In brief, leaf powder (20 g) was dissolved and extracted in 100 mL of methanol (70%) for 48 h, and then the liquid was filtered and concentrated at 50oC using a rotary evaporator (Heidolph, Germany). The extraction samples were kept in a brown plastic bottle and refrigerated at 4°C until use. Probiotic, L. plantarum CMT1, was prepared as described by Phan et al.20 The density of L. plantarum CMT1 was adjusted to 109 CFU/mL for further use.
After leaf extract and L. plantarum CMT1, the commercial feed (containing 40% protein) was coated with 1% leaf extract and/or L. plantarum at a concentration of 108 CFU/kg using the spraying method as described by Phan et al.20
Whiteleg shrimp post-larvae (PL15) were purchased from the commercial hatchery in Can Tho city, Vietnam, and stocked in a 10 m³ composite tank under continuous aeration. During acclimatization, water quality was maintained at a salinity of 15 ppt and a pH range of 8.25–8.43.
The rearing of experimental animals and feeding administration of probiotic, prebiotic and synbiotic for 60 days
1200 whiteleg shrimp with a mean weight of 2-3 g were stocked in a 500-l tank at a density of 100 shrimp per tank. They were fed with different experimental diets, namely control, prebiotic (1% leaf extract), probiotic (108 CFU/kg L. plantarum CMT1), and synbiotic (1% leaf extract + 108 CFU/kg L. plantarum CMT1). Each treatment had three replicates, and shrimp were fed four times per day at a feeding rate of 3–5% of their body weight. During a 60-day feeding, water quality in all treatments was checked and controlled in suitable ranges for shrimp growth,21 in which temperature ranged from 24.8-25.7oC, pH 7.51-8.01, salinity: 15-16 ppt, dissolved oxygen: 5.1-5.5 mg/L, alkalinity: 120.1-126.9 mgCaCO3/L, nitrite: 0.015-0.279 mg/L, and total ammonia nitrogen: 0.068-0.327 mg/L. After 60 days of culture, survival rates in the control, PRE, PRO, and SYN groups were 79.0%, 84.7%, 85.3%, and 88.0%, respectively. The control group exhibited a significantly lower survival rate than the other treatments.
Experiment 1. Effects of salinity stress on the immune response of whiteleg shrimp fed dietary supplemented noni leaf extract and Lactobacillus plantarum CMT1
After 60 days of culture, 150 shrimp from each treatment were transferred to three 250 L tanks (50 shrimp/tank) at a salinity of 15 ppt and exposed to a low salinity of 5 ppt for 48 h. Salinity was decreased at 2 ppt/h until the desired salinity was reached (5 ppt). Each treatment was conducted with three replicates. During the exposure period, all tanks were supplied with continuous aeration, and temperature, pH, and DO were maintained at 25.5–26.1 °C, 8.23–8.34, and 5.4–5.9 mg L⁻¹, respectively. The survival rate of shrimp and immune parameters, including total hemocyte count, phenoloxidase and hemocyanin were recorded at 48 h exposure.
Experiment 2. Effects of ammonia stress on the immune response of whiteleg shrimp fed dietary supplemented noni leaf extract and Lactobacillus plantarum CMT1
This experiment was conducted as similar experiment 1. Shrimp fed different diets for 60 days was stocked into a 250 L tank with continuous aeration and exposed to a high level of TAN (40 mg/L) based on the result of Phan et al.19 TAN was prepared by adding NH4Cl until the desired concentration was reached. 100 % of water from each tank was replaced every 24 h to ensure the concentration of total ammonia nitrogen at about 40 mg/L (NH3 = 3.43 mg/L based on pH 8.2 and temperature 25.6oC). Temperature, pH, and DO ranged from 25.3 to 26.1 °C, 8.12 to 8.35, and 5.3 to 5.5 mg/L, respectively. Dead shrimp was recorded every 6 h to calculate the survival rate. After 48 h of exposure, shrimp blood was collected to determine the total hemocyte count, phenoloxidase, and hemocyanin as described in experiment 1.
Data collection
Blood samples were collected from shrimp both before and after 48 hours of exposure to salinity or ammonia stress for further analysis. Total hemocyte count (THC) was measured following the method of Le Moullac et al.22 and Huynh et al.,23 with minor modifications. In summary, shrimp blood was mixed with an anticoagulant solution (pH 7.55). The 50 µL of the mixture (hemolymph) was then placed in a Neubauer counting chamber (Germany). Hemocytes were counted under a light microscope at 40× magnification. THC (cells/mL) = (Number of cells counted x dilution factor x 104)/ Number of squares counted.
