1. Introduction
The Yesso scallops (Mizuhopecten yessoensis) (Mollusca, Bivalvia) are a large cold-water filter-feeding bivalve of significant economic value. Its fan-shaped shell houses tender, nutritious meat, particularly renowned for its high protein and glycogen, and low-fat content, making it highly favored by consumers.1 However, in recent years, persistently high post-reproductive mortality rates have severely impacted its large-scale aquaculture and the healthy development of the industry. Reproductive activities significantly deplete the energy reserves of aquatic animals, leading to physiological and metabolic disorders and reduced immunity, consequently affecting growth performance and stress resistance.2 The reproductive process represents a high energy-consumption phase in the life cycle of bivalves, involving complex physiological activities, such as gonadal development, gamete release, and energy reallocation. Bivalves exhibit significant changes in their biochemical composition during the reproductive period, accompanied by dynamic remodeling of gonadal tissue structure.3 In addition, oxidative stress induced by reproductive activities can affect their health status through changes in immune enzyme activity.4 Current research on bivalve reproduction mostly focuses on changes in single indicators, lacking a systematic integrated analysis of multi-dimensional physiological indices of Yesso scallops before and after reproduction. This makes it difficult to reveal the overall impact of reproduction on the organism and clarify the reproduction-driven molecular regulatory network, thus hindering the proposal of targeted aquaculture optimization strategies. Although energy depletion and oxidative stress during the reproductive period have been documented in bivalves, this study is the first to demonstrate in Yesso scallops (Mizuhopecten yessoensis) distinct, sex-specific physiological adaptation strategies. Key findings include: (1) sexually dimorphic energy allocation patterns, with males relying predominantly on lipid reserves—resulting in a 28.2% reduction in post-reproductive energy stores—while females experience only an 11.4% decline through diversified energy buffering; (2) sex-specific metabolic reprogramming, wherein female gonads enrich amino acid degradation pathways to support oocyte maturation, and males activate sulfur-containing compound metabolism to maintain oxidative balance during spermatogenesis; and (3) localization of primary tissue damage, with the mantle exhibiting the most severe ATP depletion and markedly reduced antioxidant enzyme activity, indicating its role as the core site of reproductive vulnerability.
These results challenge the traditional view of “homogenized energy allocation” in bivalves, offering new insights into sex-differentiated mortality mechanisms and reproduction-driven physiological adaptation strategies at the molecular level. The study’s ultimate aim was to elucidate the multidimensional impacts of reproduction on M. yessoensis physiology, providing both foundational data and theoretical perspectives to understand energy allocation during reproduction better and to inform improved broodstock management practices in aquaculture.
2. Materials and methods
2.1. Experimental materials
This study was conducted at the Key Laboratory of North Sea Aquaculture and Enhancement, Ministry of Agriculture and Rural Affairs, Liaoning Province, China. M. yessoensis were purchased from Zhangzidao Group in March 2024 and transported to the laboratory on the same day in foam-insulated boxes with crushed ice for cooling. Before acclimation, epibionts on the shells were removed with a hard-bristled brush, and all scallops were acclimated for 7 days. During acclimation, continuous aeration was maintained to ensure oxygenation, with a water temperature of 8.03 ± 0.5℃, pH 7.5–8.6, and salinity 25–35, with half the water volume exchanged daily. Scallops were fed Spirulina and Chlorella in a 3:1 ratio and a feeding density of 10 mg/L. After acclimation, 100 healthy scallops (50 females and 50 males) with natural shell-closure ability and vigorous activity were selected, with an average body weight of 174.13 ± 3.30 g. The females were divided into five parallel groups (ten individuals per group), as were the males. Based on the preliminary pre-experiment, this sample size can meet the requirements for analyzing the significance of intra-group differences.
2.2. Sample collection
After the acclimation period, two M. yessoensis were randomly selected from each of the female and male groups (resulting in ten females and ten males) for pre-reproductive sampling. Gonadal tissues were collected for nutritional component analysis, tissue sectioning, and metabolomics analysis (metabolomics samples were named Q-C-X1, Q-C-X2, and Q-C-X3 for females and Q-X-X1, Q-X-X2, and Q-X-X3 for males). Gill, hepatopancreatic, renal, gonadal, mantle, and adductor muscle tissues were collected, rapidly frozen in liquid nitrogen, and transferred to –80°C storage for subsequent component detection, enzyme activity and metabolomics analyses. Gill tissues for sectioning were stored in cryotubes containing paraformaldehyde at 4°C for subsequent use.
