1. Introduction
In aquaculture, the high-density intensive farming model is widely adopted due to its efficient use of water and land resources, along with its ability to significantly increase production yields.1 However, this farming model also poses numerous challenges, particularly the increasingly prominent stress factors faced by fish. Among these, issues such as spatial limitations, deterioration of water quality, and low-oxygen environments caused by high-density intensive farming are particularly crucial.2 Deterioration of water quality leads to the gradual decomposition of feed residues and animal excretions in the aquaculture water, which in turn results in the production and accumulation of ammonia nitrogen.3,4 Due to limited space and the input of large quantities of feed, the concentration of ammonia nitrogen in the aquaculture water under the high-density intensive farming model often remains high, posing a significant threat to the health of fish. The accumulation of ammonia nitrogen becomes an important environmental factor, inducing fish diseases.3,5
Ammonia nitrogen, as the end product of protein metabolism in fish, is widely present in aquaculture systems. Its toxic forms mainly include non-ionic ammonia (NH3) and ionic ammonia (NH4+), which transform into each other under specific environmental conditions (pH, temperature, and salinity) to maintain the dynamic balance of ammonia nitrogen in the water body.6 However, due to its high lipid solubility and non-ionic property, non-ionic ammonia can easily penetrate the biological cell membrane, especially the fish gill membrane, thereby exerting significant toxic effects on aquatic organisms.2,7
In the physiological structure of teleost fish, the gill, as the core organ for breathing and gas exchange, has a densely arranged filament supported by each gill arch, and the gill lamellae densely distributed on the gill filament are the key sites for efficient gas exchange.8 However, ammonia nitrogen exposure, as common environmental stress in aquaculture, can significantly affect the health status of gill tissue, leading to pathological changes such as swelling and hyperplasia of the gill filament, increased mucus secretion, and swelling of the respiratory epithelium of the gill lamellae, which directly weaken the breathing function of fish and thereby affecting their overall physiological functions.9–11 In addition, as an immune tissue directly exposed to the external environment in fish, the health status of the gill is directly related to the ability of fish to resist environmental stress. Studies have found that ammonia nitrogen stress not only profoundly affects the immune response of fish but also induces oxidative stress and disrupts the balance of the gill tissue and its defense system.12
T. fulvidraco, as an important freshwater aquaculture fish, has both ecological and economic values.13 However, poor water quality, particularly high levels of ammonia nitrogen poses a considerable threat to intensive yellow catfish farming.14 When the ammonia nitrogen concentration in the water environment exceeds 6.72 milligrams per liter, the growth rate of the yellow catfish will be significantly inhibited, and oxidative stress responses may also occur in its body.15 At present, the research on the impact of ammonia nitrogen on yellow catfish is relatively limited. In particular, the specific tolerance ability of yellow catfish to ammonia nitrogen and the physiological response mechanism of gills during the process of coping with ammonia nitrogen stress remain unclear. Therefore, systematically assessing the impact of ammonia nitrogen stress on the health of its gill tissue not only helps to reveal the specific manifestations of ammonia toxicity, but also provides urgently needed theoretical support for the development of targeted environmental protection measures and aquaculture management strategies.
2. Materials and methods
2.1. Experimental fish
A total of 150 healthy male T. fulvidraco were obtained from a fish farming facility in Chengdu, Sichuan Province, China. The fish had an average weight of 19.23 ± 3.65 g and an average length of 13.04 ± 0.79 cm. The fish were placed in a circular aquaculture tank (1.1 m in diameter and 2.0 m in depth) for a two-week acclimation period. During the acclimation period, dissolved oxygen was maintained above 6.5 mg/L, pH was 6.5-7.0, and the water temperature was 23-24°C. Commercial feed (Tongwei, China) was fed twice a day (at 9:00 am and 6:00 pm), with apparent satiation feeding; the tank was emptied and refilled with 1/3 of the water daily. All animal handling procedures were approved by the Animal Care and Use Committee of Sichuan Agricultural University (DKY2024102018).
