1. Introduction
Eriocheir sinensis is indigenous to the river systems of eastern China, with its primary natural habitats situated within the Liaohe, Yangtze, Yellow River, and Oujiang watersheds. Its geographical distribution spans from the Liao River in the north to the Min River in the south and extends from the Yalu River in the east to Changsha in Hunan Province. Owing to its high nutritional value and distinctive flavor, this species is widely consumed and appreciated. Consequently, the scale of its aquaculture has been expanding annually, establishing it as an economically significant cultured crab species in China. E. sinensis constitutes a commercially important aquaculture species with the capability to thrive in saline-alkaline waters, thereby enhancing its aquaculture potential and geographic adaptability.1 Cultivating E. sinensis in these environments not only promotes the efficient utilization of aquatic resources but also improves production efficiency through scientific management and technological advancements.
Variations in water salinity have significant physiological effects on crustaceans. Salinity fluctuations trigger adaptive changes in Na⁺/K⁺-ATPase activity and the abundance of mitochondria-rich cells in the gill tissues of E. sinensis, mechanisms essential for maintaining osmotic balance under different salinity conditions.2 Additionally, changes in salinity affect crustacean immune responses and antioxidant capacity. Importantly, salinity variations significantly regulate the expression of the glutamate dehydrogenase (GDH) gene in E. sinensis, which is associated with amino acid metabolism under hyperosmotic stress, highlighting GDH’s crucial role in osmoregulation.3 Simultaneously, variations in salinity lead to the reorganization of intestinal microbial communities, thereby influencing the health status of crustaceans. Changes in salinity similarly affect oxidative stress and detoxification systems, as evidenced by modifications in antioxidant enzyme activities and the expression of detoxification-related enzymes in E. sinensis, which may represent adaptive responses to environmental stressors. Additionally, fluctuations in salinity impact growth and metabolic processes, as crustaceans may need to expend more energy on osmoregulation under hyposaline conditions.4 Salinity-induced changes in fatty acid composition and protein expression also affect the biosynthesis of flavor compounds.5 Overall, variations in water salinity significantly influence the nutrient and flavor compound profiles in E. sinensis and related crustaceans, affecting both physiological performance and commercial value through changes in growth dynamics and organoleptic quality.
In aquaculture, stocking density is a critical factor affecting productivity due to its impact on growth, survival, and water quality.6 Overcrowding leads to chronic stress resulting from degraded water conditions, such as elevated ammonia levels and low oxygen, as well as social conflicts, which compromise immunity and increase disease risks. Most E. sinensis perish shortly after mating, spawning, and hatching in natural conditions,7 underscoring the necessity for balanced density management that integrates water quality monitoring and behavioral assessments to optimize both economic returns and biological welfare in farming systems. The incorporation of natural prey markedly enhances the nutritional quality of crustacean aquaculture. Research indicates that diets based on zooplankton and algae supply essential long-chain polyunsaturated fatty acids (LC-PUFAs), which are vital for the development of aquatic organisms. Natural planktonic feeds surpass artificial formulations in promoting growth, a result attributable to their superior nutritional profiles, including higher levels of protein and taurine.8 Certain algal species not only provide essential nutrients but also enhance crustacean health by regulating lipid metabolism.9 In studies involving Atlantic salmon, algal supplementation significantly increased tissue levels of omega-3 LC-PUFAs, thereby improving both fish health and product quality.10
The Yellow River Delta is significantly affected by channel migrations of the Yellow River and seawater intrusion, resulting in severe soil salinization. The vegetation is predominantly composed of reeds. Notable success has been achieved in the development of fisheries on saline-alkali lands in the Yellow River Delta region. Through the exploration and practice of various development models, the utilization efficiency of these lands has been effectively improved.11 The burgeoning aquaculture industry for E. sinensis in the Yellow River Delta benefits from regionally favorable ecological conditions, such as abundant water resources and optimal climatic parameters, which support growth and reproduction. Significant advancements in cultivation scale, yield, and husbandry models within this region have greatly contributed to local economic development, while also offering valuable insights for national aquaculture practices.12
This study compares the quality of edible tissues of E. sinensis under two aquaculture models in the Yellow River Delta, thereby establishing a foundation for optimizing farming conditions and enhancing crab quality.
2. Materials and Methods
2.1. Experimental Design and Culture Management
The experiment was conducted in Kenli District, Dongying City, Shandong Province. In this study, two aquaculture models were selected: the Extensive Culture Model (ECM) and the Intensive Culture Model (ICM). The ECM experimental pond was developed by converting a 2-hectare reed field. The conversion process entailed several steps: (1) the removal of overgrown reeds; (2) the excavation of a water retention trench measuring 1.5 meters in width and 1.2 meters in depth along the perimeter of the ECM pond, designed to replicate commercial Chinese mitten crab culture ponds. Elodea nuttallii was planted at the bottom of the trench to foster a suitable growth and habitat environment for the crabs; (3) the installation of a 0.5-meter-high escape-proof barrier around the outer edge of the experimental pond. The ICM pond, situated 5 km from the ECM site, serves as a standardized commercial culture pond for E. sinensis, managed by local aquaculture enterprises. Both experimental ponds covered an area of 2 hectares each.
Water quality parameters in both the ECM and ICM ponds were monitored monthly on the 15th of each month from May to October 2022. The pH and salinity levels were assessed using a handheld multiparameter water quality analyzer (YSI ProPlus), while alkalinity and hardness were measured using reagent kits. The water quality parameters for both ECM and ICM remained relatively stable, and the results are detailed in Table 1.