For post-reproductive sampling, two M. yessoensis were again randomly selected from each of the female and male groups (resulting in ten females and ten males). Artificial spawning induction was performed using dry-air exposure combined with temperature elevation stimulation (The broodstock shellfish are temporarily reared at a water temperature of 8℃. When their gonads develop to an appropriate stage (with a plump appearance and bright color, orange-yellow for females and milky white for males), induced spawning can be carried out at an opportune time. First, air-drying stimulation is performed, with the air-drying time being 90-120 minutes (generally 120 minutes for female broodstock and 90 minutes for male broodstock). The air-drying environment was light-free, cool, with a temperature of 8℃ and a humidity of ≥60%. After air-drying, the male and female broodstock are separated and directly put into seawater at 12-13℃ for induced spawning. Approximately 30 minutes later, they will start to release sperm and eggs. After M. yessoensis had released sperm and eggs, post-reproductive sampling was conducted, with sample processing identical to the pre-reproductive stage (given that the spawning and spermiation process is completed within 24 hours, this study focuses on the key turning points before and after reproduction.). Metabolomics samples were named H-C-X1, H-C-X2, and H-C-X3 (females) and H-X-X1, H-X-X2, and H-X-X3 (males).
2.3. Component analysis
Standard methods were used to determine the nutritional components of M. yessoensis gonads: Crude protein was measured using the Kjeldahl method, which primarily calculates protein content by determining the nitrogen content in samples; crude fat was determined using the Soxhlet extraction method, which measures fat content by extracting lipids with organic solvents; glycogen was assayed using a kit (Shanghai Sangon Biotech Co., Ltd., (China Shanghai), which quantifies sugars through enzymatic hydrolysis or chemical color reactions.
2.4. Section preparation
Tissue sections were prepared using the paraffin sectioning method. Briefly, the collected gonadal samples were fixed in Bouin’s solution for 24 h, followed by dehydration through a gradient ethanol series, were cleared with xylene, and then embedded in paraffin. Serial sections with a thickness of 5 μm were cut continuously using a microtome. The sections were then gradually dewaxed and rehydrated, stained with Hematoxylin and Eosin (H&E), and finally dehydrated and cleared. The sections were mounted with resin, preserved, and observed under a microscope (Leica DM4 B, Germany), with images taken.
2.5. Enzyme activity assays
For the enzyme activity assays, 0.1–0.15 g of the previously collected gill, renal, gonadal, mantle, and adductor muscle tissues were each placed into 2 ml centrifuge tubes. Next, 10% tissue homogenates were prepared by adding PBS at a mass-to-volume ratio of 1:9. Steel beads were added, and the samples were homogenized using a tissue homogenizer for 30 s. After homogenization, the samples were centrifuged at 4°C and 3000 rpm for 10 min. The supernatant was transferred to 1.5 ml centrifuge tubes for enzyme activity assays. Kits from Nanjing Jiancheng Bioengineering Institute were used to detect superoxide dismutase (SOD), catalase (CAT), total antioxidant capacity (T-AOC), lysozyme (LZM), lactate dehydrogenase (LDH), ATP, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), following the manufacturer’s instructions.
2.6. Metabolomics
For metabolomics analysis, 0.1–0.15 g of gonadal samples was ground under liquid nitrogen, and 800 μL of methanol-acetonitrile-water (2:2:1, v/v) solution was then added. The samples were vortexed and then sonicated twice at 4°C, each for 30 min. They were then incubated at –20°C for 1h to precipitate proteins, followed by centrifugation at 13,000 rpm and 4°C for 15 min. The supernatant was transferred to a new tube, lyophilized, and stored at–80°C until use. For mass spectrometry analysis, the lyophilized samples were reconstituted with 100 μL of acetonitrile-water (1:1, v/v) solution, vortexed at 4°C, and then centrifuged at 14,000 rpm for 15 min. The supernatant was then transferred for LC-MS/MS analysis.
For chromatographic separation, chromatographic mobile phase A comprised water + 25 mmol/L ammonium acetate + 25 mmol/L ammonia water, whereas mobile phase B was acetonitrile. The chromatographic gradient elution program. Samples were maintained at 4°C in the autosampler throughout the analysis, with an injection volume of 10 μL. Separation was performed on a Waters Acquity BEH Amide column (2.1 mm×100 mm, 1.7 μm) at a column temperature of 25°C and a flow rate of 0.3 mL/min. Quality control (QC) samples were inserted into the sample queue to monitor and evaluate system stability and the reliability of experimental data.
2.7. Statistical analysis
Data are expressed as mean ± standard error. One-way ANOVA and independent t-tests were performed using SPSS software (version 22.0) to test for differences in expression between organizations. Shapiro-Wilk test and Levene’s test were first used to test the distribution of the data and the chi-squaredness of the variance, respectively. One-way ANOVA was followed by LSD (Least Significant Difference) post hoc test. p values less than 0.05 were considered statistically significant.
