1. Introduction
The Chinese perch (Siniperca chuatsi) is a visually and olfactorily acute carnivorous species known for its rapid and efficient prey capture. Notably, this species exhibits highly selective feeding behavior.1 From first feeding, they feed on live bait and categorically refuse to eat dead bait and compound feed without prior conditioning, and even prefer to cannibalize each other rather than ingesting dead baitfish in the absence of food.2 These characteristics pose significant challenges for the aquaculture of Chinese perch, such as elevated feeding costs and unmanageable food chains. Consequently, the domestication of Chinese perch to accept artificial feed is crucial for the further development of its culture industry.
ish domestication refers to the progressive adaptation of sensory cells in the eyes and lateral line system to the characteristics and movement patterns of artificial feed. Through repeated reinforcement of the feeding response, fish gradually develop conditioned reflexes that enable consistent feeding on artificial diets.3 Furthermore, given the significant difference in palatability between fresh bait and artificial feed, it is essential to gradually acclimate Chinese perch to the new taste. This step ensures successful adaptation and consistent consumption of the provided artificial feed. Critically, the feeding strategy plays a key role in the adaptation of Chinese perch to both the form and taste of the compound feeds during domestication. Among them, the use of transitional feeds (such as chilled baitfish) is a frequently applied feeding strategy. Chilled baitfish retain the general morphological and olfactory characteristics of fish, which helps facilitate visually and olfactorily dependent feeding behaviors. However, the meat quality of the frozen fish declines, and its texture is intermediate between that of dead bait and artificial feed, which means that the use of chilled baitfish may help reduce the stress caused by food transition, thereby increasing the acceptance of subsequent artificial feed by Chinese perch. It is noteworthy that the inclusion of a chilled baitfish feeding regimen also implies an increase in the number of feeding training sessions, and the impact of this on domestication effectiveness, as well as the underlying physiological mechanisms, remains unclear. In addition, feeding rate and frequency have a direct effect on the efficiency of domestication. Feeding rate directly affects the strength of feeding response intensity as well as the body oxidative stress status.4–6 Previous studies have shown that starvation stress caused by insufficient feeding induced strong feeding motivation in wild species, which led to the domestication of wild animals into domesticated animals.7,8 This means that the strategy of increasing the feeding response of Chinese perch through moderate starvation to promote domestication is feasible. Noteworthily, most species of fish exhibit much greater starvation tolerance than high-evolved animals such as mammals. Therefore, the starvation strategy for fish needs significant adjustment. Moreover, similar to mammals, excessive starvation (e.g., prolonged underfeeding and fasting) can cause physiological damage in fish, such as oxidative stress.9,10 Studies on sobaity seabream (Sparidentex hasta) and yellowfin seabream (Acanthopagrus latus) suggested that oxidative damage from severe starvation may be irreversible, even with subsequent refeeding.11,12 This indicates that excessive starvation-induced oxidative stress may have a negative impact on domestication. In fact, although moderate starvation has a positive effect on domestication, whether it still induces a certain degree of oxidative stress remains to be confirmed. Furthermore, the appropriate level of starvation during domestication process may vary for each fish species, depending on the starvation tolerance of each species. Therefore, the moderate starvation level for each fish species is worth clarifying. Feeding frequency directly affect the body’s learning and memory for feeding behavior. Studies in zebrafish (Danio rerio) and goldfish (Carassius auratus) have shown that learning and memory allow individuals to adapt quickly and flexibly to changing environments.13 However, an increase in feeding frequency can also lead to some negative effects, such as stress caused by a significant deviation of the actual feeding frequency from the innate feeding rhythm. In existing studies, the beneficial and adverse effects caused by feeding frequency are often separated, which may lead to one-sided conclusions. This study attempts to conduct a comprehensive analysis of the impact of feeding frequency on domestication during the short-term domestication process.
Although farmers have developed a systematic domestication framework based on a sequential bait transition (dying baitfish - dead bait - chilled baitfish - artificial feed), standardized feeding strategies for each stage are lacking. This lack of defined parameters contributes to consistently low domestication rates. This study investigates the effects of key feeding strategies—including feeding rate, frequency, and duration using chilled baitfish—on the domestication rate of Chinese perch. The findings aim to identify optimal feeding parameters during the domestication process and provide data-driven support for establishing standardized domestication protocols for the species. This study also explored the physiological pathways by which feeding strategies regulate the domestication effect through moderate starvation, oxidative stress, learning, and memory, providing theoretical support for further improving the domestication effect of Chinese perch.