2.2. Acute ammonia-nitrogen stress
The acute stress of ammonia nitrogen was conducted using a semi-static method. The experiment was designed with three replicates per group to ensure statistical reliability.Different concentrations of 0, 10, 20, 30, and 50 mg/L ammonia nitrogen were set for the acute stress experiment, with 30 fish randomly assigned to each concentration group (10 fish per replicate). To ensure precise measurement of ammonia nitrogen concentration in the water throughout the experiment duration, Nessler’s reagent spectrophotometry was regularly employed to detect and determine the actual levels of ammonia nitrogen. The entire experimental period lasted for 96 hours with continuous monitoring and adjustments made to maintain the desired levels of ammonia nitrogen concentration within each group. Throughout the experiment, daily observations and recordings were made on mortality rate, clinical symptoms, and gross pathological manifestations. The half-lethal concentration (LC50) of ammonia nitrogen stress at 96 hours was calculated using the Probit Analysis method while the safe concentration (1/10 LC50) was derived based on LC50 values. No feeding occurred during the acute ammonia nitrogen stress period. Actual concentrations of ammonia nitrogen stress were measured every 12 hours and adjusted using NH4Cl solution as necessary. Water replacement involving pre-aerated water took place every 24 hours to maintain dissolved oxygen levels above 6.5 mg/L, along with pH values between 6.5-7.0 and water temperature ranging from 23℃-24℃ under a light-dark cycle lasting 12 hours per day. The daily observation and recording included clinical symptoms and mortality rates among yellow catfish in each group.
2.3. Sample collection
Eighteen T. fulvidraco were randomly selected from each concentration group, and blood was collected from the tail vein after being anesthetized with MS222 (fish were placed in an aqueous solution containing 40 mg/L MS-222). The blood of every three fish was mixed into one sample and then centrifuged at a low speed of 3000-4000 r/min for 10 min to obtain serum, which was stored at 4℃ for the detection of blood physiological and biochemical indexes. The second gill on the right side was taken and fixed in neutral formalin for histopathological analysis.
2.4. Hematoxylin and Eosin Staining
Drawing upon previous studies, the methodology was established and is detailed below16–18: the gill tissue samples, which had been fixed for 48 hours, were trimmed and placed in plastic embedding frames. They were decalcified in a decalcifying solution (40% formaldehyde 40 mL, methanol 600 mL, formic acid 150 mL, concentrated hydrochloric acid 180 mL, NaCl 8.5 g, diluted to 1 L with distilled water) for 3 days, then removed and placed in neutral formalin for 24 hours. Subsequently, they were rinsed with flowing tap water for 24 hours, followed by conventional dehydration and paraffin embedding treatments. Next, the tissue sections were cut using a 5-micron-thick blade (from Leica, Germany) and stained with the classic hematoxylin and eosin (H&E) staining. Photos were taken under a Nikon Eclipse E200 microscope (from Japan).
2.5. Assessment of pathological changes
According to the histopathological scoring system proposed by Baums et al.,19 the severity of gill lesions in different groups of T. fulvidraco was scored. The severity of the lesions was evaluated using a 1 to 7-point scoring system: 1 point indicated no change; 3 points indicated mild lesions; 5 points indicated moderate lesions; 7 points indicated severe lesions. Intermediate scores were used to assess the transitional states between various pathological conditions. The specific scoring criteria are as follows: 1 points: normal tissue, with intact gill filaments and lamellae. 3 points: mild lesions, with local edema and occasional inflammatory cell infiltration. 5 points: moderate lesions, with increased edema, hyperplasia and necrosis in multiple areas, epithelial cell shedding, aggravated inflammatory cell infiltration, and fusion of gill lamellae. 7 points: severe lesions, with widespread distribution of lesions, obvious edema, inflammation, hyperplasia and necrosis symptoms, extensive epithelial cell shedding, and severe damage to gill lamellae.
2.6. Detection of physiological and biochemical indexes of serum
Six serum samples were collected, and the levels of sodium, calcium, chloride, magnesium, potassium, total protein, albumin, and blood ammonia in the serum were determined using sodium, calcium, chloride, magnesium, potassium, total protein, albumin, and blood ammonia assay kits, respectively, following the manufacturer’s instructions (Jiancheng Institute of Biological Engineering, Nanjing, China).