In the statistical analysis of this study, the false discovery rate (FDR) correction was applied to handle multiple comparisons. Specifically, both the screening of differential metabolites in metabolomics (VIP > 1 and P < 0.05) and the inter-group comparison of enzyme activities were subjected to FDR correction to control Type Ⅰ errors, ensuring the reliability of the results.
3. Results
3.1. Changes in general nutritional components before and after reproduction
The changes in nutritional components of M. yessoensis gonadal tissues before and after reproduction are shown in Table 1. Significant differences (P <0.05) were observed in the nutritional components of M. yessoensis gonads before and after reproduction. The crude protein, crude fat, and glycogen content all decreased, with that in crude fat being particularly significant (P <0.05). After spawning, crude fat decreased by 28.2% in males and 11.4% in females, and the proportional difference in content between females and males before and after spawning increased from 11.27% to 37.25%. For glycogen, males showed a 5.93% decrease and females a 3.91% decrease after spawning, with the content difference between females and males increasing from 10.81% to 13.18% before and after spawning (Table 1).
3.2. Changes in gonadal tissues before and after reproduction
During the pre-reproductive stage, the gonads were in the mature phase, with the entire follicular lumen filled with mature germ cells and few, if any, spaces between follicles. In female follicles, mature eggs were irregularly shaped as a result of mutual compression, with yolk granules densely packed inside. The nuclear membrane and nucleolus were distinct, and immature oocytes with egg stalks remained attached to the follicular wall. In male follicles, the lumen was completely filled with spermatids and metamorphosed sperm, which were densely arranged with their heads oriented toward the follicular wall and tails toward the lumen.
After reproduction (spawning stage), female gonads showed significant changes: large and small cavities formed in follicles following the release of mature eggs, and the follicular walls thinned. In the late stage, follicles were empty other than for some disintegrating residual overmature eggs. In male follicles, sperm were arranged in bundles, with a distinct central cavity in the follicular lumen. Spermatocytes, spermatids, and sperm at different developmental stages coexisted within the follicles (Figure 1).
3.3. Comparative analysis of immune-related enzyme activities in M. yessoensis before and after reproduction
There were various changes in the activity of immune system-related enzymes (SOD, CAT, LZM, and T-AOC) in gill, hepatopancreatic, renal, gonadal, adductor muscle, and mantle tissues of M. yessoensis before and after reproduction. Under reproductive stress, SOD activity showed an upward trend in gill tissues and adductor muscles of both female and male scallops. Enzyme activity in these two tissues in males was significantly different (P<0.01) before and after reproduction. In the mantle, SOD activity decreased significantly in both sexes (P<0.01 in females and P<0.05 in males). CAT activity decreased post- compared with pre-reproduction in gill tissues, adductor muscles, and mantle of both females and males. Significant differences were observed in female adductor muscles and mantle, as well as male gill tissues (P<0.05) and male mantle tissue (P <0.01). Significant changes in T-AOC were only observed in adductor muscles, where it increased in both sexes before and after reproduction (P<0.05 in females and P<0.01 in males). LZM activity increased in gill tissues of both sexes, although only significantly in females (P<0.05) compared with pre-reproductive levels. By contrast, LZM activity decreased in mantle and gonadal tissues, but only significantly so in female mantle tissues before and after reproduction (P<0.05) (Figure 2).
Changes were also recorded in the activities of energy-related enzymes (LDH, ALT, and AST) and ATP levels in gill, hepatopancreatic, renal, gonadal, adductor muscle, and mantle tissues of M. yessoensis before and after reproduction. Significant changes were observed in LDH activity in the mantle in both females and males (P<0.01). ALT activity decreased in the gill, renal, adductor muscle, and gonadal tissues in both sexes, although significant differences (P<0.01) were only observed in the gonadal and adductor muscle tissues of both females and males. Fluctuations in AST activity were only observed in hepatopancreatic and adductor muscle tissues in both sexes, with activity significantly increasing after reproduction (P<0.05). Significant differences (P<0.01) were found in ATP levels in female renal, gonadal, and mantle tissues, as well as male gill and adductor muscle tissues compared with pre-reproductive levels (Figure 3).