2. Materials and Methods
2.1. Feeding rhythm analysis of Chinese perch
Juvenile Chinese perch (10.0 ± 0.4 g/fish) with the same genetic background purchased from Anhui Dawei Aquaculture Co. Ltd were temporarily reared for one week in a recirculating water system (220 L/tank, water temperature 25.0 ± 1.0 ℃, dissolved oxygen 7.0 ± 0.5 mg/L, and ammonia and nitrite < 0.1 mg/L) at Anhui Agricultural University. Fish were randomly distributed into three tanks (15 fish/tank), and each tank was stocked with 300 baitfish (Cirrhinus mrigala, 0.2 g/fish). Remaining baitfish were quantified every 4 h via video monitoring (Canon, R100, 4 k resolution, exposure compensation) and replenished to the initial density. The experiment lasted for 72 h under natural photoperiod (LD 14:10). The feeding intake of Chinese perch in each tank was statistically evaluated by precisely calculating the difference between the initial total quantity of baitfish and the remaining quantity. At the end of the experiment, feeding rhythm curves were plotted based on the feeding intake.
2.2. Effects of different feeding strategies on domestication of Chinese perch
2.2.1. Feeding rate
Fish underwent a standardized domestication protocol (Table 1) and were randomly allocated to five feeding regimes (triplicate tanks/group, 39 fish/tank): 5%, 8%, 11%, 14%, and 17% body weight (BW)/d. Based on satiation trials (pre-experiment), groups were classified as: high-feeding rate (17% BW/d), medium-feeding rate (11-14% BW/d), and low-feeding rate (5-8% BW/d). Feed was administered twice daily (06:00, 18:00) for 11 d. At the end of domestication, the domestication rate of Chinese perch in each tank was counted using 3 d of stable intake of soft pellet feed as the criterion of domestication success, which was expressed as follows: domestication rate (%) = number of fish feeding on feed/total number of fish.14 The feed used in this experiment was made into pellets in the laboratory through a 3 mm pellet mill. The ingredients and approximate composition of the diets are shown in Table 2. Furthermore, the total number of attacks on the feed (expel or ingest) and the amount of feed consumed by the Chinese perch in each tank were recorded using video equipment.
2.2.2. Feeding frequency
The process of domestication is referred to in Table 1. All fish were randomly divided into 3 groups (triplicate tanks/group, 39 fish/tank) and domesticated at a feeding frequency of 2 times/d (06:00, 18:00), 3 times/d (06:00, 12:00, 18:00), and 4 times/d (06:00, 10:00, 14:00, 18:00), respectively. The feeding rate for each group was maintained at 11% BW/d. The domestication experiment lasted 11 d. The number of fish that had ingested the soft pellet feed, their intake, and the total number of attacks, were counted separately in each tank at the end of domestication.
2.2.3. Feeding duration of chilled baitfish
The process of domestication is referred to in Table 1, except for the feeding duration of chilled baitfish. All fish were randomly divided into 4 groups (triplicate tanks/group, 39 fish/tank) and fed chilled baitfish for 2, 4, 6, and 8 d during domestication. Other diet phases (dying baitfish, dead baitfish, soft pellet feed) maintained equal durations. Feeding rate and frequency were 11% BW/d and 2 times/d (06:00, 18:00), respectively. The entire domestication cycle was 17 d. The number of fish that had ingested the soft pellet feed, their intake, and the total number of attacks, were counted separately in each tank at the end of domestication.
2.3. Oxidative stress parameter analysis
At the end of domestication, six fish were randomly taken from each group and deeply anesthetized with tricaine methanesulfonate (MS-222, 200 mg/L), and the liver tissues were dissected and frozen in liquid nitrogen, and then stored at -80 ℃ for oxidative stress analysis. Moreover, blood was collected from the caudal vein and then immediately centrifuged at 3000×g at 4 °C for 20 min to collect plasma for cortisol analysis. The contents of cortisol and malondialdehyde (MDA), as well as the activities of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) were detected by kits (H094-1-1, A003-1, A001-1, A007-1, and A005-1) purchased from Nanjing Jiancheng Bioengineering Institute.