2.7. qRT-PCR analysis
Total RNA was extracted from the collected gill tissues using the Animal Total RNA Isolation Kit (Foregene, China). RNA quality (OD260/280 = 1.8-2.0) was verified by Nanodrop 2000 spectrophotometry (Bio-Rad, USA) and 1% agarose gel electrophoresis. cDNA synthesis was performed with RNA using the PrimeScript™ RT Reagent Kit (TaKaRa, Japan). Species-specific primers (Table 1), including β-actin as the reference gene, were designed via NCBI Primer-BLAST. qPCR reactions (Bio-Rad CFX96) utilized SYBR® Premix Ex Taq™ II (TaKaRa) under optimized cycling conditions: 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 59°C for 30 sec. Amplification specificity was confirmed by melt curve analysis (65-95°C), and relative gene expression was calculated via the 2−ΔΔCt method.
2.8. Statistical analyses
All data were presented as the mean ± SD (standard deviation). Statistical differences were assessed using SPSS 27.0 software (IBM Corp., Chicago, USA). Graphs were created using GraphPad Prism (USA) and Adobe Illustrator (USA) software. After the normality test, one-way ANOVA analysis was used to evaluate significant differences (*, **, and *** represent p < 0.05, 0.01and 0.001, respectively).
3. Results
3.1. Detection of ammonia nitrogen concentration in aquaculture water
By diluting the ammonia nitrogen standard solution, ammonia nitrogen solutions with different known concentrations were prepared, and their absorbance was measured to draw the standard curve for ammonia nitrogen detection. As shown in Fig. 1A, there is a good linear relationship between the ammonia nitrogen concentration and its corresponding absorbance. The linear equation is y = 6.5487x – 0.1383, R2 = 0.9987. Based on this linear equation, the detection results of the actual ammonia nitrogen concentration (ACT) in the aquaculture water was extremely close to the nominal concentration (Fig. 1B). The x-axis of Figure 1B represents the measured actual concentration (ACT), while the y-axis shows the nominal concentration. The close agreement between the two values (R² = 0.9987) demonstrates the accuracy of our experimental setup.
3.2. Semi-lethal concentration of ammonia nitrogen to T. fulvidraco
The acute toxicity test results of ammonia nitrogen on T. fulvidraco are shown in Fig. 2. Under the experimental conditions, after 96 hours of acute ammonia nitrogen stress at different concentrations, the mortality rate of T. fulvidraco showed a concentration-dependent increase, that is, the higher the concentration, the higher the mortality rate. At a concentration of 50 mg/L, complete mortality of T. fulvidraco was observed within 24 hours, and as the stress time increased, the mortality rate also gradually increased. Finally, through calculation, it was determined that the 96h LC50 of ammonia nitrogen on T. fulvidraco was 28.12 mg/L.
3.3. Clinical symptoms of T. fulvidraco under acute ammonia nitrogen stress.
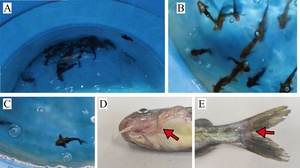
T. fulvidraco in the breeding bucket without ammonia nitrogen stress would gather at the bottom of the bucket to live. They would react quickly after being frightened, fleeing at the bottom of the water body (Fig. 3A). However, when subjected to acute ammonia nitrogen stress at different concentrations, the T. fulvidraco showed obvious poisoning symptoms, initially all scattered and floating on the surface of the water, slow in swimming, and sluggish in response after being frightened. As the duration of ammonia nitrogen stress increased, the T. fulvidraco gradually lost balance in the water and eventually died (Fig. 3B, C). The newly dead T. fulvidraco had many bleeding spots on the lower jaw (Fig. 3D), and there were symptoms such as congestion and ulceration at the base of the fin rays (Fig. 3E).