3.4. Comparative metabolomics analysis of M. yessoensis before and after reproduction
3.4.1. Classification of differential metabolites before and after reproduction
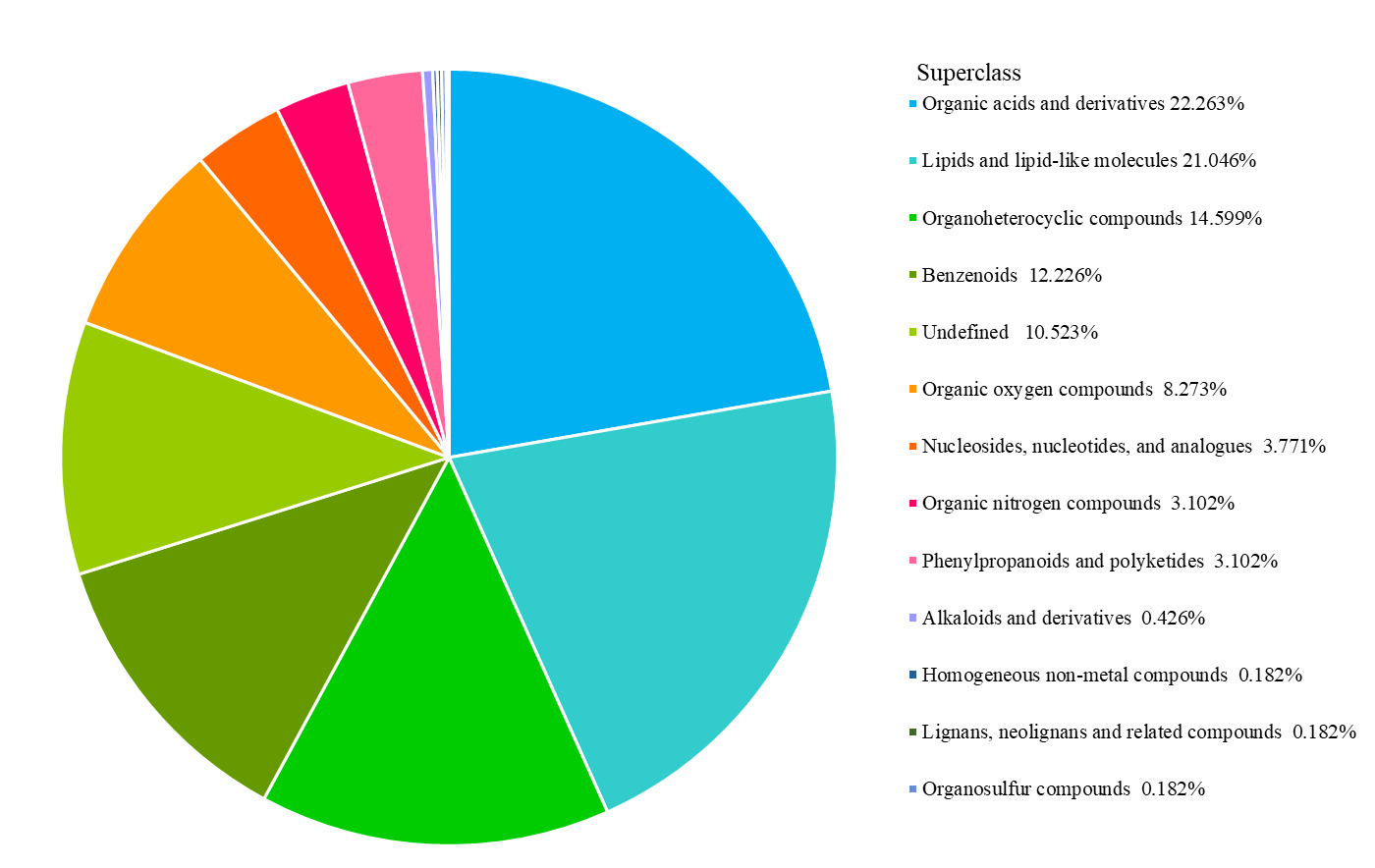
Using ultra-performance liquid chromatography-mass spectrometry (UPLC-MS), the two groups of samples were analyzed in positive (POS) and negative (NEG) ionization modes. A total of 1,644 metabolites were detected, 903 in POS mode and 741 in NEG mode. Differential metabolites were then classified, with organic acids and their derivatives, and lipids and lipid-like molecules accounting for prominent proportions of 22.263% and 21.046%, respectively (Figure 4).
3.4.2. Analysis of differential metabolites in gonadal tissues of male and female M. yessoensis before and after reproduction
3.4.2.1. Multivariate statistical analysis
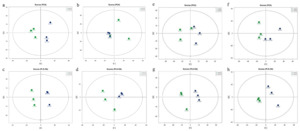
Metabolic difference analyses were conducted on the gonadal tissues of male and female M. yessoensis before and after reproduction. In POS and NEG ionization modes, principal component analysis (PCA) showed significant separation of gonadal samples before and after reproduction, indicating global reconstruction of gonadal metabolic profiles during reproduction in both sexes. Partial least squares discriminant analysis (PLS-DA) further validated this difference, with all samples within the 95% confidence interval, confirming the reliability of the data (Figure 5).