2.4. Total RNA extraction and relative quantification of mRNA
Brain tissues from six fish per group were randomly isolated based on the same operation described above. Subsequently, total RNA was extracted from brain tissues by using Trlzol Reagent (Invitrogen, China). The quantity and quality of the isolated RNA were checked by NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The ratio of absorbance at 260 and 280 nm (A260/A280) was used to assess the purity of RNA. Genomic DNA was removed from RNA sample using gDNA Eraser, then 1 µg of total RNA was used for cDNA synthesis (Takara, Japan). Target gene expression levels were determined by quantitative real-time PCR with SYBR Green (Takara, Japan) on a PCR instrument platform (Bio, USA). The reaction system included 1 µl cDNA, 0.4 µl forward and reverse primers (10 mmol/µl), 10 µl SYBR (Bio-Rad, USA), and 8.2 µl double distilled water. The primers of housekeeping gene (rpl13a), appetite-related genes (npy, agrp, cart, and pomc), and learning and memory-related genes (fosl2, egr1, c-fos, and syt4) used in the present study are listed in Table 3. The PCR cycling parameters were as follows, 95 ℃ for 30 s followed by 40 cycles at 95 ℃ for 10 s, 57 ℃ for 30 s, and melting curve from 65 ℃ to 95 ℃ (gradually increasing 0.5 ℃/s) with data acquired every 6 s. Gene expression levels were quantified relative to the expression of rpl13a using the optimized comparative Ct (2-ΔΔCt) value method.
2.5. Statistical analysis
All data were expressed as means ± SEM and the normality of data was evaluated with the Shapiro-Wilk test. All data were analyzed using one-way analysis of variance (ANOVA) with SPSS 19.0 software, followed by a Duncan test. Differences were considered to be significant if P < 0.05.
3. Results
3.1. Feeding rhythm analysis
Feed consumption of Chinese perch was recorded at 4-h intervals, revealing a significant cosine rhythm in daily feeding behavior. The peak phases of feed consumption occurred at 16:00-20:00 and 04:00-08:00 (Fig. 1), demonstrating a characteristic bimodal feeding pattern (2 meals/d).