3.4. Histopathological observation
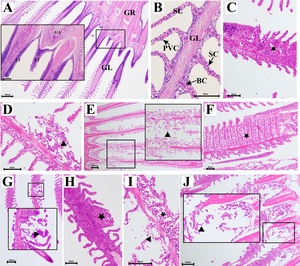
To investigate the effects of high-concentration ammonia nitrogen stress on the gills of T. fulvidraco, histopathological observations were conducted. The gill structure of the control group (0 mg/L) of T. fulvidraco was intact, consisting of three parts: the gill rakes, gill arches, and gill filaments. The gill filaments were supported by the central cartilage and attached to the gill arches, and the gill lamellae emerged from both sides of the central cartilage (Fig. 4A), the gill lamellae were supported by pillar cells, and a monolayer of pavement cell was wrapped outside, with gill blood sinuses formed between the pillar cells (Fig. 4B); at the base of the gill filaments, there were also more blue-stained gill-associated lymphoid tissues (Fig. 4A). However, under different concentrations of ammonia nitrogen stress, obvious gill damage was observed, and it intensified with the increase of concentration. At 10 mg/L, the gill filaments began to lose structural integrity, showing gill lamellae fractures, necrosis, and shedding of epithelial cells, accompanied by inflammatory cell infiltration (Fig. 4C, D). With the increase in concentration (20 and 30 mg/L), these pathological changes became more severe, manifested as severe gill lamellae necrosis, extensive epithelial cell shedding, and obvious cell proliferation of gill lamellae basal cells, resulting in the fusion and edema of gill lamellae (Fig. 4E-H). At a dose of 50 mg/L, the gill filament structure was completely disordered, gill lamellae necrosis and shedding or even disappearance, accompanied by extravasation of blood cells and extensive necrosis of gill filament basal tissue cells (Fig. 4I-J).
3.5. Ammonia nitrogen stress affects osmotic pressure regulation and excretion function
Under acute ammonia nitrogen stress, the gills of T. fulvidraco, a key organ for ammonia excretion, may be damaged, affecting the ammonia excretion function. Results show that with the increase in ammonia nitrogen concentration, the blood ammonia in T. fulvidraco accumulated significantly, especially under high concentrations of 50 mg/L (Fig. 5H). At the same time, there was a significant decrease in the content of Na+ in the serum to about half of the control group (Fig. 5A), while the content of K+ generally decreases but anomalously increases at extremely high concentrations (50 mg/L) (Fig. 5C). Other ions such as Cl-, Ca2+ and Mg2+ were stable (Fig. 5B, D, E). In addition, acute ammonia nitrogen stress has no significant effect on the content of total serum protein and serum albumin (Fig. 5F, G).