Phenoloxidase (PO) was analyzed using L-dihydrophenylalanine (L-DOPA, 3 mg/mL) as a substrate.23 Briefly, 50 µL of each substrate was incubated with 50 µL of plasma hemolymph at room temperature for 10 min, and the absorbance was measured at 490 nm using a microplate reader.
Hemocyanin was measured as described by Pascual et al.24 and Hagerman.25 For each sample, 25 µL of hemolymph was mixed with 225 µL of ultrapure water and measured at 335 nm using a microplate reader (Agilent, USA). The levels of hemocyanin (mmoL/mL) were calculated using the formula: hemocyanin (mmol/mL) = (OD₃₃₅ nm x 10)/17.26.
Statistical analysis
The Shapiro-Wilk and Levene tests were used to assess the normality and homogeneity of variances for THC, PO, hemocyanin, and survival rate. Data was analyzed using one-way analysis of variance (ANOVA), and significant differences between treatments were identified when ANOVA was performed (p < 0.05). Tukey’s multiple range tests were conducted to compare significant differences among treatments using the SPSS 22.0 (SPSS, Chicago, IL, USA). T-test was used to evaluate the significant difference in mean values pre- and post-exposure in each treatment.
Results
Total hemocyte count
Total hemocyte count of shrimp from all treatments before and after exposure to low salinity or high ammonia is presented in Fig. 1. THC levels in shrimp fed SYN diet were statistically significant compared to the control group, both before and after exposure to low salinity (p < 0.05). The values in all treatments decreased after exposure compared to pre-exposure conditions, but there was no significant difference in each treatment between these two conditions. The levels of THC in the control diet were significantly less compared to other treatments in both the conditions (pre-and post-exposure ammonia) (p < 0.05). In addition, the THC values in all treatments greatly decreased after exposure compared to pre-exposure, especially the control group, and displayed a statistically significant difference between two conditions.
Phenoloxidase activity
PO values in control treatments were significant compared to the remaining treatments under both pre- and post-exposure to low salinity (Fig. 2). PO values in all treatments slightly decreased after exposure compared to pre-exposure to low salinity, excluding the control group, and an insignificant difference was found in each treatment between the two conditions. In addition, PO levels in the control treatment decreased by nearly 50% in post-exposure conditions compared to pre-exposure conditions. In comparison to pre-exposure, PO levels in control, PRO, and SYN treatments decreased rapidly after 48 h exposure to high ammonia; it showed a significant difference in PO level from each treatment between the two conditions (p < 0.05).
Hemocyanin
The SYN treatment exhibited the highest amount of hemocyanin, whereas the control treatment showed the lowest level of hemocyanin before and after exposure to low salinity (p < 0.05) (Fig. 3). No significant difference was observed in each treatment under both conditions, except in the control treatment. As for the ammonia test, hemocyanin concentrations in the control group were significantly different as compared to PRE, PRO and SYN treatments under both conditions. The values in all treatments were lower in post-exposure compared to pre-exposure conditions, which was statistically significant under both conditions from each treatment.
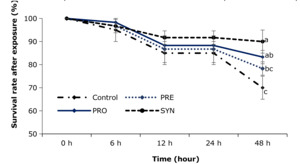
Survival rate after challenge
After 48 h of exposure to high ammonia levels, the mean survival rate of shrimp in the SYN group was 90%, while the mean values for the shrimp in PRO, PRE, and the control groups were 83.3%, 78.3% and 70%, respectively (Fig. 4). Among them, the survival rates in the PRO and SYN groups significantly increased compared to the control group (p < 0.05, Fig. 4). From 6 h to 24 h of exposure, survival rates in the PRO, PRE, and SYN groups were numerically higher than in the control group, but the differences were not statistically significant. The survival rate of shrimp in all treatments was 100% after 48 h of exposure to low salinity stress.