3.4.2.2. Screening of differential metabolites
UPLC-MS was used to analyze differential metabolites in the gonads of female and male M. yessoensis before and after reproduction. Significant sexual dimorphism was observed in metabolic reprogramming. In female gonads in POS mode, 903 differential metabolites were identified. Lipid and amino acid metabolism were among the 444 upregulated metabolites (such as 3-(2-hydroxyethyl)indole, 1-hexadecanoyl-2-octadecadienoyl-sn-glycero-3-phosphocholine, histidine, suberic acid, and sphingosine etc), whereas reduced nitrogenous compounds were among 459 downregulated metabolites. In NEG mode, 741 differential metabolites were detected, with 387 upregulated metabolites involved in energy metabolism and immune regulation, and 354 downregulated metabolites inhibiting key nodes in the tricarboxylic acid (TCA) cycle. In male gonads in POS mode: 903 differential metabolites were detected, with 586 upregulated metabolites, primarily sulfur-containing compounds(such as S-methyl-5’-thioadenosine, 1,2-pentacosanoyl-sn-glycero-3-phosphocholine, L-cysteine-glutathione disulfide, ethcathinone, glutathione, and linoleoylethanolamide etc.), and 317 downregulated metabolites, including reduced signaling lipids. In NEG mode, there were 741 differential metabolites, related to inhibited carbohydrate metabolism and enhanced oxidative stress defense (Figure 6).
3.4.2.3. Global analysis of KEGG metabolic pathways
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis showed that, after reproduction, gonadal metabolic reprogramming in female and male M. yessoensis exhibited significant sex-specific regulatory characteristics.
In female gonads, 87 differential metabolites were significantly enriched in 37 metabolic pathways. Core pathways included amino acid metabolism, such as multiple amino acid degradation pathways, and also energy metabolism regulation, such as the TCA cycle and mitochondrial oxidative phosphorylation (Figure 7).
In male gonads, 103 differential metabolites were distributed across 70 pathways. In addition to amino acid metabolism, significant enrichment was observed in neural signaling and redox regulation pathways as well as neurotransmitter synthesis, ROS scavenging, glycolytic activity, and bile secretion and mineral absorption pathways (Figure 8).
4. Discussion
4.1. Changes in biochemical composition and gonadal histology of Yesso scallops before and after reproduction
The dynamic changes in biochemical components, such as crude protein, crude lipid, and glycogen, during reproduction in M. yessoensis not only reflect its energy metabolism characteristics but also reveal differences in reproductive strategies between the sexes. This is crucial for understanding its bioenergetic allocation mechanisms and reproductive adaptability.5 Research on the Zhikong scallop (Chlamys farreri) demonstrated that, although females lost 4 kJ of overall energy during ovarian maturation, the net energy gain in the gonad reached 41.5 kJ, indicating diversified energy sources. Conversely, males experienced somatic energy loss of 21 kJ during testis maturation, with only a 17 kJ increase in testis energy, confirming significant differentiation in the pattern of reproductive energy flow between the sexes.6
In the current study, crude protein, crude lipid, and glycogen content in both male and female M. yessoensis decreased significantly after reproduction, indicating that these substances serve as core energy substrates during the reproductive process, with significant differences in demand between the sexes. Specifically, the post-reproduction decrease in lipid content was significantly higher in males (28.2%) compared with females (11.4%). This is closely related to the reliance of males on lipids from the digestive gland to sustain gonad development and reproductive activities. In terms of protein metabolism, the crude protein content in males was 9% higher than in females before reproduction, increasing to 10.16% after reproduction. This significant increase aligns with the specific protein demands of spermatogenesis. By contrast, females accumulate substantial amounts of lipids in the gonad during oogenesis to meet reproductive demands.7 Thus, the high reliance on lipids for energy during the reproductive period in male M. yessoensis could lead to a sharp decline in energy reserves post reproduction, potentially significantly reducing their survival ability. Through their diversified energy reserve and allocation strategies, individual females are better positioned to maintain their survival and ensure subsequent reproductive activities.