3.6. Exploration of the impact of ammonia nitrogen concentration on quantitative statistics of histopathological changes and blood biochemical indicators
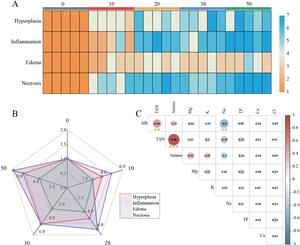
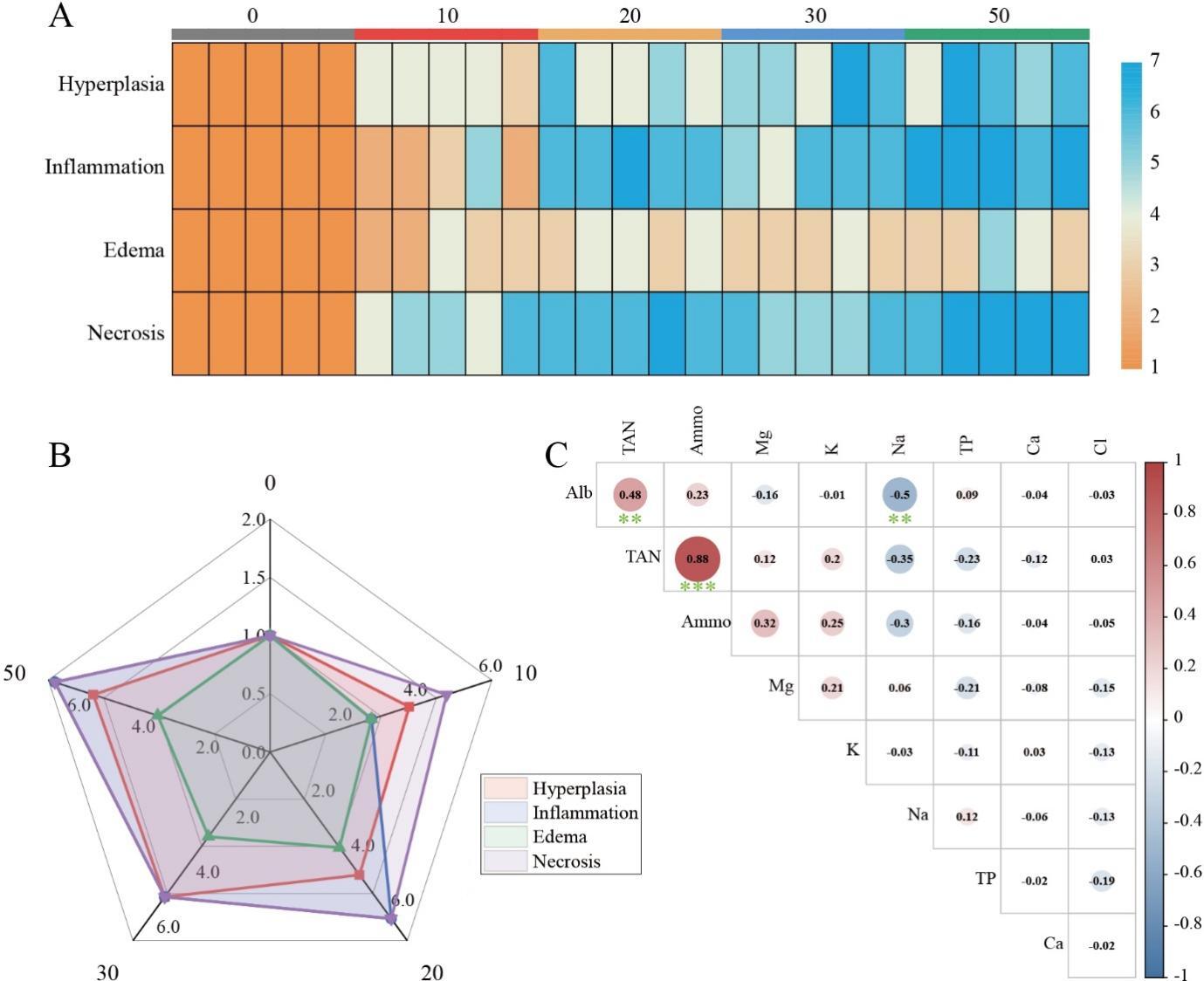
The primary pathological alterations witnessed in the tissue specimens, namely necrosis, proliferation, inflammation, and edema, were quantified and appraised utilizing a grading system. The analytical outcomes demonstrated that the scores for pathological changes in the ammonia nitrogen stress group (10-50 mg/L) were conspicuously elevated in comparison to the control group (0 mg/L). Moreover, with the escalation of ammonia nitrogen concentration, these four pathological alterations all manifested diverse levels of aggravation (Fig. 6A), particularly necrosis being the most prominent (Fig. 6B). The findings of the correlation between ammonia nitrogen concentration and osmoregulatory ions and proteins indicate that ammonia nitrogen is significantly positively correlated with blood ammonia and also possesses a strong positive correlation with albumin (Fig. 6C).
3.7. Ammonia nitrogen exposure upregulates immune-related genes and disrupts tight junction protein expression
The experimental groups exposed to varying ammonia nitrogen concentrations (0, 10, 20, 30, and 50 mg/L) exhibited distinct patterns in immune and barrier-related gene expressions in yellow catfish T. fulvidraco. Notably, the 50 mg/L group demonstrated a significant upregulation of NF-κB expression compared to the 0 and 10 mg/L groups (Fig. 7A). Similarly, TNF-α mRNA levels in the 50 mg/L group were markedly elevated relative to the 0, 10, and 20 mg/L groups (Fig. 7B). Analysis of tight junction protein genes revealed that ZO-2 expression was significantly higher in the 10, 20, and 50 mg/L groups than in the control (0 mg/L) (Fig. 7C). Furthermore, Occludin expression showed a pronounced increase in the 50 mg/L group compared to the control, while no significant differences were observed among other concentration groups (Fig. 7D).
