1. Introduction
The Dendrodoris arborescens (Collingwood, 1881) belongs to the phylum Mollusca, class Gastropoda, subclass Heterobranchia, order Nudibranchia, family Dendrodorididae, and genus Dendrodoris.1 The species was initially discovered in the southern coast of China and is currently primarily distributed in East Asia and the tropical regions of Australia.2 This species is a type of benthic nudibranch that typically inhabits shallow marine areas, with a depth range of 0 to 10 meters. Within the genus Dendrodoris, there are currently 45 valid species.3 Distinct from other gastropods, the species of this genus lack a shell and do not possess a radula; instead, they utilize a round, ring-shaped mouth for feeding on sponges. Regarding reproductive systems, these species share the characteristic of “simultaneous hermaphroditism” with other nudibranchs, functioning as both females and males during the mating process.4 It is also notable for its ability to secrete toxic substances from its head.5
D. arborescens’ research mainly focuses on taxonomy. Taxonomic and phylogenetic investigations have employed larval morphology and DNA barcoding as key tools to differentiate D. arborescens from its congeners, such as D. nigra and D. fumata.6,7 Hirose et al. utilized DNA barcoding and larval characteristics to identify five species of Dendrodoris in Japan.8 Similarly, Lee and Bae explored the complete mitochondrial genome of D.krusensternii in South Korea.9 Data on its reproductive anatomy, spawning ecology, and complete embryonic sequence are limited. Previous embryological studies on the genus Dendrodoris have been fragmentary and limited in scope. Hurst’s research provided brief records of the egg belt morphology and larval morphology of thirty Opisthobranchs,10 while Rose offered an incomplete study on the embryonic development of D. nigra.11 Additionally, Goddard investigated the embryonic development of D.nigromaculata, which exhibits a direct development pattern.12
The present study therefore had three objectives: (1) to provide the first comprehensive description of reproductive behaviour and gametogenesis in D. arborescens, (2) to document the entire ontogenetic trajectory from fertilisation to the planktonic veliger stage using histological, light-microscopic, and scanning-electron techniques, and (3) to compare developmental timing and larval morphology among D. arborescens, D. fumata, and D. nigra under identical thermal regimes. By integrating these datasets, we aim to clarify the developmental basis for shell loss in planktotrophic nudibranchs and to establish a robust framework for future comparative and experimental work on gastropod metamorphosis.
In the academic realm, nudibranchs (Nudibranchia) are often referred to as “reverse snails” due to their unique developmental trait of shedding their shells during metamorphosis. Despite this distinctive characteristic, comprehensive studies elucidating the complete embryonic development process of planktotrophic Nudibranchia species remain conspicuously absent. Consequently, investigating the reproductive mechanisms, embryonic development, and larval morphological characteristics of D. arborescens holds significant importance. It not only fills the existing research gap but also lays a foundational groundwork for future breeding efforts and investigations into the underlying mechanisms governing the shell-shedding phenomenon during their developmental process.
2. Materials and methods
2.1. Sample acquisition
In December 2023, all samples were exclusively collected from Dalian, Liaoning Province (38°52.2′N, 121°33.4′E) (Fig. 1). A total of 20 individuals were captured for breeding and embryological studies. After collection, the specimens were transported to the Key Laboratory of Mariculture and Stock Enhancement in North China Sea, Ministry of Agriculture and Rural Affairs, where four individuals of uniform size (length 4 cm) were selected for rearing in glass aquariums (volume: 36liters, length 40cm, width 30cm, height 30cm). Environmental conditions were carefully controlled to simulate natural habitats, with water temperature maintained at 22–25°C and salinity at 33‰. Water quality was managed by replacing half of the water daily, with new water pre-aerated. The diet consisted of Ulva lactuca.
Following spawning, the embryonic development and growth within each batch of egg sacs were monitored at 30-minute intervals. The developmental stages and morphological characteristics were examined using an optical microscope (LEICA DM4B), and detailed photographic documentation was obtained. The duration of each developmental stage was meticulously recorded, and changes in the embryos during each period were carefully observed.