Research on scallop gonad development stages is fundamental to understanding their reproductive patterns and holds significant importance in the field of bivalve reproductive biology. Currently, there is no unified standard for classifying gonad development stages in bivalves; instead, this relies on histological section observation combined with indicators such as follicle morphology, developmental stage of germ cells, and their abundance. For instance, through monthly monitoring of condition and gonad indices alongside histological analysis of the blue mussel (Mytilus edulis) and the hard-shelled mussel (Mytilus coruscus), Laaraj et al.8 reported that M. edulis has two main spawning seasons (spring and autumn) and one minor season (winter), whereas M. coruscus spawns year-round with a main spawning period from spring to autumn, showing a small number of sexually undifferentiated/quiescent individuals in July-August. In their study of the virgate rabbitfish (Siganus virgatus), Paraboles et al.9 showed that macroscopic examination could classify maturity stages into four phases (immature, developing, mature, and spent), while histological analysis allowed further subdivision into six stages (immature, developing, mature, spawning, spent, and redeveloping).
This study adopted the staging method proposed by Gao et al.,10 focusing on analyzing the characteristics of the mature and spawning stages in M. yessoensis gonadal development. During the mature stage, germ cells complete terminal differentiation (spermatids transform into spermatozoa, and secondary oocytes develop into ova). The spawning stage involves significant consumption of energy substances, such as crude lipids, crude protein, and glycogen, to support the release of large quantities of germ cells. Concurrently, the rupture of gonadal follicles causes tissue damage and leads to a decline in the physical condition of the scallop. This aligns closely with the observed sharp increase in mortality rates in cultured scallops after reproduction observed in aquaculture. It also provides histological and biochemical metabolic evidence supporting elucidation of the underlying mechanisms resulting in scallop mortality.
4.2. Comparative analysis of enzyme activities in Yesso scallops(Mizuhopecten yessoensis) before and after reproduction
A comparative analysis of enzyme activities in M. yessoensis before and after reproduction provides a deeper understanding of changes in physiological mechanisms, such as antioxidant defense, immunity, and energy metabolism, during its reproductive process. It also clarifies the impact of reproductive behavior on tissue function and provides a theoretical basis for elucidating the causes of high post-reproduction mortality and for formulating targeted aquaculture protection strategies.11 Philipp et al. studied the Queen scallop (Aequipecten opercularis) and found that, compared with external temperature stress, reproductive behavior itself had a more pronounced impact on metabolic rate and reactive oxygen species (ROS) levels during scallop reproduction, leading to mortality primarily caused by this stress rather than by pathogen infection.12 Consistent with the 50% decrease in SOD activity in the mantle after spawning observed in this study (P<0.01), the difference is that we found a 20% increase in SOD activity in the adductor muscle (P<0.05), suggesting a specific stress compensation in locomotor tissues. Research on the Pacific oyster (Crassostrea gigas) found that SOD activity significantly decreased in various tissues after the mass release of gametes, indicating substantial consumption of SOD proteins during reproduction.13 In terms of energy metabolism-related enzymes, our analysis of enzyme activities in gill, hepatopancreas, kidney, gonad, and mantle tissues revealed that the activity of LDH and ALT, and the ATP content, were all significantly reduced in these tissues post reproduction (P <0.05). These changes indicate that the antioxidant capacity of most tissues in M. yessoensis weakens during reproduction, leading to exacerbated tissue damage. During the reproductive period in M. yessoensis, mitochondria provide energy through oxidative phosphorylation, which involves enzymes, such as CAT, and generates ROS, including superoxide radicals. ROS has crucial roles in cellular redox signaling and apoptosis.14–16 Furthermore, as harmful substances, ROS can react with various cellular components, damaging cellular functions. Organisms primarily rely on their antioxidant enzyme system, including SOD, CAT, and T-AOC, to promptly scavenge excess ROS before they cause oxidative damage, thereby maintaining internal homeostasis. After completion of reproduction, the activities of SOD, CAT, and T-AOC in the mantle of both male and female scallops decreased. This aligns with the findings of Lian et al.17 The primary reason for this is that the mass release of gametes during reproduction leads to a significant increase in endogenous ROS, consequently reducing the activity of antioxidant enzymes in most tissues, with the mantle being particularly affected. However, SOD and T-AOC activity increased in the adductor muscle. It is hypothesized that this is an adaptive response; as a vital organ for movement and metabolism, enzyme activity is elevated in the adductor muscle to protect cells against the surge of free radicals generated in response to environmental stress during the early stages of reproduction. Moreover, their study reported that the energy supply of Chlamys farreri is dominated by glycogen, while our study reveals that the energy supply of Yesso scallops is dominated by lipids (with a 28.2% decrease in crude lipid content), reflecting interspecific differences. From an immunological perspective, the decreased lysozyme activity observed in the hepatopancreas, gonad, adductor muscle, and mantle after reproduction directly leads to a reduction in immune function. This significantly increases susceptibility to pathogen infection, which is likely a major factor contributing to post-reproduction scallop mortality. In terms of energy metabolism, the activities of LDH and ALT, as well as ATP content, all related to energy metabolism, decreased in tissues such as the mantle, gills, and gonads after reproduction. This indicates a decline in energy metabolic rate, accompanied by a corresponding reduction in tissue protein content. Similar findings have been reported in studies on other bivalves. For instance, Kraffe et al.18 studied the reproduction of the Atlantic deep-sea scallop (Placopecten magellanicus) and found that the rate of substance metabolism decreased significantly after reproductive behavior compared with before mass gamete release. Guerra et al.,19 investigated protein carbonyl levels in the catarina scallop (Argopecten ventricosus) post-reproduction, and indicated that spawning behavior intensifies oxidative damage in body tissues and reduces tissue metabolic rate after reproduction. In summary, this study found that: the ATP content in the adductor muscle decreased extremely significantly after reproduction (P<0.01), leading to the loss of locomotor ability and predator evasion capability; the SOD/CAT activity in the mantle decreased by more than 50% (P<0.01), weakening the antioxidant defense; the lysozyme activity in immune tissues (hepatopancreas/gonad) decreased by 35% (P<0.05), increasing susceptibility to pathogens. The above physiological collapse is highly consistent with the peak post-spawning mortality rate of 30-40% in aquaculture practices,10 and it is speculated to be a key contributing factor.