4. Discussion
In the intensive aquaculture system, the high stocking density and feeding rate significantly increase the accumulation of ammonia nitrogen in the water body, becoming one of the key water quality pollutants affecting the health and growth of fish. In the water environment, ammonia nitrogen exists in two forms: ionic ammonia and non-ionic ammonia.6 Among them, non-ionic ammonia is highly toxic, and the vast majority of fish are extremely sensitive to non-ionic ammonia (NH3). A large accumulation of high-concentration NH3 in the fish body will cause its death.21 Research has shown that when the ammonia level reaches 10.00 mg/L TAN (0.208 mg/L NH3), this value is much higher than the ammonia tolerance limit of many fish, such as Lota lota (0.03 mg/L NH3) and Lateolabrax japonicus (0.006 mg/L NH3).22 In addition, a recent study found that the ammonia nitrogen content of 96h LC50 of juvenile T. fulvidraco was 250 mg/L.23 However, the 96h LC50 of total ammonia nitrogen obtained under the conditions of this study is 28.12 mg/L, demonstrating variation in 96h LC50 values reported across different studies because the results obtained from acute toxicity experiments are closely related to the size, age, temperature, pH value, and water source of the experimental subjects.14
The gills are the main organs for fish to breathe, osmoregulate, filter food, and excrete ammonia nitrogen.24 The damage to the gill structure usually has an impact on the normal physiological activities of the fish gills, such as breathing, ion balance, and the excretion of nitrogen-containing waste.25 Non-ionic ammonia has the characteristics of lipid solubility and non-ionicity and can diffuse through the gill membrane, thereby causing damage to the gill tissue.6 Goldfish (Carassius auratus Linnaeus) will float on the water surface to breathe, run around randomly, swim slowly, and weaken, and the mortality rate will increase significantly under acute ammonia nitrogen stress.26 After being exposed to acute ammonia nitrogen stress, the expression of genes related to oxidative stress in the gills of Nile tilapia was up-regulated, the gill filaments and gill lamellae deformed, and the blood vessels in the gill lamellae dilated and congested.27 When the ammonia nitrogen level rises, the epithelial cells of the gill lamellae of T. fulvidraco proliferate, and the epithelial cells separate from the capillaries, forming a large gap.17 In this experiment, after being exposed to acute ammonia nitrogen stress, the gill lamellae of yellow catfish resulted in edema and the epithelial cells were floating; the obvious proliferation of the cells at the base of the gill lamellae cause the adjacent gill lamellae to adhere to each other; there were severe necrosis in the gill lamellae, and some of the gill lamellae were completely necrotic, shedding from the gill filaments, accompanied by the infiltration of inflammatory cells, and these pathological damages are positively correlated with the concentration of ammonia nitrogen stress. In addition, this study also found that the ammonia level in the blood of yellow catfish shows a significant positive correlation with the ammonia nitrogen concentration in the aquaculture water. When a large number of basal cells in the gill lamella of T. fulvidraco proliferate, the proliferating cells cause adhesion between the gill lamellae, thereby significantly increasing the path length of ammonia molecules diffusing from the body to the external environment through the gills. This structural change directly hinders the normal excretion of ammonia, causing ammonia to accumulate in the fish’s body. Therefore, we speculate that this change in the structure of the gill tissue may be the cause of the increase in ammonia concentration in the blood of T. fulvidraco.
Additionally, this study found that gill tissue damage is closely linked to the inflammatory response mediated by NF-κB. As a key transcription factor, NF-κB regulates the expression of multiple inflammatory factors and plays a critical role in both immune and inflammatory responses.28 When T. fulvidraco was exposed to acute ammonia-nitrogen stress, the NF-κB in gill tissue significantly activated, leading to the upregulation of inflammatory factors. This overexpression further exacerbates gill tissue damage. This molecular response significantly promotes physiological dysfunction in gill tissues by activating tumor necrosis factor-alpha (TNF-α) to trigger an inflammatory cascade. The inflammatory process disrupts the normal structural and functional integrity of gill tissues, resulting in a marked reduction in their key physiological functions, including gas exchange, ion regulation, and ammonia excretion. Furthermore, we observed that, concurrent with the pathological changes in the gill lamellae, the expression levels of occludin and tight junction protein ZO-2, which are associated with tight junctions, also changed significantly. These alterations may disrupt the tight junction structure, increasing permeability between the gill lamellae and thereby exacerbating the leakage of ammonia molecules. Therefore, we propose that the NF-κB mediated inflammatory response plays a significant role in gill tissue damage induced by ammonia-nitrogen stress.