2.2. Optical Microscope observation
Due to the variability in egg-laying times among individuals, each egg band was divided into three equal portions and transferred to small temperature-controlled aquariums (volume: 1.6 L; dimensions: 129 mm × 114 mm × 205 mm) for incubation. Embryonic development and growth were monitored every 30 minutes within the same batch of egg sacs. Developmental stages and morphological characteristics were observed using an optical microscope (LEICA DM4B), with detailed photographs and records maintained. The timing of each developmental stage was meticulously documented, and changes in embryos during each developmental period were observed.
Following larval hatching, a pipette was used to gently agitate the water surface to prevent larvae from adhering to the water film due to the hydrophobic properties of their cup-shaped shells. The size and morphological changes of 30 larvae at each developmental stage were recorded.
2.3. Scanning Electron Microscopy observation (SEM)
When collecting and fixing samples, the egg sacs were carefully opened to avoid mechanical damage and pressure, thereby minimizing tension and abrasion. The sampling time was kept within 1 minute. After removing the sample, it was gently washed with PBS to remove surface impurities, and the surfaces to be scanned were marked. The sample was then quickly placed in the electron microscopy fixative and fixed at room temperature for 2 hours before being transferred to 4°C for storage.
The fixed samples were rinsed three times with 0.1 mol/L phosphate buffer (pH 7.4), each for 15 minutes. Subsequently, the samples were fixed in a solution of 1% osmium acid prepared with 0.1 mol/L phosphate buffer (pH 7.4) at room temperature in the dark for 1 to 2 hours. The samples were then rinsed again three times with 0.1 mol/L phosphate buffer (pH 7.4), each for 15 minutes. Finally, the tissue was treated with isopropyl acetate for 15 minutes, followed by dehydration in a graded series of alcohol solutions (30%, 50%, 70%, 80%, 90%, 95%, 100%, and 100% alcohol) and isopropyl acetate, each for 15 minutes.13
For drying, the samples were placed in a critical point dryer. For conductive treatment, the samples were attached to conductive carbon adhesive tape and positioned on the sample stage of an ion sputtering device for approximately 15 seconds of gold sputtering. The samples were then observed under a scanning electron microscope (Hitachi SU8100).
2.4. Masson Trichrome Staining Slide Preparation
Larval samples were fixed in 10% formalin solution for 24–48 hours, then dehydrated in 70%, 80%, 90%, and 100% ethanol for 30 minutes each, followed by clearing in xylene for 20 minutes. The fixed samples were embedded in paraffin and sectioned into 5–7-micron-thick slices. They were then stained with Weigert’s iron hematoxylin for 10 minutes, rinsed with running water for 5 minutes, stained with acid fuchsin for 5–10 minutes, rinsed for 5 minutes, differentiated with phosphotungstic acid/phosphomolybdic acid for 5–10 minutes, and stained with aniline blue for 5–10 minutes. and finally rinsed again for 5 minutes. The stained sections were dehydrated in 70%, 80%, 90%, and 100% ethanol solutions for 30 seconds each, and clarified in xylene for 1 minute each step. Finally, the sections were mounted with mounting medium, and after the mounting medium had completely dried, they were observed and photographed under an optical microscope (Nikon Eclipse E100).14
3. Results and analysis
3.1. Behavioral Observations During Breeding
The specimens of D. arborescens undergoes repeated mating during cultivation, with mating occurring 8–15 times daily. Mating duration ranges from a minimum of 20 minutes to a maximum of 140 minutes. Following mating, each individual lays egg within one week (Fig. 2). Due to their similar size and morphology, individuals have difficulty distinguishing post-mating. To mark post-mating individuals, a portion of the mantle is clipped after egg-laying. Egg-laying takes approximately 2–5 hours. The female swirls clockwise and moves slowly, pressing the orange-yellow opaque adhesive egg mass firmly against the tank wall or substrate. The egg mass measures 9–14 cm in length. A 0.5 cm section of the egg mass is examined under a microscope to estimate the number of eggs, which ranges from approximately 5,400 to 6,500.