In summary, during reproduction, scallops strategically adjust their energy allocation, directing more energy toward the gonads to fuel gamete development and spawning. This regulatory mechanism results in lowered energy metabolism levels in other tissues, subsequently impairing normal scallop functions, such as reduced adduction strength and weakened immune capacity, ultimately leading to increased mortality.
4.3. Comparative metabolomic analysis of Yesso scallops before and after reproduction
A comparative metabolomic analysis of M. yessoensis before and after reproduction revealed metabolic changes associated with its reproductive process. This provides crucial insights for reducing economic losses in aquaculture and for understanding the mechanisms of reproductive regulation in scallops. Previous research suggests that, from an evolutionary perspective, the act of spawning and sperm release in aquatic animals is also a form of survival selection. However, once entering the reproductive cycle, batch spawning and sperm release in M. yessoensis cause drastic changes in body energy and metabolites, rendering reproduction itself a form of reproductive stress. Under this stress, after reproductive behavior, the endogenous antioxidant capacity of the scallop decreases, leading to ROS accumulation within cells, exacerbating cellular damage. Concurrently, the activity of enzymes related to energy metabolism decreases, slowing down the metabolic rate. Collectively, these changes result in a weakened physical constitution and reduced immune enzyme activity in scallops after the mass release of gametes, rendering them unable to cope with drastic changes in external environmental factors and predation pressure. This ultimately leads to high mortality rates post-reproduction.
This study used untargeted metabolomics to analyze the gonadal tissues of M. yessoensis before and after reproduction. The data revealed significant differences in the female gonads for metabolites such as DL-tyrosine, glycine, pyruvic acid, epinephrine, succinic acid, DL-malic acid, histidine, methylphosphonic acid, and prostaglandin A2. Significant differences were also observed in the male gonads for metabolites including 2-ketobutyric acid, DL-serine, DL-tryptophan, DL-tyrosine, glutathione (GSH), S-methyl-5’-thioadenosine, and L-lactic acid. These findings align with the dynamic metabolic adjustments observed during reproduction in C. gigas.20 It is hypothesized that these differential metabolites are associated with reproductive activities in scallops.
Succinic acid, a key intermediate metabolite in the TCA cycle, is widely involved in energy metabolism within animal cells, providing ATP support for various physiological activities.21 It is also a significantly enriched differential metabolite during the reproductive period. Its concentration changes are highly correlated with the gonad index and the maturity of germ cells. Thus, an efficient succinate-mediated energy supply might be one of the key biochemical foundations driving the final maturation of oocytes and successful release of gametes.22 Our metabolomic analysis of female M. yessoensis revealed significant upregulation of succinate dehydrogenase activity and dynamic changes in succinic acid levels in ovarian tissue after reproduction. This suggests that succinic acid is crucial for meeting the substantial energy demands of oocyte maturation and vitellogenin synthesis and accumulation, indicating immense energy consumption during reproduction. The initiation and completion of reproductive behavior in M. yessoensis does not rely on a single energy substrate. Instead, they involve the coordinated conversion of multiple substrates (sugars, lipids, and amino acids) through metabolic pathways, including the TCA cycle. Ultimately, this efficiently generates ATP via intermediates such as succinic acid. Vigorous physiological activities during the reproductive period stimulate the mobilization of energy reserves. As a hub in the TCA cycle, the metabolic flux increase in succinic acid is directly linked to the rate of ATP synthesis. This provides ample energy support for crucial stages in reproduction, such as gonad development, gamete maturation, and release, continuing until reproduction is complete.
