Introduction
Based on the current emission scenario, global atmospheric carbon dioxide (CO2) levels have been predicted to reach 1000 ppm by the end of the twenty-first century, thus resulting in an estimated drop of 0.13 to 0.42 pH in the ocean by then.1 The absorption of CO2 by the seawater results in a net increase in protons (H+) and a reduction in pH, which finally leads to what is known as ocean acidification (OA) in the earth’s oceans.2 The OA has been found to threaten ecologically and economically important mollusks by disturbing their energy metabolism and regulation, resulting in changes in growth and high mortality rates in these species.3 Therefore, understanding how these organisms respond and perhaps adapt to such changes is even more important to gain insights into their biological survival and the ecosystem balance.
The marine gastropod Brunneifusus ternatanus is widely distributed in temperate and tropical regions. It lives in subtidal beds playing a vital ecological role in the coastal community structure. Also, it is a predator that feeds on other mollusks on the muddy bottoms of mangroves or near estuaries, with a morphologic characteristic, a shell with a large and unique aperture.4 These species contain abundant protein and good nutritional value, with 20 kinds of fatty acids already identified.5 Therefore, it is marketed as a luxury food item at a high price. Unfortunately, due to habitat deterioration, the natural availability of B. ternatanus has been reduced significantly, increasing the importance of artificial breeding and aquaculture in this species.6 Coastal sources of pollution, including intensive and unregulated aquaculture, can also contribute to the production of high levels of respiratory carbon dioxide leading to changes in seawater carbonate chemistry. Therefore, it seems urgent to study sea whelks’ growth and physiological responses to future environmental changes.
The intestine is the longest portion of the digestive system, containing various enzymes involved in digestive and absorptive processes, and its morphology and structure also can affect those processes.7 The activity of digestive enzymes and metabolism-related internal organs is affected by external factors such as temperature and pH, that modify metabolic functions.8 Notwithstanding the influence of OA on the digestive efficiency and enzymatic activity of marine organisms has been reported in marine invertebrate organisms, including larvae of the sea urchin Strongylocentrotus droebachiensis,9 and the mussel Mytilus edulis10; few relevant research has been carried out in gastropods, especially related to the response of the digestive system to OA stress.11 Notably, gastropods are known to be the most diversified class in the phylum of mollusks, and second only to the insects in the number of known species.4 It is fully reasonable to select these organisms as the research object in acidification simulation experiments. Despite its economic and ecological importance, information regarding the impact of ocean acidification on the health of B. ternatanus is still scarce. This study aims to analyze the effects of OA on the growth performance, intestinal morphology, digestive enzymes, and its intestine microbiota in B. ternatanus adults, and preliminarily discuss the surviving strategies under acidification levels and the effects on its digestive functions. Findings of this study will shed light on the understanding of the effect of OA on the health of the whelks, providing valuable basic information for future studies related to the mechanism of metabolic processes in whelks.
Materials and Methods
Experimental whelks and setup
The B. ternatanus were collected from Puqian Bay (20°3′16.7″N, 110°32′57.8″E), Wenchang city, Hainan, China, and transported to the Hainan Provincial Key Laboratory of Tropical Maricultural Technology, Hainan Academy of Ocean and Fisheries Sciences (Qionghai City, Hainan). Healthy individuals of similar size (initial body weight: 174.10 ± 29.47g, shell length: 146.86 ± 15.39 mm) were randomly assigned to tank of 500L open-flow and acclimation with continuous seawater exchange at a temperature of 29 ± 2oC, salinity 30.0 ± 1.0 psu, dissolved oxygen 7.0 ± 0.5 mg L-1, and pH 8.1 ± 0.1 for a week. During the acclimation period, whelks were fed twice daily with Meretrix meretrix live clams.12 Daily food consumption was approximately 5% to 8% of the total wet weight of the whelks. Unconsumed food was removed promptly to prevent decomposition in the tanks.
The experiment was conducted on the control (C group, pH 8.1), CO2 exposure period (EP group, pH 7.3), each replicated four times. C group: Whelks submerged in seawater at 8.1 pH, representing the present natural condition were set as control. EP group: A group of whelks submerged in seawater at 7.3 pH was set as the acidification treatment, following the acidity levels predicted by IPCC (Intergovernmental Panel on Climate Change) for the year 2300. After pH treatment for 28 days, animals were randomly sampled from each tank for histological analysis, enzyme activities analysis, and DNA extraction, respectively. The intestine tissue was quickly removed and then stored at − 80◦C.