3.2. Effects of different feeding strategies on domestication
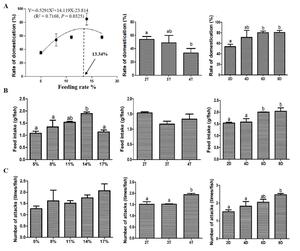
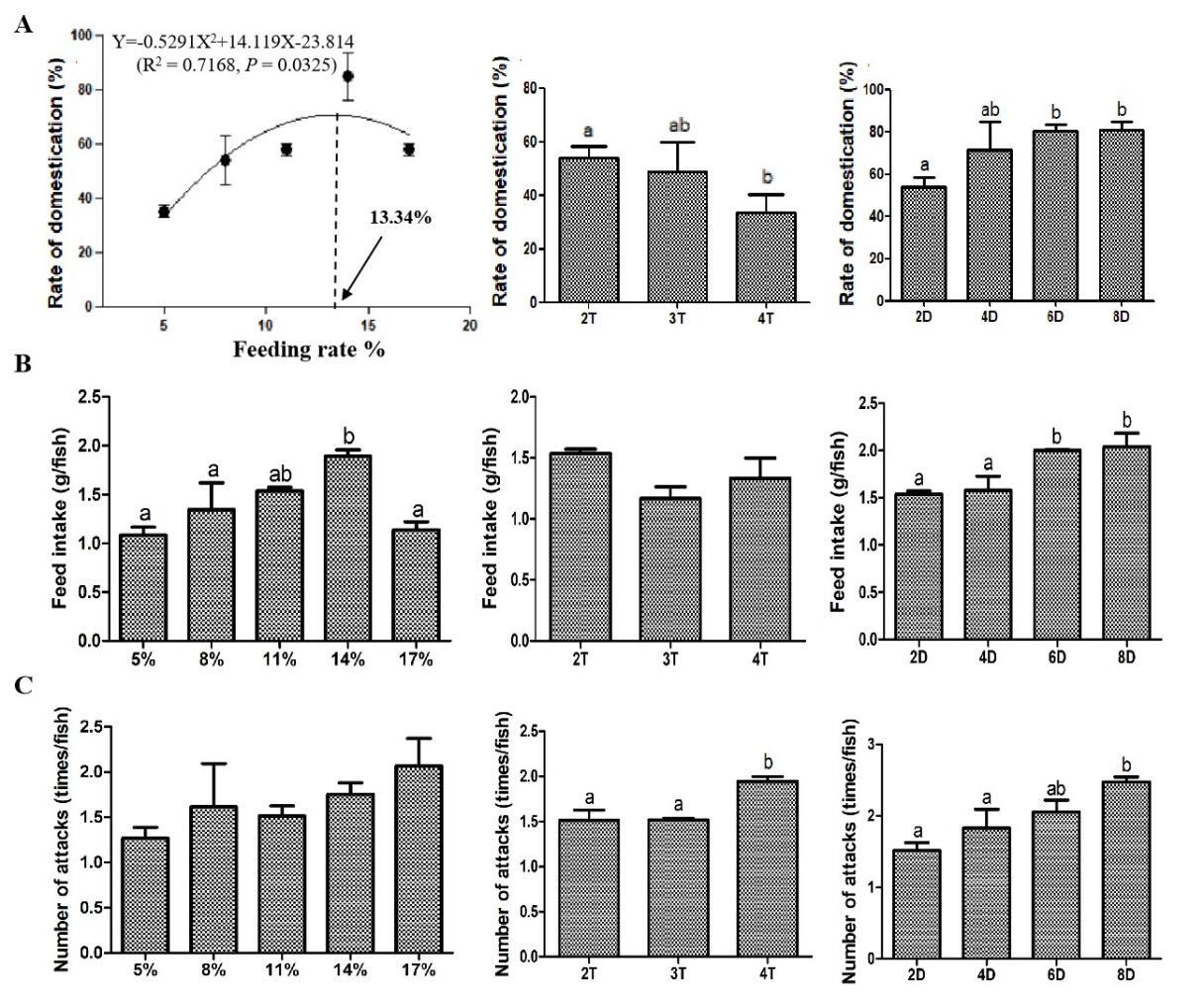
Curvilinear regression model was used to analyze the relationship between the domestication rate and the feeding rate, which was expressed as Y=-0.5291X2+14.119X-23.814 (R2 = 0.7168, P = 0.0325). The regression plot indicated that the domestication rate increased progressively from 5% to 11% feeding rates, but declined beyond 14%. The optimal feeding rate was determined to be 13.34% for the maximum domestication rate. Moreover, the 14% feeding rate group exhibited a higher feed intake compared to the other groups. The feeding frequency experiment showed that the domestication rate of the fish fed 4 times/d was significantly decreased compared with that fed 2 times/d. However, the number of attacks showed a completely opposite change trend to the domestication rate. The feeding duration experiment exhibited that the domestication rate and feed intake of the fish fed chilled fish for 6 and 8 d were significantly increased, and the number of attacks of fish fed chilled fish for 8 d was also significantly increased compared with that fed chilled fish for 2 d (Fig. 2). Above findings collectively suggested that feeding strategy dramatically affected domestication rate, and this effect might occur through different physiological pathways.
3.3. Effects of different feeding strategies on the expression of appetite-related genes
The expression level of appetite suppression-related genes cart and pomc in the low-feeding rate group (5% and 8%) and high-feeding rate group (17%) was significantly higher than that in medium-feeding rate group (11% and 14%). The expression level of cart in fish fed chilled fish for 4, 6, and 8 d was significantly lower than that in fish fed chilled fish for 2 d (Fig. 3). The data revealed that feeding rate and chilled baitfish feeding duration during domestication affected the expression of appetite-suppressor-related genes, but not appetite-promoter-related genes.