GSH, a ubiquitous tripeptide and major antioxidant in animal cells, has a fundamental role in maintaining cellular redox homeostasis, detoxification, and protecting macromolecules from oxidative damage.23 Metabolomic analyses identified GSH as a key differential metabolite in the testicular tissue of male M. yessoensis during reproduction. Changes in its concentration and the GSH/GSSG (oxidized GSH) ratio are closely related to sperm quality parameters, such as motility and acrosome integrity. Furthermore, the GSH-mediated redox balance is likely one of the core mechanisms maintaining the stability of the spermatogenic microenvironment, protecting mature sperm from ROS attacks and ensuring their functional integrity. Concurrently, neurosignaling molecules, such as dopamine, might also be involved in regulating germ cell differentiation.24 In summary, succinate accumulated significantly in female gonads and was enriched in the TCA cycle, acting as an energy hub to supply energy for oocyte maturation, but was accompanied by a ROS burst (with SOD activity decreasing by 60%). In males, however, the glutathione synthesis pathway was upregulated, which protected sperm DNA by maintaining the GSH/GSSG ratio. It is hypothesized that this imbalance in metabolism-oxidative stress coupling may be the molecular basis for the difference in mortality rates. In this study, through metabolomic analysis of male Ezo scallops before and after reproduction, we found that glutathione content in spermathecal tissues showed a significant up-regulation after reproduction. It is hypothesized that, under the oxidative stress accompanying vigorous reproductive activities and sperm release during the reproductive period in male scallops, GSH synthesis and regeneration capacity are enhanced in testicular tissue. This effectively scavenges excess ROS, protecting sperm DNA, plasma membranes, and mitochondria from oxidative damage, thereby maintaining sperm motility, movement capacity, and fertilization potential until reproduction is complete.
5. Conclusion
Reproduction leads to significant consumption of nutrients in the gonads of Yesso scallops, with crude fat and protein being the main differentially consumed substrates between males and females. Changes in enzyme activities reflect gender-specific remodeling of energy metabolism (decrease in ATPase) and antioxidant systems (fluctuations in SOD and CAT) after reproduction. Metabolomics reveals that females and males adapt to reproductive needs through lipid/amino acid metabolism and sulfur-containing compound metabolism, respectively. This study provides basic data support for understanding the energy allocation mechanism during the reproductive process of Yesso scallops and lays a theoretical foundation for exploring the reproductive metabolism regulatory network of Yesso scallops at the molecular level.
Reproduction in Yesso scallops leads to sex-specific metabolic collapse: males are more susceptible to death due to lipid-dependent energy exhaustion. Practical strategies derived from this study include: feeding females with diets rich in branched-chain amino acids before reproduction to enhance the TCA cycle (based on metabolome-enriched pathways); supplementing males with glutathione precursors (N-acetylcysteine) to alleviate oxidative damage; and reducing stocking density while increasing oxygen supply within 72 hours after spawning in both sexes to ease the recovery pressure on tissues with ATP depletion. These findings provide a theoretically driven, precision management scheme for reducing post-reproductive mortality and promoting the sustainable development of scallop aquaculture.
ACKNOWLEDGMENTS
This study was supported by the Joint Research Project of Liaoning Provincial Science and Technology Program (2024JH2/102600076) and Shellfish System of Liaoning Modern Agricultural Industrial Technology System Liaoning Key Research and Development Program (2024JH1/11700010).
AUTHORS’ CONTRIBUTION
Animal cultivation and Methodology: Ping He, Xuetao Li, Wang Xubo; Sample & Data analysis: Ping He, Ying Tian, Junxia Mao; Experimental planning: Zhenlin Hao; Conceptualization: Zhenlin Hao; Article writing: Ping He; Writing – review & editing: Zhenlin Hao.
ETHICAL CONDUCT APPROVAL - IACUC
The article adheres to the Convention on Biological Diversity and the Convention on Trade in Endangered Species of Wild Fauna and Flora Research.
CONFLICT OF INTEREST - COPE
The authors declare that they have no conflict of interest.
INFORMED CONSENT STATEMENT
All authors and institutions have confirmed this manuscript for publication.
DATA AVAILABILITY STATEMENT
The data supporting the results of this study can be acquired from the corresponding author on reasonable request.

_before_ejaculation__(b)_after.jpeg)
.jpeg)


_female_and_(c_d)_male_*m._yessoensis*_b.jpeg)


_before_ejaculation__(b)_after.jpeg)
.jpeg)


_female_and_(c_d)_male_*m._yessoensis*_b.jpeg)