Discussion
In aquaculture, environmental factors such as salinity, pH, temperature, and ammonia could alter the level of hemocytes in crustaceans.26 Presently, the intensive and super-intensive shrimp farming has been expanded in the Mekong Delta, Vietnam. These models with high stocking density have been closely related to an increase in ammonia concentration in water ponds, especially in the adult life stage, which leads to shrimp suffering environmental stress. Lin and Chen27 reported that ammonia levels in the intensive shrimp ponds can be reached over 40 mg/L. Ammonia is a harmful factor in crustacean farming, which affects the health and immune response of aquatic species, potentially resulting in mortality.28,29 In addition, heavy rain causes sudden changes in salinity in water ponds that inhibit shrimp’s immunity, making them more sensitive to pathogenic bacteria.30 Previous studies demonstrated that increased immune responses of shrimp, such as THC, hemocyanin, and PO, have mainly been linked to the supplementation of prebiotics, probiotics, and synbiotics in feed.31,32 In addition, dietary supplementation with L. acidophilus combined with moringa extract significantly increased immune response, including THC and PO in whiteleg shrimp. Chen et al.33 reported that shrimp fed SYN diet (MOS and Bacillus licheniformis) had significantly higher immune responses than those in shrimp fed the other diets after ammonia exposure. In this study, the THC levels in shrimp fed SYN were significantly elevated in comparison to the control treatments both before and after exposure to ammonia or salinity conditions. This finding is consistent with the report of Hamsah et al.,34 wherein the supplementation of synbiotics (Pseudoalteromonas piscicida and MOS) enhanced THC values in whiteleg shrimp compared to other treatments, both before and after the challenge test. In the current study, THC values in all treatments decreased after exposure to ammonia stress, and hence, a low THC indicates a greater susceptibility to pathogens.35 According to Liu et al.,9 the apoptosis of hemocytes was increased under ammonia exposure, leading to decreased THC values of shrimp.
Phenoloxidase activity is used as an indicator of immune response in penaeid shrimp, which is associated with environmental stress.36 PO of shrimp in all treatments significantly decreased after the ammonia or salinity challenge test. Compared with the control group, all treatments presented better PO values, with the synbiotic treatment showing the most significant improvement in both pre-exposure and post-exposure with ammonia or salinity. This suggests that the addition of synbiotics to the diet could enhance PO activity in shrimp, which resulted in less susceptibility to disease infection.37 Hemocyanin plays an important physiological role in crustaceans, including aerobic respiration, innate immune response, and molting,38 which are also used as indicators of gas exchange in crustacean species.39 In the present study, hemocyanin levels of post-exposure shrimp in all treatments were lower than those of pre-exposure shrimp, especially under high ammonia stress. These results align with the study of Zhao et al.,40 where the level of hemocyanin in whiteleg shrimp greatly decreased under high ammonia stress. Additionally, shrimp fed synbiotic treatment notably enhanced hemocyanin compared to the control treatment, which is consistent with the role of noni leaf extract in promoting immunity and increased immune gene expression.41 The enhanced levels of hemocyanin in the synbiotic treatment emphasize the synergistic effects of Lactobacillus sp. and noni leaf extract in increasing the immune response of L. vannamei.
In conclusion, our findings suggest that the supplementation of synbiotics derived from L. plantarum and noni leaf extract might enhance shrimp’s capacity to resist high ammonia and low salinity stress. These results provide useful information and insights for improving shrimp health in super-intensive and intensive culture under climate change conditions. Further research should be needed to confirm gene expression and explore the underlying mechanisms involved.
Acknowledgments
Phan Thi Cam Tu was funded by the Postdoctoral Scholarship Programme of Vingroup Innovation Foundation, code VINIF.2024.STS.33.
Authors’ Contribution
Conceptualization: Phan Thi Cam Tu (Equal), Tran Thi Thanh Hien (Equal). Methodology: Phan Thi Cam Tu (Equal), Huynh Truong Giang (Equal), Tran Thi Thanh Hien (Equal). Formal Analysis: Phan Thi Cam Tu (Equal), Nguyen Thi Kim Lien (Equal). Writing – original draft: Phan Thi Cam Tu (Lead). Writing – review & editing: Phan Thi Cam Tu (Equal), Nguyen Thi Kim Lien (Equal), Huynh Truong Giang (Equal), Tran Thi Thanh Hien (Equal). Supervision: Tran Thi Thanh Hien (Lead), Funding acquisition: Phan Thi Cam Tu (Lead).
Competing of Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
This experiment was carried out in accordance with national guidelines on the protection of animals and experimental animal welfare in Vietnam (Law on Animal Health, 2015). (Reference: Law on Animal Health, 2015. Vietnam National Assembly, No. 79/2015/QH13)
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.