3.4. Effects of different feeding strategies on the oxidative stress parameter
Compared to the medium-feeding rate group (11% and 14%), low-feeding rate group (5% and 8%) exhibited a significant increase in MDA and cortisol content, and a significant decrease in the activities of SOD and CAT. Compared with the fish fed 2 and 3 times/d, the fish fed 4 times/d exhibited a significant increase in the content of cortisol. Additionally, compared with the fish fed chilled fish for 2 d, the fish fed chilled fish for 6 and 8 d exhibited a significantly lower MDA content, and the fish fed chilled fish for 6 d exhibited a significantly higher CAT activity (Fig. 4). Cortisol content also showed a similar trend to MDA (Fig. 5). As the feeding duration with chilled fish during the domestication increased, cortisol content gradually decreased, and there was a significant reduction in cortisol content of fish fed chilled fish for 6 and 8 d. Existing results indicated that low-feeding rate and high feeding frequency during the domestication induced oxidative damage, and that extending the feeding duration of chilled fish to more than 6 d alleviated the oxidative stress associated with domestication.
3.5. Effects of different feeding strategies on the expression of learning and memory-related genes
Compared with the fish fed 2 and 3 times/d, the fish fed 4 times/d exhibited a significant increase in the expression level of fosl2. Additionally, compared with the fish fed chilled fish for 2, 4, and 6 d, the fish fed chilled fish for 8 d exhibited a significant increase in the expression level of syt4 (Fig. 6). The data uncovered that learning and memory might mediate the effects of different feeding frequencies and chilled baitfish feeding duration on domestication.
4. Discussion
4.1. Effects of feeding rate on domestication
Multiple factors influence fish domestication, with feeding rate representing a critical factor. In this study, the relationship between feeding rate and domestication rate was expressed as Y = -0.5291X2 + 14.119X - 23.814 (R2 = 0.7168, P = 0.0325), and the optimal feeding rate for Chinese perch during domestication was determined to be 13.34%. The feeding response intensity observed across different groups supported the reliability of the aforementioned data. CART/POMC neurons and NPY/AgRP neurons serve as key central appetite-regulating neurons. CART/POMC neurons inhibit feeding behavior by releasing anorexigenic neuropeptides (e.g., α-MSH, CART), while NPY/AgRP neurons stimulate feeding via releasing pro-appetite neuropeptides or neurotransmitters (e.g., GABA).15,16 Consequently, their expression levels directly reflect fish feeding motivation. It was found that fish species such as Ctenopharyngodon Idella and Carassius auratus tend to simultaneously regulate the expression levels of NPY/AgRP and CART/POMC to rapidly adjust their appetite.17,18 In the present study, the medium feeding rate groups (11% and 14%) exhibited the lowest expression levels of the appetite suppression-related genes cart and pomc, indicating that moderate feeding rates enhance feeding response for domestication. In contrast, both low (5% and 8%) and high (17%) feeding rates significantly increased cart and pomc expression, indicating reduced feeding motivation. These data suggest a strong correlation between the domestication rate under the influence of feeding rate and the anorexigenic neurons rather than the orexigenic neurons. The discovery that the feeding response could be regulated merely by adjusting the anorexigenic neurons or the orexigenic neurons, rather than jointly regulating both sets of genes, was frequently observed in species such as Micropterus salmoides and Rachycentron canadum.19,20 This may be attributed to the refined energy management exhibited by these species (including Chinese perch) as high-intensity predators, which tend to prioritize the inhibition of CART/POMC neurons to conserve energy, as neuronal inhibition is more energy-efficient than activation in terms of energy utilization.15,16
Starvation stress has been identified as the underlying mechanism through which feeding rate affects domestication.7,8 Moderate starvation stress can significantly enhance fish appetite, facilitating dietary habit transition. This theory aligns with our observation of a higher domestication rate in the medium feeding rate group, which experienced moderate starvation. However, the low feeding rate group showed a significant decrease in domestication rate, which might be directly related to oxidative stress caused by starvation stress. MDA is a marker of oxidative stress and its level directly indicates the degree of oxidative damage to tissues or cells, whereas SOD and CAT are crucial antioxidant enzymes protecting cells from oxidative damage.21,22 Previous studies collectively reported that short-term starvation induced oxidative stress while significantly increasing appetite, indicating that the stress induced by short-term starvation did not deterministically cause appetite suppression.23,24 This is attributed to the extremely strong starvation tolerance of most fish, for example, many fish species can survive an entire winter without feeding. The difference is that the Chinese perch has an extremely poor ability to endure starvation. It is manifested that juvenile Chinese perch will cannibalize each other within 2-3 d after food deprivation.14 Therefore, an 11-day low feeding rate means excessive starvation, which may cause significant oxidative damage to the Chinese perch. In the present study, it was explicitly observed that MDA content was significantly increased, and SOD and CAT activities were significantly decreased in the low feeding rate groups compared to the medium feeding rate groups, verifying the occurrence of oxidative damage. The results detailed previously indicated that excessively low feeding rate induced substantial oxidative damage, ultimately reducing the domestication rate.