To ensure genetic uniformity of crab juveniles between the two culture systems, the ECM pond was stocked with E. sinensis juveniles sourced from the same aquaculture company that manages the ICM pond, with females averaging 13.85 ± 2.18 g/individual and males averaging 15.51 ± 1.52 g/individual (n=20). Stocking densities were maintained at 0.75 individuals/m² for the ECM pond and 3 individuals/m² for the ICM pond. Both ponds were provided with identical complete formulated feed for E. sinensis (36% crude protein, 3% crude fat, 12% crude fiber, 18% crude ash, 0.6% total phosphorus, 1.8% Lys, 12% moisture), produced by the same manufacturer.
Between April and October 2022, manual broadcast feeding was conducted twice daily at 08:00 and 16:00. Between August and September, frozen trash fish were incorporated into the formulated feed to enhance fattening, with feeding ceasing upon the attainment of market size in October. The final harvest specifications for adult crabs under both culture models are detailed in Table 2.
2.2. Sample Collection
Sampling occurred in November 2022, during which crabs were randomly harvested from both experimental ponds using benthic traps. The captured crabs were secured and transported to the laboratory in foam insulation boxes containing ice packs. In the laboratory, low-temperature anesthesia was administered. The crabs were carefully restrained by securing all appendages to ensure stability. Measurements of carapace length and width were obtained using a vernier caliper. Each crab was then weighed on an electronic balance with a capacity of 500 g and an accuracy of 0.01 g, and the stabilized weight was recorded as the body weight. Dissections were conducted in an ice bath, where the hepatopancreatic and gonadal tissues were meticulously isolated using forceps and precisely weighed with an electronic balance to calculate the hepatosomatic index (HSI) and gonadosomatic index (GSI). Muscle tissues were subsequently separated using dissecting scissors and forceps to determine muscle yield (MY). The total edible yield (TEY) was calculated using the formula: TEY = HSI + GSI + MY. The condition factor (CF) was derived from measurements of body length and weight.
The condition factor (CF), hepatosomatic index (HSI, %), gonadosomatic index (GSI,%), meat yield (MY, %), and total edible yield (TEY, %) of the crab was calculated using formulas 1–5:
CF(g/cm3)=W/L3
HSI(%)=WH/W×100
GSI(%)=WG/W×100
MY(%)=WM/W×100
TEY(%)=GSI+HSI+MY
where W is the body weight (g), L is the carapace length (cm), WH is the hepatopancreas weight (g), WG is the gonadal weight (g), and WM is the muscle weight (g).
A total of 30 crabs (comprising 15 females and 15 males) were collected from the ECM pond, and an equivalent number from the ICM pond, for dissection aimed at calculating the previously mentioned indices. Subsequently, specimens that conformed to specific size criteria (females: 100 ± 10 g/individual, males: 150 ± 10 g/individual; 6 females and 6 males from each pond) were selected for nutritional quality assessment. The hepatopancreas, gonad, and muscle tissues from each crab were meticulously labeled, cryopreserved, and subjected to freeze-drying to determine moisture content. Post-lyophilization, the samples were ground into a homogeneous powder for subsequent nutrient composition analysis.
This study also involved the collection of stable isotope samples from potential food sources for crabs in both experimental ponds. These sources included artificially supplied inputs (formulated feed, corn, and frozen trash fish), dominant aquatic plants (Elodea nuttallii and reeds), and surface sediment organic matter (SOM). The primary collection methods were as follows: dominant aquatic plants were manually gathered from the pond edges, while formulated feed, corn, and frozen trash fish were sourced from the pond storage facility. Surface sediments (0–5 cm) and benthic organisms were collected using a Petersen grab with an opening area of 1/32 m² and were stored in sealed bags. In the laboratory, muscle tissue from crabs, formulated feed, corn, frozen trash fish, and filter membrane samples were oven-dried at 60 °C until a constant weight was achieved. Sediment and terrestrial plant samples were air-dried at room temperature. Sediment samples were then sieved through a 100-mesh sieve to eliminate large particulate matter such as sand, gravel, and root debris. All samples were subsequently ground using a grinding machine in preparation for instrumental analysis.
2.3. Proximate composition analysis
The moisture content of the edible tissues in E. sinensis was quantified by applying the freeze-drying method. The crude protein content was assessed utilizing the Kjeldahl method, while the crude fat content was extracted via the Soxhlet extraction technique. Furthermore, the crude ash content was determined by incineration at 550°C.