3.2. Early Embryonic Development
The embryonic development process is divided into 12 distinct periods (Table 1). At 22-23°C, development from the fertilisation to the planktonic veliger stage required 9–10d. The stage was grouped into into five major phases: cleavage, blastula, gastrula, larval development, and planktonic veliger.
Cleavage (0–22 h post-fertilisation, hpf).No polar-body extrusion was detected.
1–3.3 hpf: 2-cell stage. Two unequal blastomeres (174 ± 8.1 µm)(Fig.3-A,B).
3.3–5 hpf: 4-cell stage. One large and three small blastomeres arranged in two planes (175 ± 7.3 µm)(Fig. 3-C).
5–13 hpf: 8-cell stage. Four large and four small blastomeres; asynchronous cleavage (175 ± 5.9 µm) (Fig. 3-D).
13–15 hpf: 16-cell stage. Blastomere volume decreased; embryo enlarged to 179 ± 5.1 µm(Fig.3-E).
15–22 hpf: Morula. Cell boundaries indistinct; no blastocoel (182 ± 7.6 µm)(Figure 3-F).
Blastula (1.5 d post-fertilisation, dpf). Ciliated blastula exhibiting weak rotational movement (186 ± 5.8 µm) (Fig.3-G).
Gastrula (1.5–3 dpf). Clockwise rotation continued; diameter unchanged (186 ± 5.8 µm) (Fig.3-H).
3–4 dpf: Trochophore with incipient heart-shaped velum (203 ± 8.7 µm) (Fig.3-I).
larval development 4–6 dpf: Early veliger (210 ± 9.2 µm)(Fig.3-J).6–7 dpf: Late veliger fully formed (Fig.3-K).
Hatching 9–10 dpf: ; shell length 192 ± 10.7 µm(Fig.3-L).
Monozygotic twins: one embryo reached the veliger stage, the other arrested at blastula (Fig.4-A).
Monozygotic triplets: only one embryo completed development; the remaining two exhibited cleavage arrest (Fig.4-B).
Polyspermy was observed. Multiple male pronuclei fused, yielding supernumerary chromosome sets that segregated aberrantly, producing multipolar mitoses and subsequent developmental arrest (Fig.4-C).
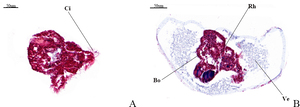
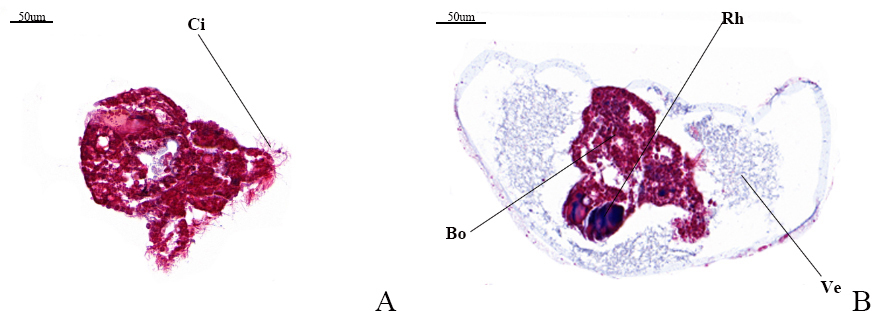
3.3. Masson staining observation
Under Masson’s trichrome staining, the veliger larva exhibits a blue hue, indicative of a rich collagen fiber matrix, while the worm body displays a red coloration, reflecting its composition of muscle fibers. Notably, the rhinophore has not yet developed in the observed specimens, which in adults typically evolves into a rod-shaped structure. As a prominent external anatomical feature of the head in marine gastropod nudibranch molluscs (Nudibranchia, Aplysiomorpha, Sacoglossa), the rhinophore plays a significant role in sensory perception. Fig.5-A illustrates a young larva equipped with a rudimentary set of cilia, preceding the formation of the veliger structure.
3.4. Observation of eggs under Scanning Electron Microscope(SEM)
As illustrated in Fig.6-A, a fertilised egg is depicted with a spherical profile, an intact egg membrane (EM), and localized minor damage. Beneath the egg membrane, a conspicuous layer of lipid droplets (LD) can be discerned, poised to enter the division stage. In Fig.6-B, the same egg has progressed to the first cleavage division, evidenced by the appearance of a distinct cleavage furrow.
3.5. Observation of disc larvae under the Optical Microscope(OM)
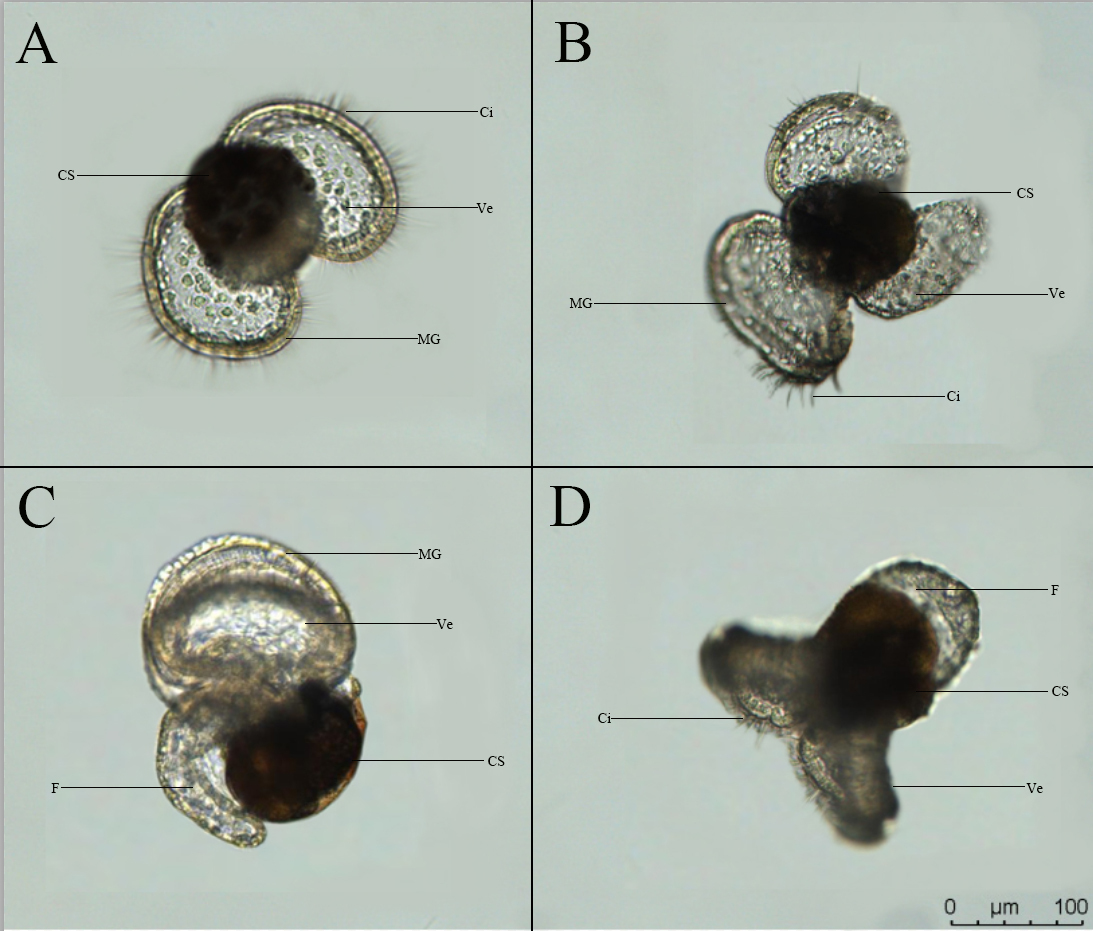
Fig.7 shows the morphological characteristics of the veliger larva of D.arborescens. The shell is yellowish-brown, translucent and cup-shaped, with a vivid orange marginal band. The internal organs are compactly enclosed within the shell, and the dense pigmentation in the shell makes it difficult to directly observe the specific location and development of the internal organs. The apex is white and lacks an operculum. The larva has two symmetrically distributed elliptical ciliated wing-like membrane structures, the velum, which it uses for swimming and floating. When fully extended, the larva can reach a maximum diameter of about 220 μm. The outer edge of the disc is surrounded by a wide, yellowish translucent marginal band that supports the ciliated structures. The interior of the disc is blue and scattered with white vacuolar round cells. When viewed from the side (Fig.7-C), the larva has a large, transparent foot structure with a thick row of granules along the bottom edge, and the foot bends around the posterior part of the shell. The larva swims rapidly through its surface and well-developed cilia. When swimming, the surface plate contracts and folds, then unfolds again (Fig.7-D). Compared to most planktonic larvae, the veliger larva of this species is morphologically unique. Each larva lacks an operculum structure and has an unusually developed apex that cannot be retracted into the shell.
Only two larvae have developed to the shellless larva stage. The larvae shed the yellowish-brown cup-like shell and cilia on the velum (Fig.8-A), at which point they appear transparent, with the statocyst visible (Fig.8-B), swim slowly, and die after 6d.
4. Discussion
The mating behavior of D. arborescens is similar to the simultaneous hermaphrodite mating mechanism described by Burn (2006),15 but there are key differences: individuals of D.arborescens do not exhibit obvious male dominance competition during mating, but rather tend to engage in mutual fertilization, thereby increasing reproductive success. This strategy may confer a significant advantage in low-density populations, avoiding reproductive failure due to mate scarcity. However, the specific mechanisms of sperm competition remain to be further investigated, such as whether phenomena like sperm precedence or sperm storage exist.16,17
Identical twins, identical triplets, and multiple spermatozoa entering eggs were observed. In Thompson’s (1958) study of nudibranchs, identical twins were observed in Onchidoris fusca, Doris pseudoargus, Jorunna tomentosa, and Adalaria proxima, but identical triplets were observed for the first time in nudibranch research.18 Compared to the relatively common phenomenon of identical multiple embryos in vertebrates, identical multiple births in mollusks are rare, and their formation mechanisms are associated with abnormal early embryonic cleavage or special environmental adaptive strategies.19 This discovery is of great significance for understanding the diversity of reproductive strategies in mollusks. It may represent an unrecognized adaptive strategy, warranting further investigation through larger sample sizes and molecular approaches to elucidate its underlying mechanisms. Some gastropods adjust their reproductive strategies under fluctuating salinity conditions,20 but whether nudibranchs regulate embryonic development to respond to environmental stress remains to be verified through controlled experiments (e.g., temperature/pH gradient designs) combined with molecular markers (e.g., DNA methylation detection). In particular, the Hox gene cluster and Wnt signaling pathway play a key role in establishing embryonic polarity in mollusks.21 Future studies could explore the role of these pathways in polyembryony through single-embryo transcriptomic analysis.
Eggs (173-181μm diameter) and first-hatched larvae veligers (181.3-202.7 μm shell length) of D. arborescens were significantly larger than the values reported (121-129 μm of egg diameter and 153 μm of shell length of larvae) under the culture conditions of 22-23 °C.7 This difference stems from morphological variation among geographic populations or subtle differences in culture conditions. Compared to the results of Brodie and Calado (2006) at higher temperatures (26-27°C) (egg diameters 121.4-141.7 μm, first hatch larval shell lengths in the range of 142.6-159.4 μm), the egg diameter and first hatch larval shell lengths of D.arborescens still showed a larger size,10 and the time of incubation at higher temperature (26-27°C) The incubation time at higher temperatures (26-27℃) was about 30% shorter than that at 22-23℃, which is in line with the general pattern of development of metazoans, and this phenomenon further supports that temperature is a key environmental factor affecting the early development of marine invertebrates.22
While the closely related species D. fumata showed the characteristic of “small eggs and large larvae” (egg diameter 112.2-121.8 μm, larval shell length 206.1-233.9 μm), D.nigra was the smallest in overall size (egg diameter 71.2-76.8 μm, larval shell length 129-141 μm), and the difference may be related to its larval ecological adaptation strategies, e.g. D. fumata may improve survival competitiveness in the planktonic phase through larger larval size,23 while miniaturization of D.nigra may facilitate rapid development or adaptation to specific ecological niches.24
The developmental patterns of 26 species of Dendrodorididae worldwide. Among these, 54% of the groups exhibit planktotrophic development, 4% exhibit lecithotrophic development, and 42% exhibit direct development.12 Planktotrophic development is more common in Dendrodorididae.