4.2. Effects of feeding frequency on domestication
In intensive aquaculture, food intake of fish depends largely on farmer-imposed feeding strategies. Appropriate feeding frequency can strengthen the feeding response, thereby having a positive effect on domestication.25 Previous study on juvenile hybrid grouper (Epinephelus fuscoguttatus × E.lanceolatus) reported that the optimal domestication effect was achieved at a feeding frequency of 4 times/d.26 However, the domestication rate of Chinese perch gradually decreased with the increase of feeding frequency and showed a significant decrease at a feeding frequency of 4 times/d compared to 2 times/d, which was also reflected in the decreased expressions of the appetite-promoting related gene agrp and increased expressions of the appetite suppression-related gene pomc at a feeding frequency of 4 times/d. It was reported that the optimal feeding frequency was closely related to feeding rhythms, and a reasonable feeding frequency should match the natural feeding rhythms of the species in order to maximize growth efficiency, feed intake, and reduce stress.27,28 Therefore, the optimal feeding frequency of Chinese perch during domestication may largely depend on the natural feeding rhythm. Our observation of significant daily cosine rhythmicity in feed consumption, with peaks occurring between 16:00-20:00 and 04:00-08:00, demonstrates a characteristic bimodal feeding innate feeding rhythm (2 meals/d). A previous study reported that the retinal structure of Chinese perch was adapted to the low-light environment, mainly relying on the “scotopic system” (sensitive to low light) rather than the “photopic system” (sensitive to strong light).14 This special retinal structure enables the Chinese perch to clearly perceive prey under low-light conditions, thereby occupying the ecological niche of “dawn/dusk predator” and determining its innate feeding rhythm of 2 times/d.14 This supports the logical inference that a feeding frequency of 2 times/d aligns best with innate feeding rhythm, yielding superior domestication effects in Chinese perch.
Learning and memory can effectively enhance fish feeding ability and efficiency.13 Previous study has shown that increased feeding frequency may deepen learned feeding responses, aiding adaptation to new feeding practices.13,29 The gene fosl2 is one of the typical learning memory-related genes, which belongs to the immediate early genes (IEGs), and its expression level increases in response to the activation of neurons associated with long-term memory.30 Our preceding study on the relationship between domestication and learning memory demonstrated that two taming cycles of Chinese perch significantly up-regulated the fosl2 expression level and were accompanied by a shorter response time to ingesting the artificial diet compared to one taming cycle.13 In the present study, the expression level of fosl2 was up-regulated at the feeding frequency of 4 times/d compared to 3 times/d. It means that the feeding memory of Chinese perch for artificially fed food is strengthened with more training, which may result in a stronger feeding response. The significantly increased number of attacks observed at the feeding frequency of 4 times/d supports the above finding. In general, an increase in the number of attacks implies an increased probability of feeding. However, the learning and memory-reinforced feeding responses did not translate into improved domestication in this experiment. Studies in Takifugu rubripes and Megalobrama amblycephala have reported that a significant and prolonged discrepancy between the frequency of feeding and innate feeding rhythms led the body to remain in a state of persistent stress.31,32 In this study, a feeding frequency of 4 times/d led to a significant increase in content of cortisol, suggesting the presence of stress. Considering the negative impact of stress on domestication rate, it is inferred that stress caused by the deviation of a 4-times/d feeding frequency from the innate feeding rhythm has a greater effect on domestication compared to the newly formed feeding memory during the short period of domestication. Although the number of attacks on artificial feed increased, the rise in stress led to a decrease in the acceptance of artificial feed by Chinese perch, resulting in the failure to truly ingest the artificial feed.