2.4. Fatty Acid Analysis
Freeze-dried samples (0.1 g) were homogenized using a chloroform/methanol mixture (2:1, v/v) for the purpose of total lipid extraction. Following the extraction process, 1 mL of a 0.9% NaCl solution was introduced, and the mixture was subjected to vortex mixing. Upon phase separation, the lower organic layer was isolated and evaporated under a nitrogen stream. The resulting lipid residue underwent methyl esterification through the addition of 1 mL of a 5% sulfuric acid-methanol solution (v/v) and incubation at 100°C for 1 hour. Fatty acid methyl esters (FAMEs) were subsequently analyzed utilizing a gas chromatography-mass spectrometry system (Agilent 7890A-5975C) equipped with an HP-88 capillary column (100 m × 0.25 mm ID, 0.2 μm film thickness; Agilent 112-88A7). High-purity helium was employed as the carrier gas at a column flow rate of 1.5 mL/min. The injector and flame ionization detector (FID) temperatures were maintained at 250°C and 280°C, respectively. The oven temperature program was as follows: an initial temperature of 100°C held for 5 minutes, followed by an increase to 180°C at a rate of 10°C/min with a 10-minute hold, then an increase to 200°C at a rate of 1°C/min with a 10-minute hold, and finally an increase to 230°C at a rate of 10°C/min with a 10-minute hold.13
2.5. Amino Acid Analysis
Freeze-dried samples (0.05 g) were accurately weighed and transferred into 25 mL hydrolysis tubes, to which 10 mL of a 6 mol/L hydrochloric acid solution was subsequently added. The tubes were purged with nitrogen gas for 15 minutes before being sealed. Hydrolysis was conducted at 110°C for 24 hours in an electrically heated air-blowing incubator. Following hydrolysis, the samples were cooled to room temperature, and the resulting hydrolysate was transferred to a 50 mL volumetric flask. The volume was adjusted with distilled water, ensuring thorough rinsing of the hydrolysis tube with distilled water three times during the transfer process. A 1 mL aliquot of the solution was then transferred to a chromatography vial, dried under a nitrogen stream, and reconstituted in 1 mL of distilled water. Prior to analysis, the solution was filtered through a 0.22 μm aqueous-phase membrane filter. Analysis was performed using an A300 amino acid analyzer (MembraPure GmbH, Germany).14
2.6. Free Amino Acid Analysis and Taste Activity Value:
For the analysis, freeze-dried samples (0.3 g) were homogenized with three volumes of distilled water. A 0.3 g portion of the homogenate was combined with 0.15 mL of a 25% sulfosalicylic acid solution and then refrigerated at 4°C for 60 minutes. The mixture was centrifuged at 15,000 g for 15 minutes. Subsequently, 200 μL of the supernatant was diluted with 800 μL of distilled water. The solution was filtered through a 0.22 μm aqueous-phase membrane filter using a syringe, transferred to a brown headspace vial, and analyzed using an A300 amino acid analyzer (membra Pure GmbH, Germany).15
Analysis of Free Amino Acids and Calculation of Taste Activity Value: The Taste Activity Value (TAV) is determined using the formula: TAV = C/T, where C denotes the concentration of the flavor substance in the sample, and T denotes the threshold concentration of that flavor substance.
2.7. Stable Isotope Analysis
Carbon and nitrogen stable isotopes were analyzed using a Carlo Erba EA1110 elemental analyzer coupled with a Delta Plus Finnigan isotope ratio mass spectrometer. The results were expressed in δ notation, with the stable isotope ratio calculated using the following formula:
δX (‰) = [(R_{Sample} / R_{standard}) - 1] × 1000 \tag{6}
where X represents either ¹³C or ¹⁵N, RSample is the isotope ratio of the sample, and Rstandard is the isotope ratio of the standard reference material (δ¹³C/¹²C for carbon and δ¹⁵N/¹⁴N for nitrogen). The δ value reflects the ratio of heavy to light isotopes in the sample. The standard reference materials for carbon and nitrogen stable isotopes were Vienna Pee Dee Belemnite (VPDB) and atmospheric N₂, respectively. Three standard reference materials were inserted for every 15 samples to ensure accuracy, with the analytical precision of isotope measurements being approximately ± 0.3‰.16
2.8. Statistical Analysis