Seawater chemistry
Seawater in the experimental tanks was changed every day, and to maintain the pH 7.3 at a relatively stable level during the 28-day exposure period, CO2 gas was added as required. To lower the pH of the seawater to 7.3, CO2 gas was added to the aquaria using a pH regulation unit, which measured the pH levels to an accuracy of 0.01 pH units. The pH levels in each aquarium were checked three times a day throughout the experiment using a pH meter (Hanna Instruments, USA) calibrated with NBS standard buffers. Aeration was bubbled into the experimental aquaria through air stones for all group. During the experimental period, the dissolved oxygen concentration was monitored in all aquaria, and animals were visually inspected daily to check for mortality. All treatments were fed with the same diet as in the acclimation period.
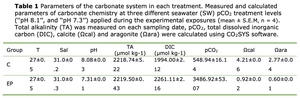
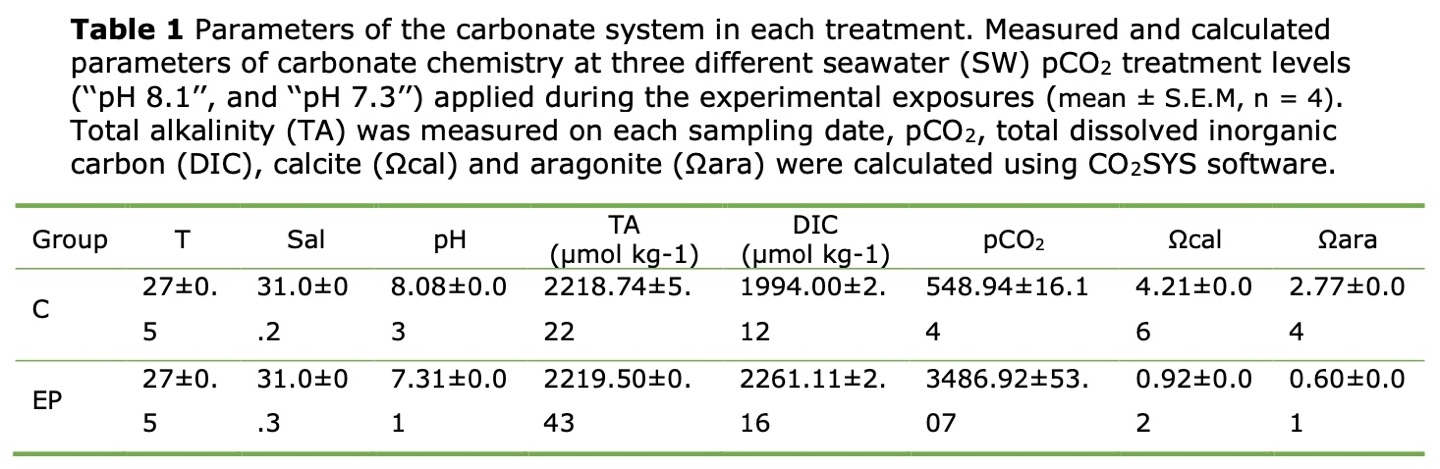
Seawater chemistry parameters including Total alkalinity (TA),Dissolved inorganic carbon (DIC), partial pressure of CO2 in seawater (pCO2), the CaCO3 saturation state for calcite (Ωcal) and aragonite (Ωara) are calculated by referring to Liu et al., 2017 method.
Growth and survival of whelks
To obtain the average weight (Wt) of the whelks, extra water from surface of the animal was removed from paper towels and weighed on a digital balance (0.01g sensitivity; LE2002E; METTLER TOLEDO). The weight gain rate (WGR), specific growth rate (SGR), and survival rate (SR) were calculated using the following formulae:
where is the mean body weight (g) of live B. ternatanus on day 0, t in each tank, respectively. t (days) is the experiment period. is the number of live whelks on day 0, t in each tank, respectively.
Histopathological observation
Paraffin section, haematoxylin, and eosin staining (H&E)
Four whelks from each treatment were anesthetized and dissected, and the whole intestine was surgically removed from each individual. Before anesthesia, animals are first incubated in fresh seawater and placed in a dark, vibration-free place. Anesthesia can begin when the animal has returned to its natural state. Then whelks were anesthetized with an overdose of tricaine methanesulfonate (MS-222, Sigma-Aldrich, St. Louis, MO, USA) before dissection. Dissected samples were immediately fixed in 4% PFA and later dehydrated in an ascending graded series of ethanol (70, 90, 95, and 100%) for 1 hour each and then cleared in 3 changes of xylene within 24 hours. The cleared samples were then embedded in melted paraffin wax. Paraffin-embedded specimens were sectioned at 4 µm thickness using a pathological microtome. Later, sections were deparaffinized and stained with haematoxylin and eosin to analyze general morphology. The photographs at 200× and 600× magnification were taken with a computer-aided microscope (Eclipse E100, Nikon, Tokyo, Japan).
Ultrastructure observation via transmission electron microscope (TEM)
Fragments (1mm length approx.) of intestine from each experimental group (n = 4) were collected and fixed overnight at 4 oC for 2-4 hours in 2.5% (v/v) glutaraldehyde. The intestine samples were washed in 0.1 M phosphate buffer (PB, pH 7.4) three times, for 15 min each, then fixed with 1% OsO4 in 0.1 M PB (pH 7.4) for 2 hours. After removing OsO4, the tissues were rinsed in PB 3 times, 15 min each. Followed by tissue dehydration in a graded series of ethanols, cleared in propylene oxide, and then embedded in Epon resin for 48 h at 60 oC. Ultrathin sections (60-80 nm thin) were fished onto the 150 meshes cuprum grids with formvar film and double-stained with 2% uranium acetate and 2.6% lead citrate using conventional techniques. The epithelium and cilium modifications of the intestinal mucosa were assessed by histomorphometric analyses using electron micrographs on a transmission electron microscope (TEM, H-7800, HITACHI, JAPAN).
Enzyme activities assay
To analyze the enzymatic activity in the intestine of whelks, the gut of four individuals of B. ternatanus from each group of previously frozen samples were thawed, weighed, and homogenized. Homogenates were then centrifuged at 3000 ×g for 15 min at 4 °C to precipitate large particles. The supernatants were collected for the enzyme analysis in triplicate. The digestive enzyme activities of amylase (AMS, No. C016-1-1, EC 3.2.1.1), trypsin (TRY, No. A080-2-2, EC 3.2.4.4), lipase (LPS, No. A054-2-1, EC 3.1.1.3), and lysozyme (LZM, No. A050-1-1, EC 3.2.1.17) were detected using commercial kits (Jian Cheng Bio-engineering Research Institute, Nanjing, P. R. China). All the enzymatic activities were expressed in units (U/mg prot). Each unit of trypsin (U/mg prot) is adopted as the change of 0.003 in absorbance by trypsin in 1 mg of protein at 37 °C, pH 8.1; Each unit of amylase (U/mg prot) is adopted as 1 mg of protein that hydrolyzed 10.0 mg starch in 30 min at 37°C; the activity of lipase (U/mg prot) is adopted as 1 mg of protein that hydrolyzed 1 μmol of the substrate in 1 min at 37 oC; Each unit of lysozyme (U/mg prot) is adopted as the change in transmittance of 0.001 each minute at 37 oC.7
Intestinal microbiota analysis
The bacterial genomic DNA from intestine samples (50-100 mg) was extracted using E.Z.N.A. ®Stool DNA Kit (D4015-02, Omega, USA) following the manufacturer’s instructions. Quantification of DNA was carried out by ultraviolet spectroscopy and agarose gel electrophoresis for further analysis. The V3 - V4 region of the bacterial 16S rRNA gene was PCR-amplified with forward primer 341F: (5’-CCTACGGGNGGCWGCAG-3’) and reverse primer 805R: (5’-ACTACHVGGGTAT CTAATCC-3’). All PCR reactions were performed in the 25 μL reaction system containing 25 ng of template DNA, 12.5 μL PCR Premix, 2.5 μL of each primer, and PCR-grade water to adjust the volume (20). The PCR condition was as follows: initial denaturation at 98 °C for 30 s and 35 cycles of 98°C for 10 s, annealing at 54 °C for 30s and elongation at 72 °C for 45 s, and then final elongation at 72 °C for 10 min. The amplicon products were confirmed with 2% agarose gels and purified by AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA), and then quantified by Qubit (Invitrogen, USA). Subsequently, sequencing libraries were constructed using Agilent 2100 Bioanalyzer (Agilent, USA) and Library Quantification Kit for Illumina (Kapa Biosciences, Woburn, MA, USA) sequenced using paired-end on Illumina NovaSeq PE250 platform.
The data obtained from the sequencing were preprocessed by the paired-end reads and overlap relationship and then analyzed with QIIME 2.0.13 Paired-end reads were assembled using FLASH software with default and quality-filtered using fqtrim (v0.94) to obtain the high-quality clean tags. Further filtering of chimeric sequences was conducted by the software Vsearch (v2.3.4). After, the effective sequences were used in the final analysis. Feature table and feature sequence were obtained by dereplication using DADA2. Depending on the SILVA (release 132) classifier, the characteristic abundance was performed with normalization by the relative abundance of each sample. Lastly, subsequent analyses of alpha diversity were calculated by QIIME2, and all were performed based on these normalized output data. Alpha diversity, including OTUs, Chao1 index, Shannon index, and Simpson index, was used to estimate the microbial diversity of intestinal samples. Beta diversity was carried out using principal coordinates analysis (PCOA), and the ANOSIM was used to perform a hypothesis test of the microbiome.14
Data analysis
The results were analyzed by independent t-test at a 5% significance level using SPSS version 18.0 (SPSSInc, Chicago, IL, USA). Data were presented as arithmetic means with standard error of the mean (S.E.M) and were statistically significant when P < 0.05.
Results
Seawater chemistry
The seawater physicochemical parameters measured during the experiment are shown in Table 1. The TA values ranged from 2218 μmol kg-1 to 2219 μmol kg-1 throughout the experiment. The salinity and the temperature levels were 31.1 ± 0.3 psu and 28.0 ± 0.8 ◦C respectively. The pH in the normal oceanic was 8.08 ± 0.02, and the pH in the acidification condition was 7.31 ± 0.01.
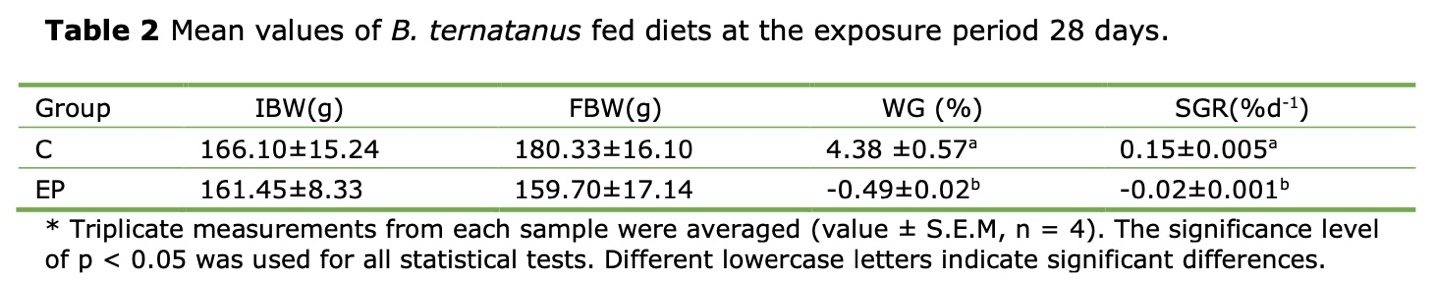
Growth performance
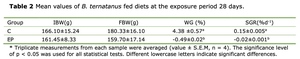
After 28 days, the WGR and SGR of the whelks subjected to high acidity condition (pH 7.3) were negative, -0.49% ± 0.02% and -0.02% ± 0.001% respectively. These values were significantly lower than 4.38% ± 0.57% and 0.15% ± 0.005% in the control group (pH8.1, p < 0.05). During the experimental period, neither mortality nor visible disease signs were observed in the whelks exposed to OA (Table 2).
Gut tissue structure of B. ternatanus
The H&E staining of histological sections of the guts revealed that the whelks subjected to high acidity conditions (EP) exhibited alterations in their morphology. (Figure 1A, 1B). In the control group, the intestinal mucosal epithelium was intact and with well-organized microvilli (Mv) (Figure 1A). The guts exhibited intestine degeneration, swelling, and slight tissue detachment (Figure 1B). Some regions of the intestine also suffer the absence of cellular structure compared to controls, including enterocyte necrosis, rupture, and falling into the lumen on day 28 (Figure 1C, 1D). Further observations of the gut under TEM revealed differences in the cellular structure between the EP and RP groups (Figure 1E, 1F). The rough endoplasmic reticulum (RER) was swollen and degranulated in the EP groups. The microvilli (MV) were thicker and shorter, and some areas were sparse and disordered. EP was more severe than the control, with partial rupture of the mitochondrial membrane and loss of structure (Figure 1E, 1F).
Intestine enzyme activity analysis
The activity of the digestive enzymes trypsin (TRS), lipase (LPS), amylase (AMS), and lysozyme (LZM) exhibited dissimilar levels among the whelks that were cultured under different conditions.
Whelks from the EP group exhibited a significant reduction in their TRS activity (154.42±6.94 U/ mg prot) compared to the control (251.58 ± 11.94 U/ mg prot). The LPS activity in whelks cultured in normal and low acidity seawater was 60.09 ± 3.36 U/ mg prot, and 27.11 ± 2.04 U/ mg prot, respectively. The AMS activity in the EP group (0.55 ± 0.04 U/ mg prot) was significantly lower than the control group (0.55 ± 0.04 U/ mg prot). Conversely, the activity level of LZM in the EP group (15.20±1.08 U/ mg prot) was significantly higher than that in the control group (5.11±0.57 U/ mg prot) (Table 3).
Gut community diversity analysis
In the microbiome survey, 621,421 sequences were analyzed from high-quality 16S rRNA readers in 8 samples, with a median of 78,790 reads per sample. The obtained sequences were analyzed and classified into different taxonomic units of bacteria with a phase sequence similarity of 97%. Venn diagram showed the core gut taxa of whelks before and after acidification (Figure 2).
171 OTUs were shared in all groups. The 487 OTUs in the acidified group were much higher than the 294 OTUs in the normal group. The Ace and Chao index and Shannon and Simpson diversity index showed that there were no significant differences in the intestine microbiota community richness between experimental groups (p>0.05) (Table 4). PCoA analysis based on the relative abundance of OTUs found that samples from the same group were clustered together (Figure 3). Meanwhile, Anosim and Adoni analyses showed significant differences in the gut flora of the EP group samples compared to the C group (P < 0.05, R = 0.45), as shown in Table 5.
The gut microbiota in the whelks subjected to different conditions exhibited variability among all experimental groups. In the EP group, 97.90% of the phylotypes were occupied by five core phyla, all of which had a mean relative abundance of >1%, including Tenericutes (41.37%), Proteobacteria (35.61%), Bacteroidetes (19.11%), Bacteroidota (1.13%, Figure 4A). Tenericutes was also found to be the most representative phylum in the acidified state, and the value was significantly decreased in the natural conditions (p<0.05; Figure 4B). Similarly, two core phyla were identified in the C group, including Proteobacteria (82.42%), and Spirochaetes (14.06%).
Thus, the dominant phylum was Proteobacteria, which significantly increased compared to the acidified group (p<0.05). Moreover, Firmicutes/Bacteroides ratio declined from 4.38 in the control group to 1.25 in the EP group (Figure 4C)
Meanwhile, above 1% of mean relative abundances at the genus level were classified as core genera. 75.21% of the phylotypes were occupied by four core genera in the C group, namely Burkholderia-Caballeronia-Paraburkholderia (66.78%), Sphingopyxis (4.1%), Phreatobacter (2.81%), Delfti (1.52%). As with Group C, the four core genera were Mycoplasma (41.37%), Burkholderia-Caballeronia-Paraburkholderia (28.47%), Bacteroides (2.47%), Sphingopyxis (1.95%), and were included in 74% of the phylotypes (Figure 5A). Furthermore, the relative abundance of Burkholderia-Caballeronia-Paraburkholderia as the dominant bacterium in the C group increased significantly (p<0.05) relative to the EP group. Also, Mycoplasma as the dominant bacterium in the EP group showed a dramatic increase in relative abundance relative to the C group (p<0.05) (Figure 5B).
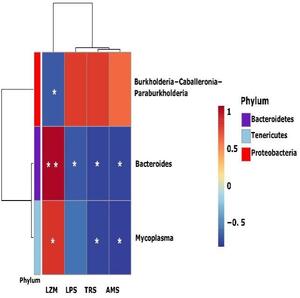
Correlation analysis between the bacterial abundance and enzymes
The correlation between the microbiota of B. ternatanus and digestion-related enzymes was analyzed by Spearman correlation analysis (Figure 6). The dominant bacteria (relative abundance >1%) were Mycoplasma, Bacteroides and Burkholderia-Caballeronia-Paraburkholderia showing significant correlations with the enzymes (p < 0.05). Among them, Mycoplasma had the highest abundance in the EP group, followed by Bacteroides, which all showed a positive correlation with LMZ activity. Also, they were negatively correlated with the activities of the digestive enzymes TRS, AMS and LPS. Although the correlation with LPS was not significant in the genus Mycoplasma, it also showed a down-regulation trend. However, Burkholderia-Caballeronia-Paraburkholderia only had a negative correlation with LZM and no significant correlation with the other enzymes.
Discussion
Previous studies have demonstrated that OA had a significant impact on marine organisms, with negative effects on the structure of the digestive gland in the blue mussel Mytilus edulis.15 However, the effects of OA exposure on digestive physiology and gut microbiota remained unclear. In this study, we first observed that a short period of exposure to pH 7.3 would cause histological damage and alter intestine digestive enzyme levels and inter-group differences of gut flora in adult sea snails.
Our study observed moderate alterations in whelks subjected to low acidity condition (pH 7.3). These observations indicate that OA can damage the digestive function of adult B. ternatanus by causing inflammation. Combined with the growth data described in this study, it is possible that the inhibition of WGR and SGR after pH changes might be caused by intestinal function damage. A similar observation was reported in the intestine of adult sea urchins, showing that morphology and histology of intestines were prominently altered in all OA-treated, accompanied by a reduction in their growth. Meanwhile, there are increasing reports of physiological indicators of shellfish health from the digestive glands when adult shellfish are exposed to acidified environments.7,15 Therefore, to observe the structural changes of the digestive glands caused by acidification, it is important to obtain information on enzyme activity in the gut. Digestive enzyme patterns can not only reflect the digestive capacity and indirectly reflect the metabolism of various substances in the mussels.16 Besides, it can be used as a biomarker in environmental monitoring since it can indicate the metabolic status of animals and the degree of adaptation to the environment.17 In the present study, TRS, LPS, and AMS were significantly suppressed as a result of pH changes, indicating that a long-term exposure to OA may affect the absorption of multiple nutritional components by altering the metabolic efficiency of carbohydrates and proteins in B. ternatanus. Most studies have reported that the activity of the digestive enzymes in marine animals could be inhibited by hypercapnic conditions (excessive CO2). For example, in Solea senegalensis, digestive enzyme activity is efficiently inhibited by OA in both pancreatic and intestinal enzymes of larvae through morphological and physiological impairment in different pH gradients.18 In adults of the mussel M. edulis,15 exposure to acidifying seawater changed the inner pH of the digestive gland and made it unfavorable for the activation of the digestive enzyme (AMS, LPS, protease). Although the possible mechanisms by which these phenomena occur have not been fully discussed, studies of a possible relationship with metabolic inhibition have been proposed.15 It is possible to consider that the digestive capacity of B. ternatanus is sensitive to ocean acidification and might induce changes in its eating behavior. In addition, a decrease in pH in the digestive glands can damage its tissue structure, involving not only excessive death of abnormal epithelial cells but also changing the integrity of the endoplasmic reticulum, resulting in the inactivation of digestive enzymes.9,15 This negative effect on the digestive capacity was consistent with a similar study that described the responses of the crab Poryunus trituberculatus to an elevated pCO2 condition.19 Although, earlier studies show that in mussels, oysters, and clams, digestive enzymes were moderately or not affected at an elevated pCO2 condition.20 The differences in these results may be due to the different species such as clams and oysters can experience extreme hypercapnia and acidification in their habitats with increased CO2 up to 10,000μatm and pH below 7,21 thereby resulting in well adaptation to ocean acidification. In general, the intestine would be damaged after four weeks of exposure to low pH, thus resulting in a significant alteration of digestive enzyme activity and intestine tissue.