4.3. Effects of feeding duration of chilled baitfish on domestication
Chilled baitfish serves as a crucial transitional feed from live bait to an artificial diet during domestication. On the one hand, chilled baitfish retain the general morphology of the fish, but still undergo some degree of change compared to live and dead bait. The conditioning reflex established by feeding chilled baitfish may diminish the reliance on vision during feeding, thereby lowering the requirements for food morphology.13,14 On the other hand, the texture of chilled baitfish is intermediate between that of dead bait and artificial feed, which indicates that the use of transitional feed may help reduce the stress caused by food transition, thereby increasing the acceptance of subsequent artificial feed by Chinese perch. In this experiment, the levels of cortisol and malondialdehyde (MDA) significantly decreased and the activity level of catalase (CAT) significantly increased after 6 and 8 d of chilled baitfish feeding, indicating a reduction in stress. This is attributed to the enhanced adaptability to artificial feed resulting from the exercise of longer transitional bait feeding duration for Chinese perch. Furthermore, the appetite suppression-related gene cart gradually decreased, and the feed intake and domestication rate gradually increased with the increase in feeding duration of chilled baitfish. These indicators showed significant changes after 6 and 8 d of chilled baitfish feeding, suggesting a connection between the feeding duration of chilled fish and oxidative stress as well as the domestication effect. It is noteworthy that the period of chilled baitfish feeding during which significant changes in oxidative stress levels and domestication rate occurred was completely consistent. Meanwhile, stress has been reported in other fish species as an important factor or pathway influencing domestication.33,34 Therefore, it could be inferred that the duration of chilled baitfish feeding might improve domestication effects via reducing stress cause by food transition.
In addition to reducing oxidative stress, a longer duration of chilled baitfish feeding implies an increased number of training sessions for feeding on artificially fed food. Similar to increasing feeding frequency, this may exert a certain effect on learning and memory phenotypes. The results of this study verify the aforementioned inference. Feeding chilled baitfish for 8 d significantly up-regulated the expression level of the learning and memory-related gene syt4. SYT4 is a membrane transport protein that affects learning and memory by dynamically regulating neurotransmitter release and influencing synaptic plasticity.33 syt4 knockout mice exhibited impaired performance in the social transmission of food preference test compared to the wild types.34 Learning and memory triggered changes in syt4 expression, which were similar to the result in this experiment. The significantly increased number of attacks on artificial feed further confirmed the positive impact of the feeding duration of chilled baitfish on the learning and memory phenotypes. These results collectively indicated that an enhancement in the feeding duration of chilled baitfish could reinforce Chinese perch feeding responses through learning and memory, thereby influencing the domestication effect.
Collectively, the above findings suggest that an optimized domestication strategy for Chinese perch, comprising a feeding rate of 13.4%, a feeding frequency of 2 times/d, and chilled baitfish feeding duration exceeding 6 d, achieves a higher domestication effect. Moderate starvation, learning, and memory can effectively drive the domestication of Chinese perch.
Acknowledgments
This work was financially supported by Natural Science Foundation of Anhui Province (2308085QC109), Natural Science Research Projects of Colleges and Universities in Anhui Province (2023AH051033), Research Funds of Joint Research Center for Food Nutrition and Health of IHM (2023SJY01), and key technology research and development of Hefei city “unveiled” project (hx23118).
Authors’ Contribution
Conceptualization: Yanpeng Zhang, Xiaochen Yuan; Methodology: Ting Chu; Formal analysis and investigation: Junhao Wang; Writing - original draft preparation: Yanpeng Zhang; Writing - review and editing: Xiaochen Yuan; Funding acquisition: Yanpeng Zhang, Xiaochen Yuan; Resources: Min Wu, Yanou Yang, Shengzhen Jin; Supervision: Yucheng Liu, Ziwen Wang.
Competing of Interest – COPE
No competing interests were disclosed.
Ethical Conduct Approval – IACUC
The animals and experiments were conducted in compliance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, China). The study was approved by the Institutional Animal Care and Use Ethics Committee of Anhui Agricultural University. All efforts were made to minimize suffering.
Informed Consent Statement
All authors and institutions have confirmed this manuscript for publication.
Data Availability Statement
All are available upon reasonable request.

.png)

_in_the_liver_of_chinese_perch_with_differe.jpeg)


.png)

_in_the_liver_of_chinese_perch_with_differe.jpeg)