All data were expressed as mean ± standard deviation (Mean ± SD) and analyzed using SPSS 26.0 software. Homogeneity of variances was assessed using Levene’s test. For data that did not meet the assumption of homogeneity, percentage values were transformed using arcsine or square root transformations. Differences in measured indices between the two models of river crabs were evaluated using an independent samples t-test, with statistical significance defined as p<0.05 and high statistical significance defined as p<0.01. To estimate the proportional contributions of various food sources to the diet of E. sinensis, a Bayesian mixing model was applied using the “simmr” package within the JAGS environment in R version 4.1.0.
3. Results
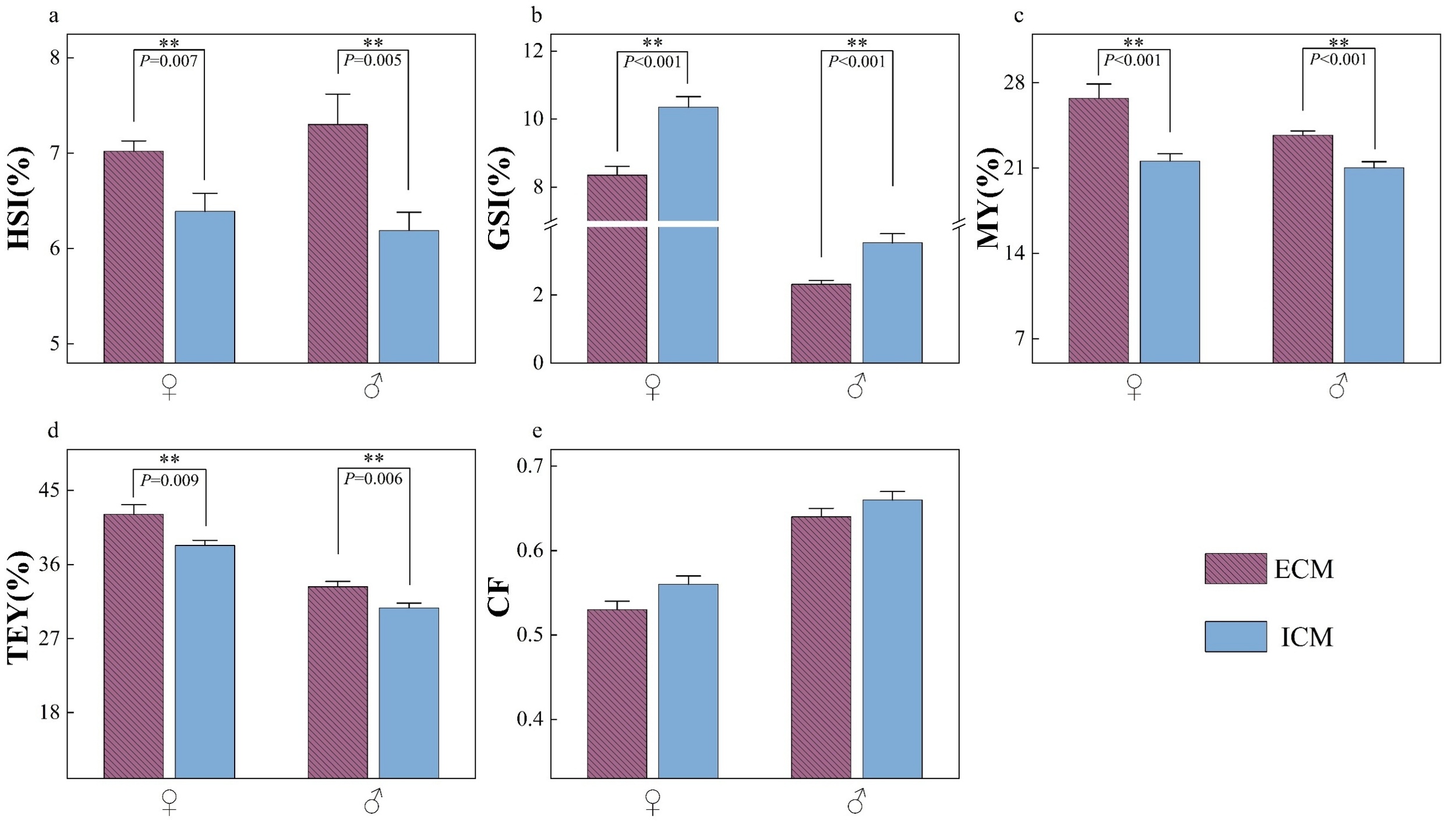
3.1. Tissue Indices、final growth characteristics and survival rate
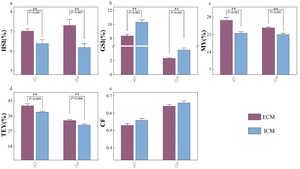
Figure 1 illustrates the tissue indices of E. sinensis subjected to two different models. Independent of gender, the hepatosomatic index (HSI), muscle yield (MY), and total edible yield (TEY) were significantly elevated in crabs from the ECM compared to those from the ICM (p<0.05). Conversely, the gonadosomatic index (GSI) was significantly lower in ECM crabs relative to ICM crabs (p<0.05). No significant difference was observed in the condition factor (CF) between the two farming models (p>0.05).
The survival rate of E. sinensis was 62.8% in the ECM pond and 53.3% in the ICM pond. The average marketable size of crabs under the two culture models at harvest is presented in Table 2.
With the exception of the carapace width of female crabs, no significant differences were observed in other size parameters (p > 0.05).
3.2. Proximate Composition
Figure 2 presents the proximate composition of the edible parts of E. sinensis under the two farming models. With respect to moisture content, the hepatopancreas moisture content in female ECM crabs was significantly reduced compared to female ICM crabs (p<0.05), whereas the gonad moisture content was significantly increased in ECM crabs compared to ICM crabs (p<0.05). The muscle moisture content in male ICM crabs was significantly higher than that in male ECM crabs (p<0.05). In terms of crude protein content, the hepatopancreas crude protein content was significantly greater in ECM crabs than in ICM crabs (p<0.05), while the gonad crude protein content in female ECM crabs was significantly lower than that in female ICM crabs (p<0.05). The crude protein content in the muscle tissue of male ECM crabs was significantly lower than that of male ICM crabs (p<0.05). Furthermore, the crude fat and crude ash content in the muscle tissue of male ECM crabs were also significantly lower compared to their ICM counterparts (p<0.05).
3.3. Fatty Acid Composition
Figure 3 illustrates the comparative fatty acid composition and nutritional evaluation indices in the edible tissues of E. sinensis reared under two different aquaculture systems.
Crabs from the ECM group exhibited a significantly higher ΣMUFA content in the hepatopancreas compared to those from the ICM group (p<0.05). In contrast, the ΣPUFA and ΣHUFA contents were significantly lower in ECM crabs (p<0.05). Specifically, the levels of LA were increased in the hepatopancreas of ECM crabs (p<0.05), whereas LNA, ARA, EPA, DHA, Σn-3PUFA, and the n-3/n-6 PUFA ratio were significantly reduced compared to the ICM group (p<0.05).
In the female gonads, levels of linoleic acid (LA) and total n-6 polyunsaturated fatty acids (Σn-6PUFA) were significantly elevated in crabs from the ECM group compared to those from the ICM group (p<0.05). In contrast, male crabs from the ECM group exhibited lower levels of LA and total n-3 polyunsaturated fatty acids (Σn-3PUFA) than their ICM counterparts (p<0.05). Furthermore, female ECM gonads demonstrated reduced concentrations of alpha-linolenic acid (LNA), arachidonic acid (ARA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), Σn-3PUFA, and a decreased n-3/n-6 PUFA ratio compared to female ICM gonads (p<0.05).
In the muscle tissues of female crabs, ICM specimens showed significantly lower levels of LNA, DHA, and n-3/n-6 PUFA ratios than ECM specimens (p<0.05), whereas ARA and Σn-6PUFA levels were higher in ICM females (p<0.05). Male ICM crabs exhibited higher contents of LA, ARA, and Σn-6PUFA in their muscle tissues compared to ECM males (p<0.05). Conversely, ECM males had reduced levels of LNA, DHA, Σn-3PUFA, and lower n-3/n-6 PUFA ratios relative to ICM males (p<0.05).