The fluctuation of the lysozyme activity is often regarded as a typical humoral defense factor in the immune responses of bivalve mollusks.22 Similarly studies reported that a low pH will cause a significant rise in lysozyme activity in bivalves.7 Similar results were found in the present study when LMS was greatly increased in exposure time. Although it is not clear how pH affects the immune responses in whelks, the results reported in the present study indicated that CO2 exposure can induce clear alterations in B. ternatanus. Thus, it is possible to speculate that a significant alteration of LMS represents a part of the immune function that is activated, which is a defense against the effects of a low-acidity environment on the gut.
Environmental stress, including seawater temperature, hypoxia, food species and microplastics, can alter the microbial community diversity in the gut of aquatic animals and has been closely related to the host health status.23 In this study, the bacterial α-diversity in the whelk’s gut was not transformed by OA-stress but it causes changes in the composition of gut microbiota. A similar finding has been reported in the Pacific oyster Crassostrea gigas.24 Disruption of the gut microbiota can enhance host susceptibility to infection by invertebrate pathogens .25 Exposure to disorganized biota in ocean acidified snail gut microbiota may promote organismal disease probability and reduce viability.
At the phylum level, the relative abundance of gut microbiota was altered in the control and acidified groups. The relative abundance of Tenericutes was found high in the exposed to low pH group whereas the ratio of Firmicutes/Bacteroidetes decreased. Previous research reported that Tenericutes also was observed in the gut microbial community in fish, and that was significantly increased after feeding with plant ingredients, which exerted negative effects on intestinal health in the golden pompano fish.26 Evidence suggests that the ratio of the Firmicutes/Bacteroides is correlated positively with body weight in mammals.27 Hence, we suggest that a reduction of Firmicutes/Bacteroidetes ratio in the acidified group indicates that whelk intestinal fat decreases under OA stress, which might be due to abstention from feeding or inadequate food intake.
At the genus level, Mycoplasma (Phylum Tenericutes, Class Mollicutes) was found in higher numbers in the digestive tract of whelk B. ternatanus in the EP group, which might be sensitive to environmental stress. Although Mycoplasma is considered a parasite or pathogen in vertebrates, its function is unknown in some mollusks, such as the snail R. venosa.28 Furthermore, this change in abundance was similar to the increase in the relative abundance of mycoplasma in oysters subjected to acidifying conditions.24 Meanwhile, the dominant Mycoplasma and Bacteroides showed a strong positive correlation with LZM and a negative correlation with other enzymes under acidification conditions. In contrast, Burkholderia-Caballeronia-Paraburkholderia with reduced relative abundance only showed a significant positive correlation with LZM. Therefore digestion-related enzymes were inhibited, and immune enzymes were activated in the EP group. These results suggest that changes in digestion-related enzymes are influenced by decreased pH. OA exposure can alter the composition of the microbiota, suggesting that the gut microbiota plays a very important role in OA stress in B. ternatanus. Furthermore, acidified conditions in artificial simulations may make animals more sensitive to disease than natural seawater, so it needs further confirmation. More research is necessary to determine how the pH reduction directly affects the gut samples’ bacterial populations.
In conclusion, seawater acidification causes structural damage and inhibition of enzymatic activity in the gut and affects the composition of the microbial community. An increase in functional taxa, including Tenericutes, and Bacteroidetes, can inhibit the absorption of nutrients from the animal’s gut, increase the growth of potential pathogens, and increase the susceptibility of organisms to disease. These effects may result in reduced energy intake of shellfish, thereby reducing their growth rate and thus posing a health challenge to whelks.
Acknowledgments
The Science Foundation of Hainan Province funded this research grant number 320QN366, the Key Research and Development Project of Hainan Province grant number ZDYF2021XDNY182, and grant number ZDYF2020176.



_of_the_bacterial_community_of_*b.ternatanus*_(n___4).jpeg)




_of_the_bacterial_community_of_*b.ternatanus*_(n___4).jpeg)