Additionally, the large foot structure of D.arborescens larvae indicates its adaptation to a long-term planktonic lifestyle. Large feet may enhance swimming ability or assist in later attachment. In summary, the morphological characteristics of D.arborescens larvae reflect multiple adaptive strategies for the planktonic life stage, including potential light-regulated mechanisms for shell pigmentation and specialized locomotor organs. Unfortunately, the larvae failed to complete metamorphosis, possibly due to the absence of key environmental inducers. Similar chemical signal-dependent metamorphosis triggering mechanisms have been reported in various marine invertebrates.25,26
5. Conclusion
-
D.arborescens is a simultaneous hermaphrodite species, and both individuals will lay eggs after mating. Noteworthy phenomena include monozygotic twins, monozygotic triplets, and multiple spermatozoa entering eggs. The phenomenon of identical triplets and multiple spermatozoa in eggs was first documented in the species Nudibranchus.
-
The embryonic development of D. arborescens follows the sequence of all gastropods and is divided into five main stages: cleavage, blatula, gastrula, intracapsular larval development, and planktonic veliger larval stage. Hatching occurs after 9-10 d at a temperature of 22-23°C.
-
D. arborescens larvae possess a yellowish-brown cup-like shell with an apex that cannot be retracted into the shell, an absence of an operculum, and a notably large foot adapted for a prolonged planktonic phase. Notably, the completion of metamorphosis is contingent upon the induction of a specific inducing substance. Without a suitable inducing substance, it is not possible to move on to the next stage of development
ACKNOWLEDGMENTS
This research was supported by the Science and Technology Major Projects in Liaoning Province (2025); National Natural Science Foundation of China (42076101); the Central Government Subsidy Project for Liaoning;Liaoning Province Science and Technology Plan Joint Research Project (2024JH2/102600076);Liaoning Province Modern Agricultural Industry Technology System Shellfish System;Liaoning Province Key Research and Development Program Project (2024JH1/11700010)
AUTHORS’ CONTRIBUTION
Methodology: Linxuan Cai (Equal), Yida Han (Equal). Writing – original draft: Linxuan Cai (Equal), Yida Han (Equal). Data curation: Menghao Jia (Equal), Wenjia Li (Equal). Conceptualization: Zhenlin Hao (Lead). Writing – review & editing: Zhenlin Hao (Equal), Ying Tian (Equal). Formal Analysis: Ying Tian (Lead).
ETHICAL CONDUCT APPROVAL - IACUC
The article adheres to the Convention on Biological Diversity and the Convention on Trade in Endangered Species of Wild Fauna and Flora Research.
CONFLICT OF INTEREST - COPE
The authors declare that they have no conflict of interest.
INFORMED CONSENT STATEMENT
All authors and institutions have confirmed this manuscript for publication.
DATA AVAILABILITY STATEMENT
The data supporting the results of this study can be acquired from the corresponding author on reasonable request.

.jpeg)


__monozygotic_triplets_(b)__and_multiple_spermatozoa_entering_eggsc.jpeg)


.jpeg)
.jpeg)


__monozygotic_triplets_(b)__and_multiple_spermatozoa_entering_eggsc.jpeg)

.jpeg)