3.4. Amino Acids Composition
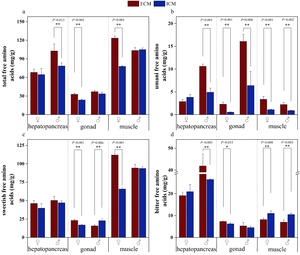
Figure 4 presents a comparative analysis of the concentrations of seven essential amino acids and the indices for amino acid nutritional evaluation in the edible tissues of E. sinensis cultivated under two distinct aquaculture models. In the female hepatopancreas, the levels of phenylalanine (Phe) and lysine (Lys) were significantly lower in crabs from the extensive culture model (ECM) compared to those from the intensive culture model (ICM) (p<0.05). The gonadal tissues of ECM crabs exhibited significantly higher total amino acid (TAA) content, essential amino acid (EAA) content, and EAA/TAA ratio than those of ICM crabs (p<0.05). Notably, female ECM gonads had reduced Phe levels compared to their ICM counterparts (p<0.05), whereas male ECM gonads showed increased Phe content relative to ICM males (p<0.05). Furthermore, the concentrations of Lys, threonine (Thr), valine (Val), isoleucine (Ile), and leucine (Leu) were significantly higher in ECM gonads than in ICM gonads (p<0.05). Muscle tissues of ECM crabs demonstrated significantly higher TAA and EAA contents compared to those of ICM crabs (p<0.05). Additionally, male ECM crabs exhibited a higher EAA/TAA ratio in muscle tissue than ICM males (p<0.05). Moreover, ECM muscle tissues showed elevated levels of Lys, Thr, Val, Ile, and Leu compared to those of ICM crabs (p<0.05).
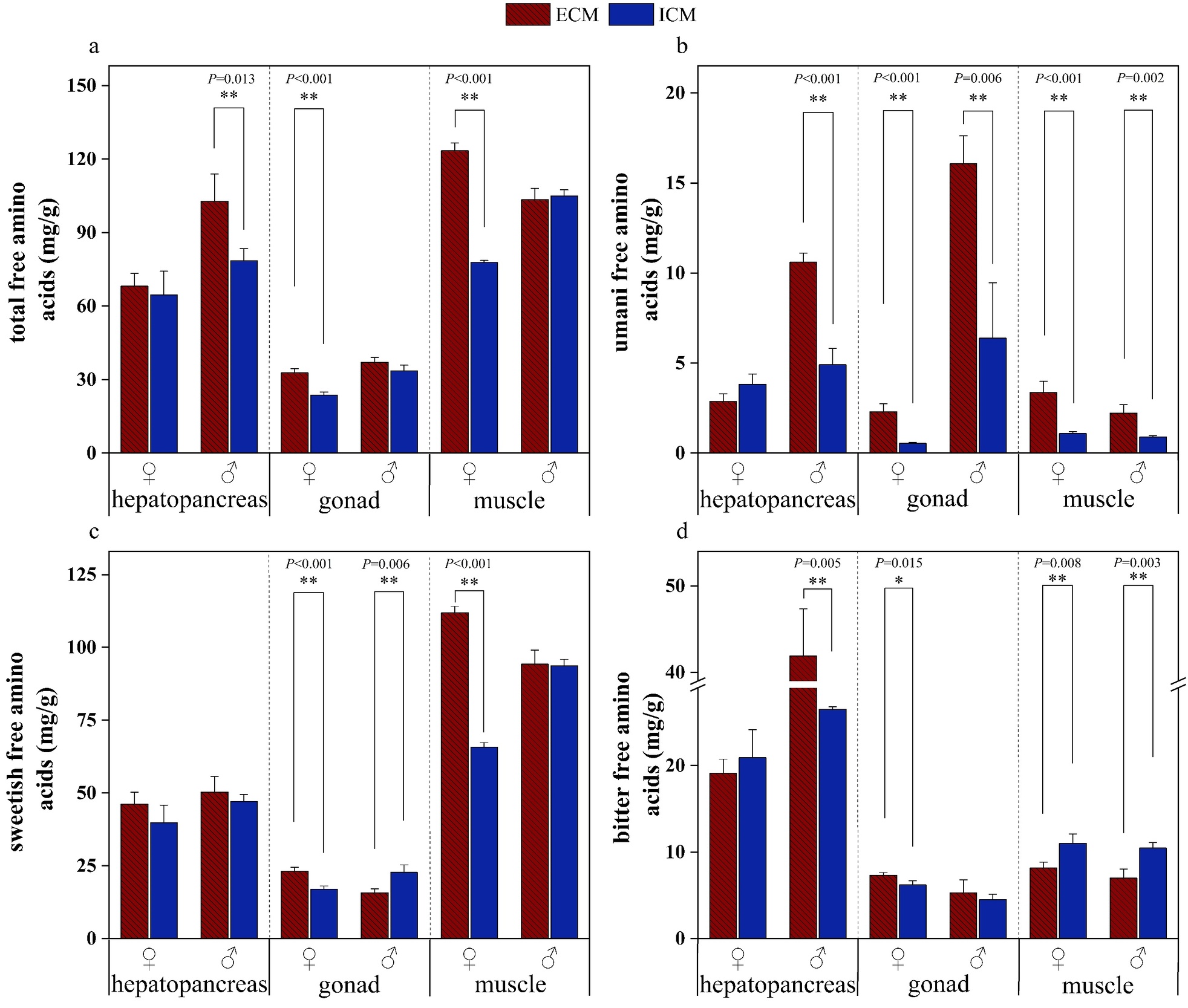
3.5. Free Amino Acids Composition and Taste Activity Value
Figure 5 illustrates the profiles of free amino acids in the edible tissues of E. sinensis cultivated under two distinct aquaculture models, whereas Figure 6 depicts the taste activity values (TAVs) of individual free amino acids (FAAs). The total free amino acid (TFAA) and umami-related free amino acid (UFAA) concentrations were significantly elevated in all tissues of crabs from the extensive culture model (ECM) compared to those from the intensive culture model (ICM), irrespective of sex (p<0.05). The sweetish free amino acid (SFAA) levels were notably higher in all edible tissues of ECM crabs than in ICM crabs (p<0.05), with the exception of the male gonads. The bitter free amino acid (BFAA) content in the hepatopancreas and gonads of male ECM crabs was significantly greater than that in ICM males (p<0.05), whereas BFAA levels in ECM muscle tissues were significantly lower than those in ICM males (p<0.05).