Fish can effectively regulate and transfer ions through their gills. This process mainly relies on the precise control of the crystal osmotic pressure of the organism to achieve osmotic balance with the external environment.29 Studies have found that in freshwater fish, mainly Na+, Cl-, K+, Mg2+, Ca2+, and other ions constitute the osmotic pressure in their bodies, while marine fish also include urea and trimethylamine oxide in addition to these ions, among which the content of Na+ and Cl- is the highest.30 Wood et al.9 found that exposing oscar (Astronotus ocellatus) to acute hypoxic stress, the Na+ efflux rate in the gills rapidly decreased to 55%, and the ammonia excretion, net loss rate of K+, and water efflux rate also decreased by 55-75%, which may be due to the rapid closure of transcellular channels during acute hypoxia. The concentration of Na+ and Cl- in spiny dogfish (Squalus acanthias) decreased significantly under acute lead stress.10 This indicates that the ion concentration in fish can be affected by a variety of external environments. In this study, the content of serum ions in T. fulvidraco under acute ammonia nitrogen stress was detected, and it was found that after being exposed to acute ammonia nitrogen stress, it mainly affected the osmotic pressure of T. fulvidraco, manifested in a significant decrease in Na+ content, and the K+ content in the 50 mg/L ammonia nitrogen stress group increased significantly compared to other groups. This may be due to the obvious necrosis of the gill tissue of T. fulvidraco caused by ammonia nitrogen stress, destroying the normal histological structure of the gills, leading to the massive loss of ions in the body, thereby affecting the osmotic pressure in its body and causing the death of T. fulvidraco.
While this study sheds light on the impacts of acute ammonia nitrogen stress on yellow catfish, its laboratory setting may not fully mirror real aquaculture conditions, and the focus on short-term effects overlooks potential long-term consequences. Future research should validate findings in the field, monitor chronic ammonia nitrogen impacts for sustainable aquaculture, delve deeper into molecular toxicity mechanisms like NF-κB interactions, and explore sustainable solutions such as probiotics or bioremediation to reduce ammonia nitrogen levels.
5. Conclusion
In this study, we conducted an acute ammonia nitrogen stress experiment on T. fulvidraco, and determined that its 96hLC50 is 28.12 mg/L. Observations showed that T. fulvidraco exhibited poisoning symptoms under ammonia stress, such as rapid breathing and sluggish movement, accompanied by severe pathological damage to the gill tissue, including edema, hyperplasia, and necrotizing gill inflammation, which is associated with NF-κB mediated inflammatory responses and altered expression of tight junction proteins (occludin and ZO-2), leading to increased gill permeability and ammonia leakage. These injuries were not only significantly positively correlated with ammonia nitrogen concentration, but also caused a significant increase in blood ammonia levels, further indicating the direct effect of ammonia nitrogen concentration on the physiological state of T. fulvidraco. In addition, ammonia nitrogen stress also caused imbalance disorders, especially the loss of Na+ and K+, which affects the osmoregulation of T. fulvidraco. Overall, this study determined the LC50 and elucidated the impacts of ammonia nitrogen stress on gill function, blood ammonia levels, and osmoregulation in T. fulvidraco. However, further investigation is necessary to elucidate the specific damage mechanism.
Acknowledgments
This research was funded by the Opening Fund of Key Laboratory of Sichuan Province for Fishes Conservation and Utilization in the Upper Reaches of the Yangtze River (No. NJTCSC09)
Authors’ Contributions
Hongli Liu, Jun Wang contributed the work equally. Hongli Liu, Liang Zhong: Formal analysis, Investigation, Visualization, Writing - original draft. Jun Wang: Formal analysis, Investigation. Sha Liu: Data curation, Writing - reviewing & editing. Jun Wang, Xiaoli Huang: Supervision, Conceptualization, Writing - reviewing & editing, Project administration. All authors read and approved the final manuscript.
Competing of Interest – COPE
The authors declare that they have no competing interests.
Ethical Conduct Approval – IACUC
The animal research protocol was approved by the Animal Care Advisory Committee of Sichuan Agricultural University (Chengdu, Sichuan, China), No. DKY2024102018.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.