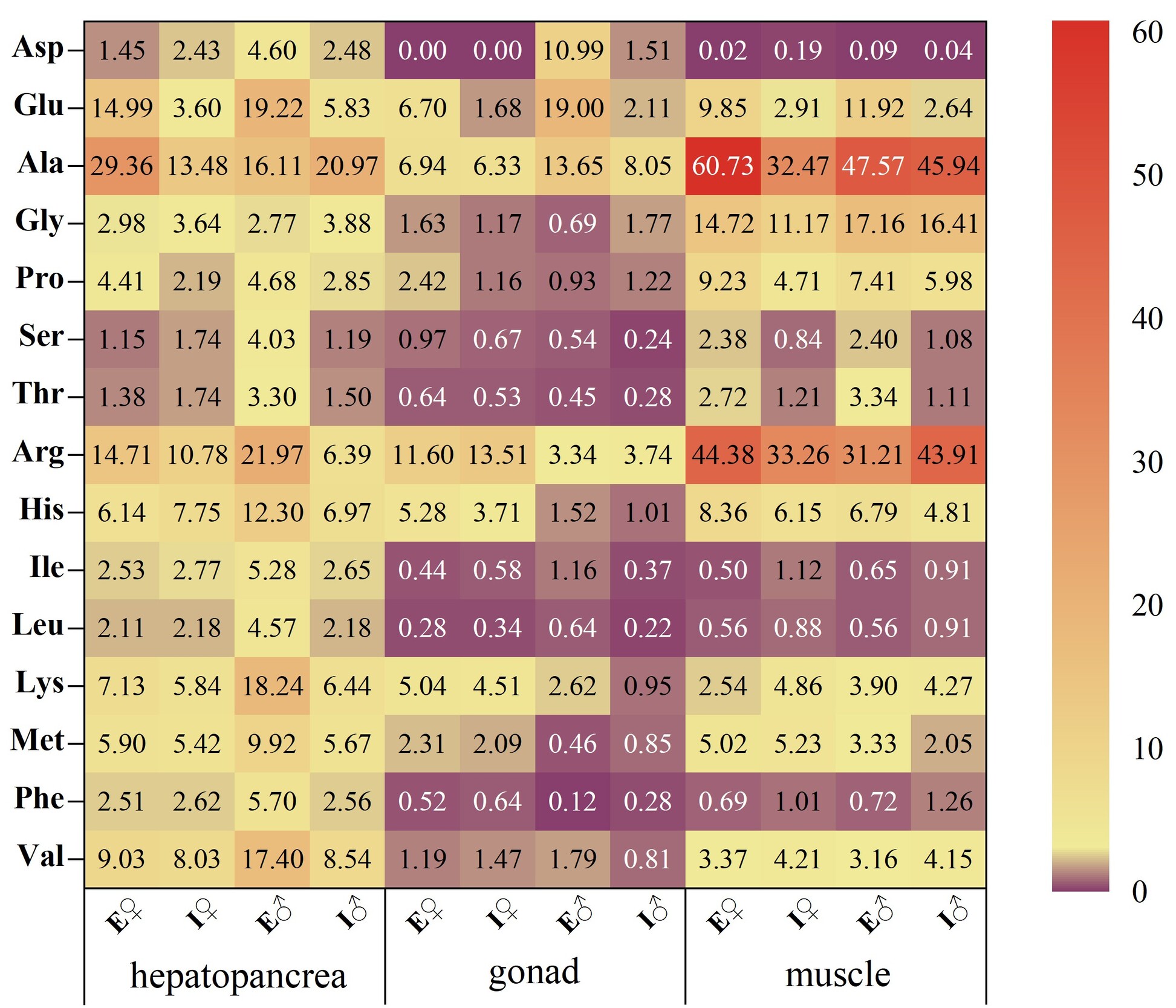
Glutamic acid (Glu), alanine (Ala), arginine (Arg), and histidine (His) were identified as the predominant flavor-active amino acids in both aquaculture models. ECM crabs displayed higher TAVs for all FAAs in the hepatopancreas, suggesting a more complex flavor profile. The gonads of ECM crabs exhibited pronounced umami characteristics, with elevated TAVs across FAAs, indicating enhanced flavor richness. Female ECM muscle tissues demonstrated stronger umami and sweetness attributes, while male ECM muscle tissues showed intensified umami characteristics. Overall, ECM muscle exhibited a more diverse flavor profile compared to ICM counterparts.
3.6. Stable Isotope Analysis
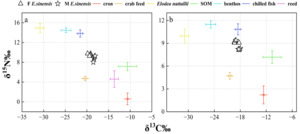
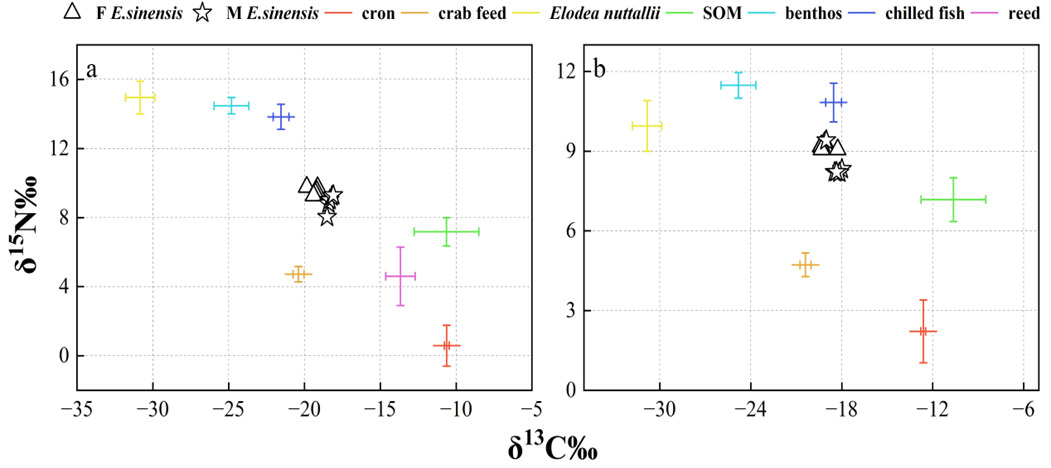
No significant difference was observed in the δ13C (‰) values of muscle tissue from female crabs between the two development and utilization models, whereas a significant difference was found in 15N (‰) values (t-test: δ13C, F = 0.665, P = 0.276; δ15N, F = 2.306, P = 0.013) (Figure 7). In contrast, no significant differences were detected in the isotopic values of muscle tissue from male crabs (t-test: δ13C, F = 1.758, P = 0.686; δ15N, F = 0.005, P = 0.245).
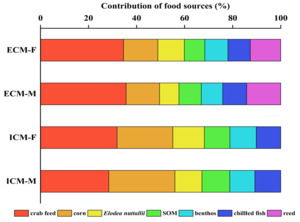
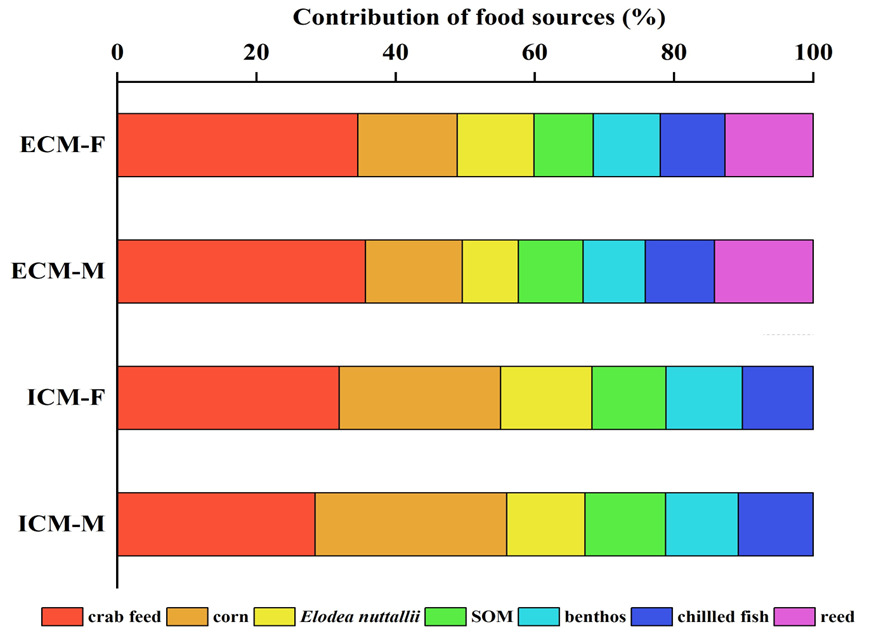
The δ¹³C isotopic values for the dietary sources of ECM crabs exhibited a range from -30.83‰ to -10.62‰, resulting in a span of 20.21‰. Concurrently, the δ¹⁵N values varied from 0.58‰ to 14.95‰, with a total range of 14.37‰. In female crabs, the predominant dietary source was formulated feed, contributing 34.6% to their diet, followed by corn (14.3%), reed (12.6%), Elodea nuttallii (11.0%), benthic animals (9.6%), frozen trash fish (9.3%), and sediment organic matter (SOM) (8.5%). Male crabs also primarily consumed formulated feed, accounting for 29.6% of their diet, with subsequent contributions from corn (17.9%), reed (14.5%), Elodea nuttallii (9.7%), frozen trash fish (9.5%), SOM (9.5%), and benthic animals (9.3%).
For ICM crabs, the δ¹³C isotopic values of their food sources similarly ranged from -30.83‰ to -10.62‰, maintaining a difference of 20.21‰ (Figure 8). The δ¹⁵N values for this group spanned from 2.21‰ to 11.47‰, with an overall range of 9.26‰. In female ICM crabs, formulated feed was the primary dietary component, comprising 31.9% of their intake, followed by corn (23.2%), Elodea nuttallii (13.1%), benthic animals (11.0%), SOM (10.6%), and frozen trash fish (10.2%). Male ICM crabs predominantly consumed formulated feed as well, which constituted 28.4% of their diet, with additional contributions from corn (27.5%), SOM (11.5%), Elodea nuttallii (11.3%), frozen trash fish (10.7%), and benthic animals (10.5%).
4. Discussion
Variations in the salinity of aquatic habitats significantly influence the osmotic pressure regulation in aquatic organisms, thereby impacting their growth and metabolic processes.17 Under conditions of alkalinity stress, E. sinensis requires an increased intake of nutrients to sustain its fundamental physiological metabolism. Consequently, the organism reduces its energy expenditure and reallocates internal nutrient resources to support essential metabolic functions.18
Eriocheir sinensis cultured in saline-alkaline environments exhibits improved gonadal development, edible yield, coloration, and nutritional quality.19 The influence of gonadal development on lipid and fatty acid metabolism in E. sinensis represents a complex physiological process, characterized by intricate interactions among multiple factors, and significantly impacts protein metabolism and amino acid synthesis. During gonadal maturation, notable changes in lipid metabolism occur within the crab’s physiology, potentially fulfilling the energy demands and structural requirements of the developing gonads.20 These metabolic shifts in lipid pathways may subsequently influence protein metabolism due to the established crosstalk between lipid and protein metabolic networks.21 Furthermore, gonadal development has been shown to modulate antioxidant capacity and immune function in E. sinensis. This alteration may be linked to the regulation of protein metabolism, given that protein-derived metabolites can function as antioxidants and immunomodulatory agents. Exposure to saline-alkaline stress is associated with behavioral abnormalities and damage to neurosecretory cells, along with a significant downregulation of pathways involved in detoxification metabolism, neurotransmitter synthesis, and antioxidant defense.22 During acclimation to salinity, the activities of key osmoregulatory enzymes, such as Na+/K+-ATPase and V-type H+-ATPase, undergo adaptive modifications.23 Notably, crabs reared in saline-alkaline conditions display superior nutritional and flavor profiles compared to those cultured in freshwater, indicating that salinity and alkalinity may positively influence product quality. Additionally, fluctuations in salinity affect gene expression and enzymatic activity, particularly during migratory phases, where osmoregulatory mechanisms are crucial for physiological adaptation.23 In saline-alkaline environments, the hepatic and muscular tissues of E. sinensis demonstrate substantial increases in DHA, EPA, and total essential amino acids, suggesting enhanced nutritional enrichment under such conditions.19Crustaceans accumulate amino acids and their derivatives, such as alanine and proline, to mitigate hyperosmotic stress in high-salinity environments. Variations in salinity also influence gene expression and metabolic pathways, with a notable upregulation of the glutamine synthetase (GS) gene expression, which correlates with increased glutamine concentrations under elevated salinity conditions.24 These adaptive responses imply that salinity-induced modifications in amino acid metabolism contribute to the regulation of osmotic balance.
Increased stocking density has been shown to adversely impact growth performance, antioxidant capacity, and nutrient metabolism in crustaceans. High-density culture conditions generally lead to reduced growth rates, decreased survival, and diminished disease resistance.25 In the current study, a highly significant difference was observed in the proportion of tissues within the edible parts of river crabs across both culture models (p<0.01), alongside a significant difference in the fatty acid composition of most edible parts (p<0.05). These findings can primarily be associated with the impact of high culture density on the metabolic pathways of triglycerides, which subsequently influences the energy supply from glycerol and fatty acids. Furthermore, high-density culture conditions impair immune function in Cyprinus carpio and increase fatty acid levels in muscle tissues.26 In biofloc systems for Litopenaeus vannamei, high stocking density is generally associated with negative biological, microbial, and physiological effects.27
In this study, significant differences were observed exclusively in the muscle δ15N (‰) isotope values of female crabs (p<0.01), despite the use of identical feeds across the two culture models. This phenomenon is hypothesized to arise from the ingestion of varied natural baits by river crabs in distinct environments. It has been documented that different aquaculture environments provide diverse natural baits.28 Variations in natural baits across different cultural environments may significantly influence the nutritional quality of E. sinensis. Natural diets have been shown to improve growth performance, immunity, and antioxidant capacity in crustaceans. For example, the inclusion of dietary astaxanthin has been found to significantly enhance antioxidant activity, immune response, and ammonia tolerance in E. sinensis.27 Similarly, supplementation with Vitamin D3 has been observed to promote growth as well as immune-antioxidant functions.29 The composition of gut microbiota in E. sinensis, which is intricately linked to environmental conditions, can be modulated through natural diets to optimize adaptability and growth. Furthermore, specific dietary components are known to regulate signaling pathways and metabolic processes, thereby influencing both growth and reproductive success.30
5. Conclusion
This study demonstrates that crabs from extensive culture models (ECM) exhibit superior edible yield, essential amino acid content, free amino acid content, and umami and sweet flavor intensity compared to those from intensive pond culture models (ICM).
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No.2023YFD2400505), the Key Research and Development Program of Hubei Province: (No.2023BBB084) and the Yellow River Delta Industry Leading Talent Project (No. DYRC20200215). The authors thank Ms. Jun Men and Ms. Yanxia Zuo (the Center for Instrumental Analysis and Metrology, Institute of Hydrobiology, Chinese Academy of Science) for technical assistance in the measurement of Amino Acids, Free Amino Acids and Fatty Acids.
Authors’ Contribution
Methodology: Wang Li (Equal), Hao Li (Equal), Ruojing Li (Equal). Formal Analysis: Wang Li (Equal), Jiajun Wu (Equal). Investigation: Wang Li (Equal), Jiajun Wu (Equal). Writing – original draft: Wang Li (Lead). Writing – review & editing: Wang Li (Equal), Tanglin Zhang (Equal). Resources: Wei Li (Equal), Tanglin Zhang (Equal). Conceptualization: Tanglin Zhang (Lead). Funding acquisition: Tanglin Zhang (Lead).
Competing of Interest – COPE
No competing interests were disclosed
Ethical Conduct Approval – IACUC
The animal experiment was approved by the Animal Ethical and Welfare Committee of Institute of Hydrobiology, Chinese Academy of Sciences (Approval No. IHB/LL/2022048).
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.
Abbreviations
The following abbreviations are used in this manuscript: